Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A strained alkyne-containing bipyridine reagent; synthesis, reactivity and fluorescence properties†
Sam Forshaw a,
Richard C. Knighton
a,
Richard C. Knighton a,
Jami Rebera,
Jeremy S. Parker
a,
Jami Rebera,
Jeremy S. Parker b,
Nikola P. Chmel
b,
Nikola P. Chmel a and
Martin Wills
a and
Martin Wills *a
*a
aDepartment of Chemistry, The University of Warwick, Coventry, CV4 7AL, UK. E-mail: m.wills@warwick.ac.uk
bEarly Chemical Development, Pharmaceutical Sciences, IMED Biotech Unit, AstraZeneca, Macclesfield, SK10 2NA, UK
First published on 6th November 2019
Abstract
We report the synthesis of a bipyridyl reagent containing a strained alkyne, which significantly restricts its flexibility. Upon strain-promoted alkyne-azide cycloaddition (SPAAC) with an azide, which does not require a Cu catalyst, the structure becomes significantly more flexible and an increase in fluorescence is observed. Upon addition of Zn(II), the fluorescence is enhanced further. The reagent has the potential to act as a fluorescent labelling agent with azide-containing substrates, including biological molecules.
Introduction
Zinc is a vital trace element and is required in the basic cellular function of many biological organisms.1 In humans, proteins that bind zinc account for ca. 10% of all proteins,2 and zinc is the only transition metal found across all classes of enzymes.3 It is essential for important processes such as gene expression,4 neurotransmission,5 regulation of apoptosis6 and in reproduction.7 Maintenance of the correct intracellular levels of zinc is clearly requisite for critical cell functions. Indeed, misregulation of zinc in the brain is implicated in a range of neurological disorders such as epilepsy and Alzheimer's disease.4 Abnormal zinc levels are also intrinsically linked to cancer and are an important factor in apoptosis of malignant tumours.8 These factors have driven research into treatments or diagnostic tools for Zn(II), through the design of complexation agents and sensors. Molecules which modulate their fluorescence upon undergoing a reaction have the potential to selectively indicate the location of specific functional groups or analyte, for example azide- or alkyne-containing proteins or other synthetic molecules.9 Azido coumarin 1 is an example of a reagent which increases significantly in fluorescence upon cycloaddition with an alkyne.9a In cases where the partner is a strained alkyne,10 the cycloaddition reaction can take place without the need for a copper catalyst,11 which is highly advantageous in a biological setting due to its bioorthogonality (Scheme 1).11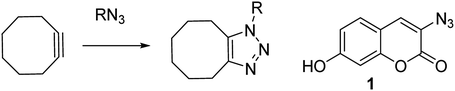 | ||
| Scheme 1 Uncatalysed reaction of azide with strained cyclooctyne, and a coumarin reagent which fluoresces upon undergoing a cycloaddition. | ||
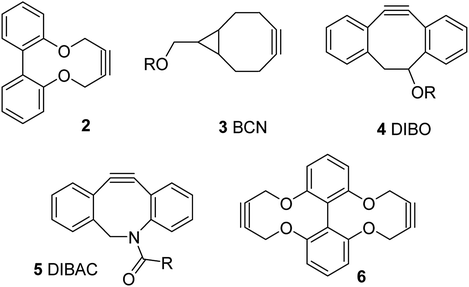
We, and others, have recently reported the synthesis and reactions of a strained alkyne of general structure 2, derived from a biphenol backbone (Fig. 1).12 Although not as reactive as the more strained commercial reagents such as 3–5,10,11 it is simple to prepare via a short sequence, reacts with azides without a metal catalyst and has been demonstrated to be capable of forming synthetically-useful derivatives. We have also reported a dialkyne version of this reagent, i.e. 6.12d
Although strained alkynes such as 3–5 can be functionalised with a fluorescent group,13 we felt that it would be advantageous to design a reagent which could potentially gain fluorescence upon undergoing the cycloaddition reaction. Toward this end we designed reagent 7, which contains a bipyridyl functional group, reasoning that its out-of-plane locking of the aryl rings by the inflexible alkyne would prevent fluorescence and eliminate the potential for metal chelation between the pyridiyl nitrogen atoms. However, upon cycloaddition with an azide, the rotation barrier about the biaryl C–C bond should be decreased, increasing the propensity to become co-planar and thus become weakly emissive, which has the potential to increase further upon chelation of a suitable metal ion such as Zn(II), which are known to be highly emissive.14 Related reagents based on the same dipyridyl scaffold have been reported,15 providing some precedent for our synthetic approach.
Results and discussion
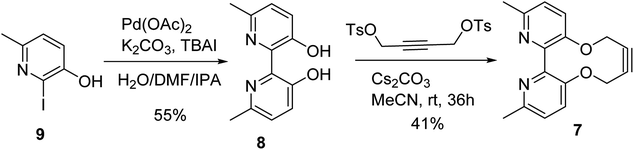
The target reagent, 7, was prepared through a two-step process by first forming the known 2,2′-bipyridyl-3,3′-diol 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 from iodopyridine 9 using a new protocol, and then cyclising the reagent through reaction with but-2-yne-1,4-diyl bis(4-methylbenzenesulfonate), following our established protocol, to give 7 in 41% yield. The 1H NMR spectrum of 7 exhibited diastereotopic CH2 resonances indicating conversion between atropisomers is slow on the NMR timescale (500 MHz, CDCl3, 298 K). Correspondingly, it was found that compound 7 was not an effective bidentate ligand, likely due to the large C–C biaryl torsional angle; our previous solid-state studies of biphenol strained alkynes evidence significantly non-planar C–C biaryl bond dihedral angles (Scheme 2).12a
16 from iodopyridine 9 using a new protocol, and then cyclising the reagent through reaction with but-2-yne-1,4-diyl bis(4-methylbenzenesulfonate), following our established protocol, to give 7 in 41% yield. The 1H NMR spectrum of 7 exhibited diastereotopic CH2 resonances indicating conversion between atropisomers is slow on the NMR timescale (500 MHz, CDCl3, 298 K). Correspondingly, it was found that compound 7 was not an effective bidentate ligand, likely due to the large C–C biaryl torsional angle; our previous solid-state studies of biphenol strained alkynes evidence significantly non-planar C–C biaryl bond dihedral angles (Scheme 2).12a
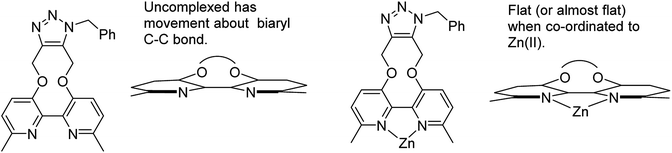
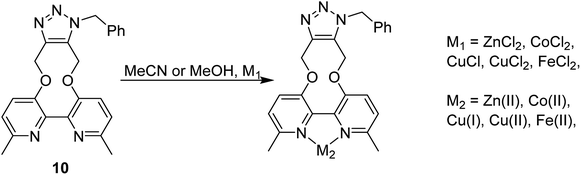
The cycloaddition of 7 with benzylazide was studied (Scheme 3), using 1H-NMR to follow the changes in situ. The reaction took place without the requirement for a copper catalyst, generating the cycloadduct 10 in high yield over several days. The adduct 10 was qualitatively more fluorescent than the 2,2′-bipyridyl strained alkyne 7, presumably due to the reduced rotational restriction upon cycloaddition. The cycloaddition in the presence of ZnCl2 was slightly faster than in its absence and gave a significantly emissive adduct. It is unclear as to how the Zn(II) accelerates the reaction however it is unlikely to chelate to the two pyridyl rings due to the likely wide torsion angle in the strained alkyne (vide supra).
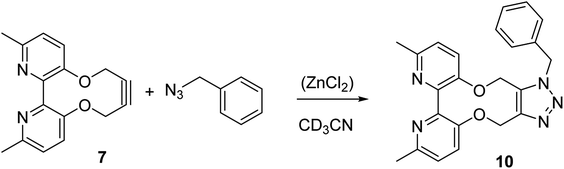 | ||
| Scheme 3 Cycloaddition of bipyridyl strained alkyne 7 with benzyl azide. The reaction was run with and without ZnCl2 in order to gauge its effect on the rate. | ||
The NMR spectra, recorded at time intervals, of the cycloaddition without ZnCl2 are overlaid in Fig. 2; the product CH2 resonances are broad, indicating that the detected hydrogen atoms are alternating between different conformations due to restricted rotations on the NMR time scale, as previously observed for cycloadducts of this type.12 A variable temperature 1H NMR study of 10 (500 MHz, CDCl3) revealed that the slow exchange of the atropisomers is reached at 243 K, contrasting 7 which is resolved at ambient temperature, indicating greater flexibility of 10, however coalescence was not reached at 323 K (ESI†).
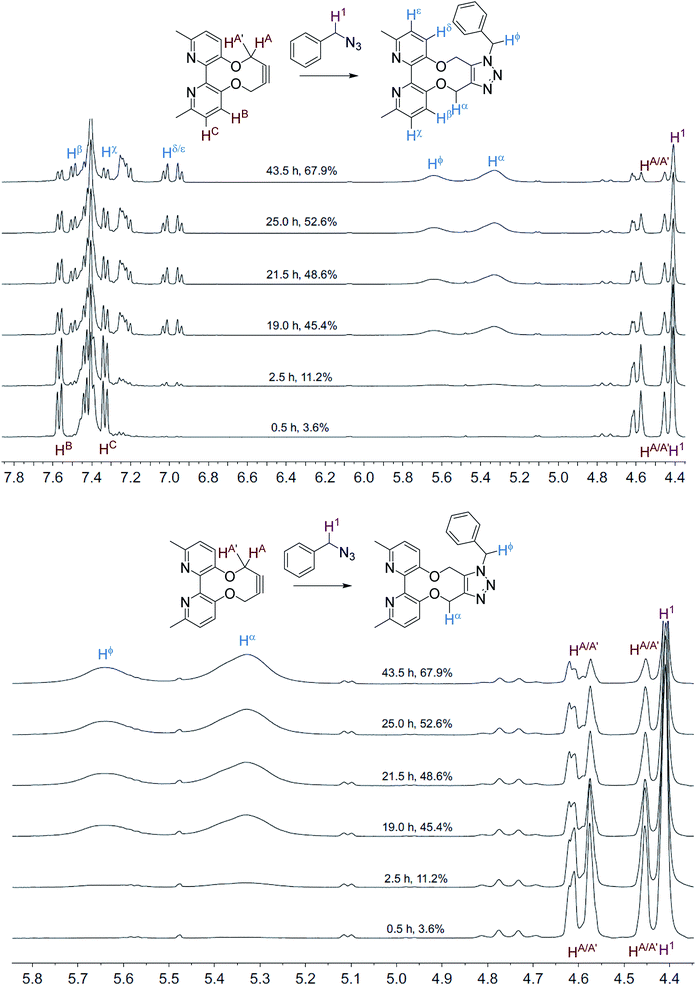 | ||
| Fig. 2 Overlaid 1H NMR spectra (400 MHz, CD3CN, 298 K) for the cycloaddition reaction in Scheme 3 without added ZnCl2. | ||
The NMR spectra recorded, at each time interval, of the ZnCl2-complexed cycloaddition reaction are similarly overlaid in Fig. 3. In this case the product peaks were sharp, with three singlets being formed, corresponding to each of the CH2 groups in the product. In an independent test, ZnCl2 (1.0 eq., CD3CN) was added to the uncomplexed NMR sample, resulting in sharp NMR peaks similar to the Zn(II)-complexed product 10 (Fig. 4).
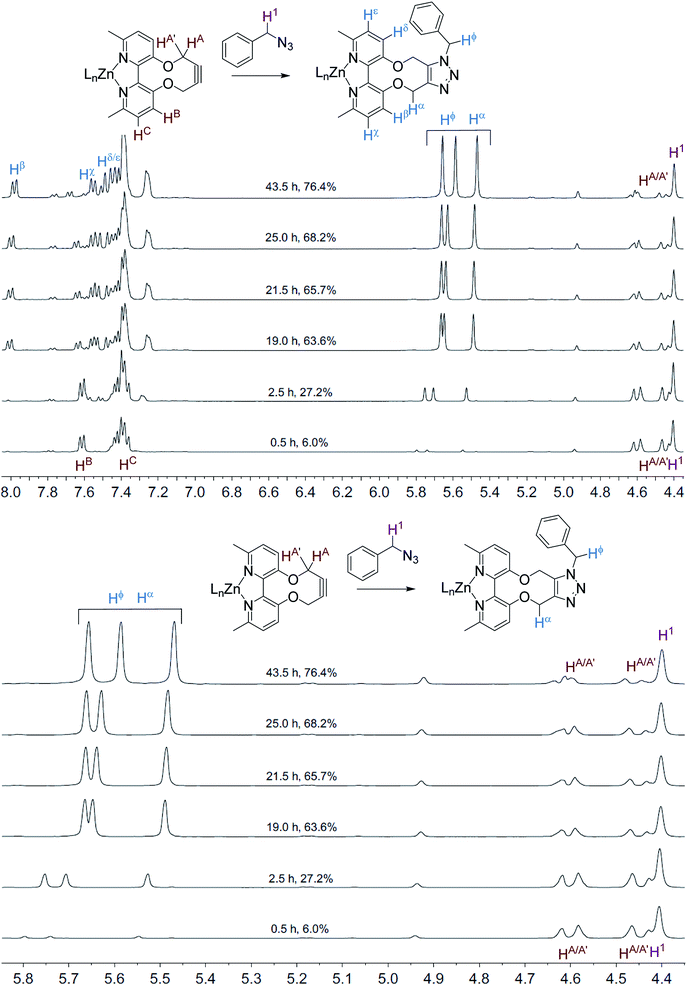 | ||
| Fig. 3 Overlaid 1H NMR spectra (400 MHz, CD3CN, 298 K) for the cycloaddition reaction in Scheme 3 with added ZnCl2. | ||
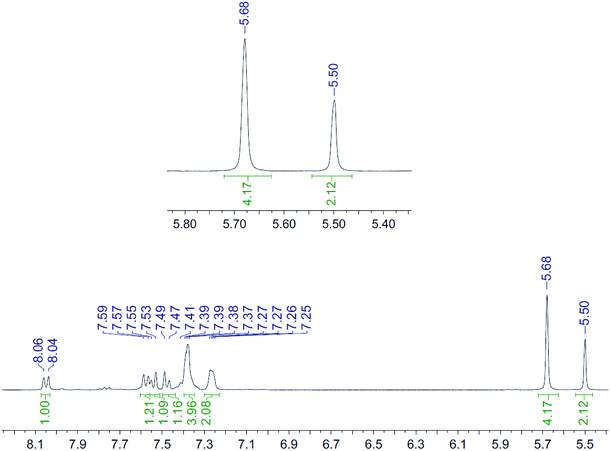 | ||
| Fig. 4 1H NMR spectrum (400 MHz, CD3CN, 298 K) for the uncomplexed addition product 10 after adding ZnCl2. | ||
It is unclear why the compound which had Zn(II) added before the cycloaddition reaction has three singlet peaks that each integrate to two hydrogens, while the compound which had Zn(II) added after the cycloaddition reaction has two singlet peaks, one of which integrates to four hydrogens and the other which integrates to two hydrogens. Since Zn(II) is able to form complexes with up to three bipyridyl compounds, this could be due to a different ratio of Zn(II) to bipyridyl in each sample.14 The observations indicate that the Zn(II) complexation is likely to be responsible for the change in appearance of the NMR spectrum, possibly due to the flattening of the ring and reduction of the degrees of freedom (Fig. 5).
Whilst this Zn(II) complexation with a bipyridyl strained alkyne cycloaddition adduct is novel, Zn(II) complexation with bipyridyl compounds, to create highly fluorescent adducts, has been previously studied in detail.14 Zn(II) complexation has been shown to increase fluorescence intensity, a property which could potentially be beneficial for fluorescence-based reagents. The effect of Zn(II) complexation on fluorescence was also observed on both the cycloaddition bipyridyl product 10 and the precursor bipyridyl diol 8. All samples were made to the same concentration (10−5 M), and values were recorded at the same parameters on the same instrument (Fig. 6). The strained alkyne reagent 7 was also studied for comparison purposes.
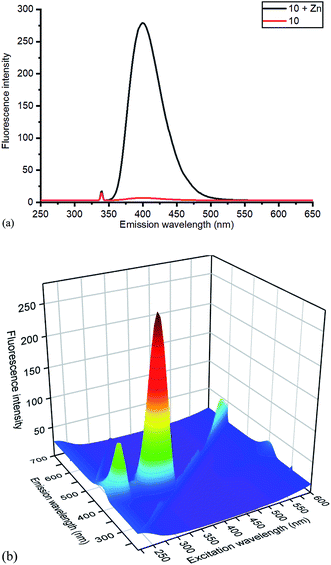 | ||
| Fig. 6 (a) Emission spectra of 10 without and with added ZnCl2, with excitation at 340 nm, and (b) 3D excitation vs. emission spectrum of 10/ZnCl2. The 3D spectrum of uncomplexed 10 is given in the ESI.† | ||
Based on relative intensity, cycloaddition product 10 showed a significant increase in fluorescence upon addition of ZnCl2 (Fig. 6) In contrast, addition of Zn(II) to the solution of 7 resulted in quenching of the fluorescence (ESI†). This indicated that chelation of the metal was required for increased fluorescence, since the rigid structure of 7 prevents this. After observing the increase in fluorescence with Zn(II) for cycloaddition bipyridyl product 10, it was complexed with several other metal salts to investigate the selectivity of the system. The 3D fluorescence spectra were recorded for each of the metal complexes (Scheme 4, Fig. 7) The 3D and contour excitation vs. emission spectra are given in the ESI†.
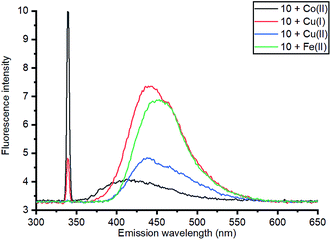 | ||
| Fig. 7 Comparison of emission of complexes of 10 with a range of metals at 340 nm excitation; 3D and contour excitation vs. emission spectra are given in the ESI.† | ||
The Zn(II)-complexed bipyridyl 10 exhibited a significantly higher fluorescence intensity compared to the other metal complexes; none of the other metals enhanced the fluorescence of the bipyridyl 10; moreover, the other metal complexes had lower emission intensities than even the uncomplexed bipyridyl 7. Table 1 summarises the combination of excitation and emission wavelength that gave the highest response as determined by 3D fluorescence spectroscopy, and the Stokes shifts. For comparison, the fluorescence of the ZnCl2-complexed and uncomplexed bipyridyl diol precursor 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 was also measured using 3D fluorescence spectroscopy (Fig. 8).
17 was also measured using 3D fluorescence spectroscopy (Fig. 8).
| Excitation λmax (nm) | Emission λmax (nm) | Intensity | Shift/nm | |
|---|---|---|---|---|
| a Spectra were recorded at a concentration of 10−5 M in MeCN. | ||||
| Uncomplexed 10 | 295 | 379 | 23 | 85 |
| 10 Zn II complex | 341 | 400 | 279 | 59 |
| 10 Cu I complex | 340 | 439 | 7 | 99 |
| 10 Cu II complex | 345 | 438 | 5 | 93 |
| 10 Co II complex | 325 | 450 | 6 | 75 |
| 10 Fe II complex | 335 | 450 | 8 | 115 |
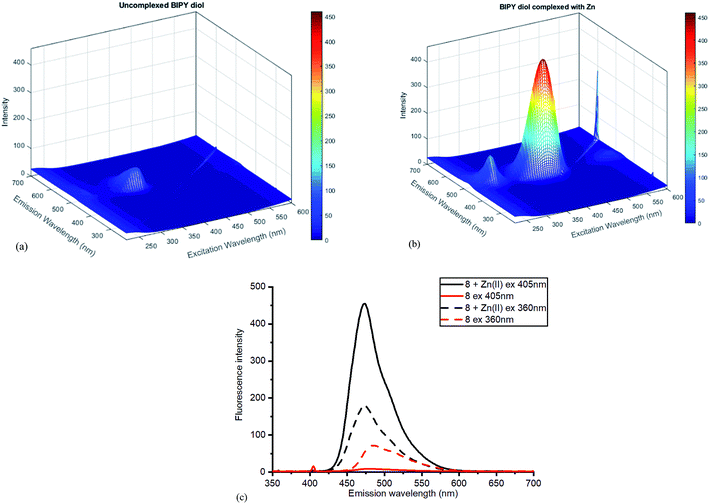 | ||
| Fig. 8 Fluorescence spectra for diol 8 (a) without added ZnCl2 (b) with added ZnCl2. (c) Contrasting emissions at 405 and 360 nm excitation with and without added Zn(II). | ||
Once again, the Zn(II) complexation caused a dramatic increase in the fluorescent intensity of the compound, although the selection of excitation wavelength was crucial in establishing the sharpest difference between the complexed and uncomplexed emission. With excitation at 405 nm, sharply contrasting emission results were obtained compared to those at 360 nm excitation, where little difference was observed (Fig. 8c). The high fluorescence reflects efficient chelation of the Zn(II) by diol 8.
Conclusion
In conclusion, we have prepared a new strained alkyne based on a bipyridyl dioxy backbone and have applied this to strain-promoted cycloaddition reactions with benzyl azide. Metal complexation is precluded on the alkyne compound 7 due to the rigidity and non-coplanarity of the bipyridine. Upon triazole formation, product 10 increases in conformational flexibility, allowing coordination to a range of transition and alkaline earth metals, as evidence by photophysical studies. Importantly, the fluorescence intensity increases significantly when Zn(II) salts are added to it, which is selective across the ranges of metals studied.Data sharing statement
The research data (and/or materials) supporting this publication can be accessed at http://wrap.warwick.ac.uk/.Conflicts of interest
The authors declare no conflicting interests.Acknowledgements
We thank the EPSRC for support of RCK (EPSRC Project Grant EP/M006670/1) and EPSRC and Astrazeneca for support of SF through a National Productivity Investment Fund studentship. The authors thank Professor Mike Ward for helpful discussions.References
-
(a) S.-H. Choi, K.-L. Lee, J.-H. Shin, Y.-B. Cho, S.-S. Cha and J.-H. Roe, Nat. Commun., 2017, 8, 15812 CrossRef
; (b) J. M. Goldberg, F. Wang, C. D. Sessler, N. W. Vogler, D. Y. Zhang, W. H. Loucks, T. Tzounopoulos and S. J. Lippard, J. Am. Chem. Soc., 2018, 140, 2020–2023 CrossRef CAS
.
- C. Andreini, L. Banci, I. Bertini and A. Rosato, J. Proteome Res., 2006, 5, 196–201 CrossRef CAS
.
- M. R. Broadley, P. J. White, J. P. Hammond, I. Zelko and A. Lux, New Phytol., 2007, 173, 677–702 CrossRef CAS
.
- J. Peng, W. Xu, C. L. Teoh, S. Han, B. Kim, A. Samanta, J. C. Er, L. Wang, L. Yuan, X. Liu and Y.-T. Chang, J. Am. Chem. Soc., 2015, 137, 2336–2342 CrossRef CAS
.
- Y.-N. Wang, D. Figueiredo, X.-D. Sun, Z.-Q. Dong, W.-B. Chen, W.-P. Cui, F. Liu, H.-S. Wang, H.-W. Li, H. Robinson, E.-K. Fei, B.-X. Pan, B.-M. Li, W.-C. Xiong and L. Mei, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 2508–2513 CrossRef CAS
.
- P. Makhov, K. Golovine, R. G. Uzzo, J. Rothman, P. L. Crispen, T. Shaw, B. J. Scoll and V. M. Kolenko, Cell Death Differ., 2008, 15, 1745–1751 CrossRef CAS
.
- K. Kerns, M. Zigo, E. Z. Drobnis, M. Sutovsky and P. Sutovsky, Nat. Commun., 2018, 9, 2061 CrossRef
.
-
(a) R. B. Franklin and L. C. Costello, J. Cell. Biochem., 2009, 106, 750–757 CrossRef CAS
; (b) J. S. Cristóvão, V. K. Morris, I. Cardoso, S. S. Leal, J. Martínez, H. M. Botelho, C. Göbl, R. David, K. Kierdorf, M. Alemi, T. Madl, G. Fritz, B. Reif and C. M. Gomes, Sci. Adv., 2018, 4, 1702 CrossRef
.
-
(a) M. T. Gokmen, J. Brassinne, R. A. Prasath and F. E. Du Prez, Chem. Commun., 2011, 47, 4652–4654 RSC
; (b) J.-J. Shie, Y.-C. Liu, J.-C. Hsiao, J.-M. Fang and C.-H. Wong, Chem. Commun., 2017, 53, 1490–1493 RSC
; (c) C. Favre and F. Friscourt, Org. Lett., 2018, 20, 4213–4217 CrossRef CAS
; (d) E. Decuypère, M. Riomet, A. Sallustrau, S. Bregant, R. Thai, G. Pieters, G. Clavier, D. Audisio and F. Taran, Chem. Commun., 2018, 54, 10758–10761 RSC
.
-
(a) J. Dommerholt, F. P. J. T. Rutjes and F. L. van Delft, Top. Curr. Chem., 2016, 374, 16 CrossRef
; (b) C. G. Gordon, J. L. Mackey, J. C. Jewett, E. M. Sletten, K. M. Houk and C. R. Bertozzi, J. Am. Chem. Soc., 2012, 134, 9199–9208 CrossRef CAS
.
-
(a) Y. Gong and L. Pan, Tetrahedron Lett., 2015, 56, 2123–2332 CrossRef CAS
; (b) E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974–6998 CrossRef CAS
; (c) P. Thirumurugan, D. Matosiuk and K. Jowiak, Chem. Rev., 2013, 113, 4905–4979 CrossRef CAS
; (d) C. S. McKay and M. G. Finn, Chem. Biol., 2014, 21, 1075–1101 CrossRef CAS
.
-
(a) A. Del Grosso, L.-D. Galanopoulos, C. K. C. Chiu, G. J. Clarkson, P. B. O'Connor and M. Wills, Org. Biomol. Chem., 2017, 15, 4517–4521 RSC
; (b) A. Mistry, R. C. Knighton, S. Forshaw, Z. Dualeh, J. S. Parker and M. Wills, Org. Biomol. Chem., 2018, 16, 8965–8975 RSC
; (c) T. Harris, G. d. P. Gomes, S. Ayad, R. J. Clark, V. V. Lobodin, M. Tuscan, K. Hanson and I. V. Alabugin, Chem, 2017, 3, 629–640 CrossRef CAS
; (d) R. C. Knighton, K. Sharma, N. S. Robertson, D. R. Spring and M. Wills, ACS Omega, 2019, 4, 2160–2167 CrossRef CAS
; (e) T. Harris and I. V. Alabugin, Mendeleev Commun., 2019, 29, 237–248 CrossRef CAS
.
-
(a) M. Boudiemeline, C. D. McNitt, T. A. Singleton, V. V. Popik and A. P. Kostikov, Org. Biomol. Chem., 2018, 10, 363–366 RSC
; (b) J. Dommerholt, S. Schmidt, R. Temming, J. J. A. Hedriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422–9425 CrossRef CAS
; (c) J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS
; (d) X. Ning, J. Guo, M. Wolfert and G.-J. Boons, Angew. Chem., Int. Ed., 2008, 47, 2253–2255 CrossRef CAS
.
-
(a) S. N. Ghosh, Inorg. Nucl. Chem. Lett., 1969, 5, 841–843 CrossRef
; (b) W. L. Turnbull and L. G. Luyt, Chem.–Eur. J., 2018, 24, 14539–14546 CrossRef CAS
; (c) L. Zhu, A. H. Younes, Z. Yuan and R. J. Clark, J. Photochem. Photobiol., A, 2015, 311, 1–15 CrossRef CAS
.
-
(a) K. Takaishi, J. Suzuki, T. Yabe, H. Asano, M. Nishikawa, D. Hashizume, A. Muranaka, M. Uchiyama and A. Yokoyama, Org. Lett., 2015, 17, 4098–4101 CrossRef CAS
; (b) T. Shimada, A. Kina and T. Hayashi, J. Org. Chem., 2003, 68, 6329–6337 CrossRef CAS
.
- A. Reynal, J. Etxebarria, N. Nieto, S. Serres, E. Palomares and A. Vidal-Ferran, Eur. J. Inorg. Chem., 2010, 1360–1365 CrossRef CAS
.
- G. Ulrich, F. Nastasi, P. Retailleau, F. Puntoriero, R. Ziessel and S. Campagna, Chem.–Eur. J., 2008, 14, 4381–4392 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR and fluorescence spectra. See DOI: 10.1039/c9ra06866j |
| This journal is © The Royal Society of Chemistry 2019 |