An investigation into the factors governing the oxidation of two-dimensional Ti3C2 MXene†
Yoonjeong
Chae
a,
Seon Joon
Kim‡
bc,
Soo-Yeon
Cho
de,
Junghoon
Choi
d,
Kathleen
Maleski
b,
Byeong-Joo
Lee
a,
Hee-Tae
Jung
 de,
Yury
Gogotsi
b,
Yonghee
Lee§
de,
Yury
Gogotsi
b,
Yonghee
Lee§
 *a and
Chi Won
Ahn§
*a
*a and
Chi Won
Ahn§
*a
aGlobal Nanotechnology Development Team, National Nano Fab Center (NNFC), Daejeon 34141, South Korea. E-mail: cwahn@nnfc.re.kr; yhlee@nnfc.re.kr
bDepartment of Materials Science and Engineering, and A.J. Drexel Nanomaterials Institute, Drexel University, Philadelphia, PA 19104, USA
cWearable Platform Materials Technology Center, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, South Korea
dDepartment of Chemical and Biomolecular Engineering (BK-21 Plus), Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, South Korea
eKAIST Institute for the NanoCentury, Daejeon 34141, South Korea
First published on 29th March 2019
Abstract
Two-dimensional (2D) transition metal carbides (MXenes) exhibit outstanding performances in many applications, such as energy storage, optoelectronics, and electrocatalysts. However, colloidal solutions of Ti3C2Tx MXene flakes deteriorate rapidly under ambient conditions due to the conversion of the titanium carbide to titanium dioxide. Here, we discuss the dominant factors influencing the rate of oxidation of Ti3C2Tx MXene flakes, and present guidelines for their storage with the aim of maintaining the intrinsic properties of the as-prepared material. The oxidation stability of the Ti3C2Tx flakes is dramatically improved in a system where water molecules and temperature were well-controlled. It was found that aqueous solutions of Ti3C2Tx MXene can be chemically stable for more than 39 weeks when the storage temperature (−80 °C) is sufficiently low to cease the oxidation processes. It was also found that if the Ti3C2Tx flakes are dispersed in ethanol, the degradation process can be significantly delayed even at 5 °C. Moreover, the oxidation stability of the Ti3C2Tx flakes is dramatically improved in both cases, even in the presence of oxygen-containing atmosphere. We demonstrate practical applications of our approach by employing Ti3C2Tx in a gas sensor showing that when oxidation is inhibited, the device can retain the original electrical properties after 5 weeks of storage.
Introduction
In the past decade, two-dimensional (2D) materials have received increased attention because of their outstanding performances in electronic devices such as gas sensors and1,2 energy storage3–5 and as transparent conductive electrodes.6,7 MXenes are a new family of 2D materials that consist of a few atomic layers of transition metal carbides or carbonitrides and are selectively etched from the bulk MAX phase in an acid etchant solution; MAX (Mn+1AXn) consists of M, which is an early transition metal, A, which is a group of 13 or 14 elements, and X, which is carbon and/or nitrogen.8–12 To obtain Ti3C2Tx from the Ti3AlC2 MAX phase, the Al atoms are removed by wet chemical etching with HF or LiF/HCl or with an etchant containing hydroxides.3,13–15 The surfaces of MXenes are terminated by –OH, –F, or –O groups, which is denoted as Tx in the formula Mn+1XnTx. In general, MXenes are stored in deionized (DI) water because exfoliated individual flakes are hydrophilic and easily form an aqueous colloidal suspension. However, it has been experimentally confirmed that when Ti3C2Tx is stored in water for long periods, oxygen dissolved in water and/or in air acts as an oxidizing agent that induces the conversion of titanium carbide to its oxidized form (titanium dioxide, TiO2).16–18 The chemical stability of Ti3C2Tx MXene limits its potential in applications where longevity of materials is required. Although it has been demonstrated that serious problems arise due to the oxidation of electronic materials, including MXenes, investigations into the mechanism of oxidation and methods to prevent Ti3C2Tx MXene oxidation are necessary to understand and control the degradation. Previous reports have shown that storing Ti3C2Tx MXene solutions in organic solvents, Ar-filled bottles, and polymer matrices, and at lower temperatures (≤5 °C or freeze dried) slows the oxidation process, hypothesized to be because the solutions exposed to less dissolved oxygen were stable longer.16–19 Recently, it has been shown that the stability of Ti2CTx and Ti3C2Tx was improved when materials were dispersed in isopropyl alcohol (IPA), displaying that dissolved oxygen (O2) may not be as detrimental to oxidation as reactions with water, revealing that the mechanism is more complex than that was originally proposed.16In this study, we investigated the dominant factors governing the oxidation of two-dimensional Ti3C2Tx MXene. We present an effective storage system where the water and temperature environment were controlled, and the oxidation rate was significantly slowed. Due to the previous reports showing that the oxidation reaction rate is slower at low temperatures,20 we followed these protocols by storing the Ti3C2Tx MXene solutions at low temperatures. In addition, we developed two methods for the storage of MXene that do not require degassing or purging and that also minimize the influence of water. First, we kept air-filled bottles of Ti3C2Tx aqueous solution in a refrigerator and at controlled temperatures of 5 °C, −18 °C, and −80 °C (samples named D@5, D@-18, and D@-80; D meaning DI water). The water molecules were quickly frozen and converted to ice at the cryogenic temperature (5 °C is a normal cold storage temperature, −18 °C is a normal freezing temperature, and −80 °C is a typical cryogenic temperature). In another method, the MXenes were stored at 5 °C in anhydrous ethanol (sample named E@5, E meaning ethanol). Furthermore, we examined the effects of humidity and temperature on the solidified Ti3C2Tx prepared from these two stored solutions by fabricating films through vacuum-assisted filtration. We also assessed the stability of the Ti3C2Tx colloids and films by performing scanning electron microscopy (SEM), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), energy dispersive spectrometry (EDS), and dynamic light scattering (DLS). To probe the longevity of an electronic device, we evaluated Ti3C2Tx MXene gas sensor device performance tests over the course of 5 weeks.
Results and discussion
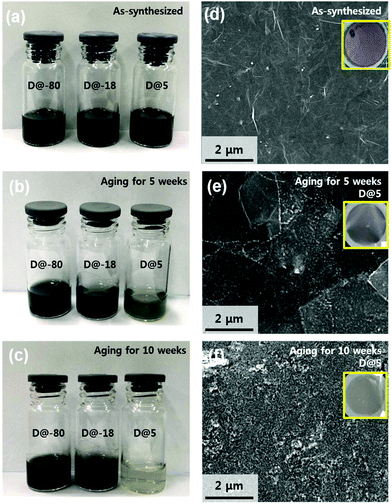
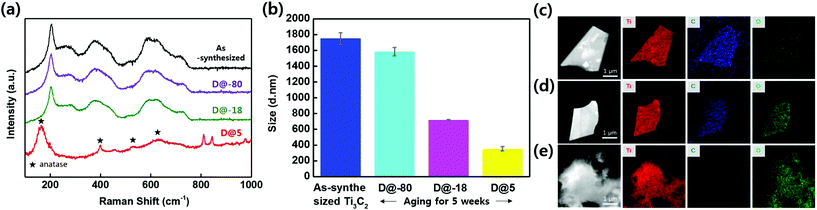
Fig. 1a shows an optical image of the as-synthesized Ti3C2Tx solutions that display a dark colour. The Ti3C2Tx solutions were stored in air at 5 °C, −18 °C, and −80 °C. Fig. 1b shows an optical image of aged Ti3C2Tx solutions stored at 5 °C, −18 °C, and −80 °C for 5 weeks; the colour of solutions stored at −18 °C, and −80 °C retained their dark colour, suggesting that oxidation was minimized. On the other hand, the colour of the solution stored at 5 °C starts to change, suggesting that oxidation has started and the concentration of Ti3C2Tx has started to decrease (Fig. S1†). Furthermore, Fig. 1c shows an optical image of aged Ti3C2Tx solutions stored at 5 °C, −18 °C, and −80 °C for 10 weeks; the colour of the solution stored at 5 °C has become almost transparent. Scanning electron microscopy (SEM) of vacuum filtered films was utilized to compare the extent of oxidation under each experimental condition (Fig. 1d–f, inset shows films). Fig. 1d displays the expected morphology of a vacuum filtered Ti3C2Tx film. As shown in Fig. 1e, the solution stored at 5 °C has undergone oxidation, i.e. the filtered film has a light green film morphology (inset Fig. 1e) and SEM reveals that the flakes have white edges (TiO2 particles forming). As shown in Fig. 1f, the solution D@5 for 10 weeks was heavily oxidized and the SEM exposes a rough film surface. From these observations, it can be seen that the oxidation of D@5 solution proceeds slowly from the as-synthesized solution, degrading over the course of 10 weeks; however, the materials stored at D@-18 and D@-80 appear different.Fig. 2a displays the integrated Raman spectra of the as-synthesized solution and solutions stored for 5 weeks at −80 °C, −18 °C, and 5 °C. The Ti3C2Tx monolayer modes at 228 cm−1 and 599 cm−1 correspond to the out-of-plane stretching vibrations of Ti2C and C, respectively.21 Please note that the measurement was based on the 5 weeks of storage when the oxidation began to appear.
In particular, the storage for 5 weeks of the solution at 5 °C results in the formation of anatase, as is evident from the peak at approximately 156 cm−1, and the three other peaks at 399 cm−1, 528 cm−1, and 630 cm−1, which are assigned to the Eg(1), B1g(1), and A1g and Eg(3) vibrational modes of TiO2, respectively.19 Thus, the Raman analysis contains peaks due to TiO2 and disordered carbon.22Fig. 2b shows the hydrodynamic sizes of the flakes of Ti3C2Tx in the solutions after storage for 5 weeks; these results confirm that the flake size decreases from approximately 1700 nm to 300 nm when the Ti3C2Tx flakes are oxidized. TiO2 particles form on the edges of the flakes, and then the particles gradually spread over the surface as oxidation proceeds. The oxidized components gradually detach from the original flakes and the flakes become smaller. Therefore, our measurements of the flake size demonstrate that the Ti3C2Tx flakes stored at 5 °C are oxidized and that the flakes stored for 5 weeks at −80 °C undergo oxidation more slowly. The as-prepared Ti3C2Tx flakes typically have an intact morphology and uniform distributions of Ti, C, and O elements, as shown in Fig. 2c. After storing Ti3C2Tx for 5 weeks at −80 °C, the flakes were examined with EDS to identify any changes in the chemical composition. The TEM image in Fig. 2d shows that the surface of this Ti3C2Tx sample is clean and that the elements Ti, C, and O are still evenly distributed.19 However, Fig. 2e shows that the Ti3C2Tx stored at 5 °C for 5 weeks has undergone severe oxidation and that agglomerated particles have formed on its surface. The titanium and oxygen elements are preserved during this process, but the carbon is slowly removed through the conversion of Ti3C2Tx to TiO2.23
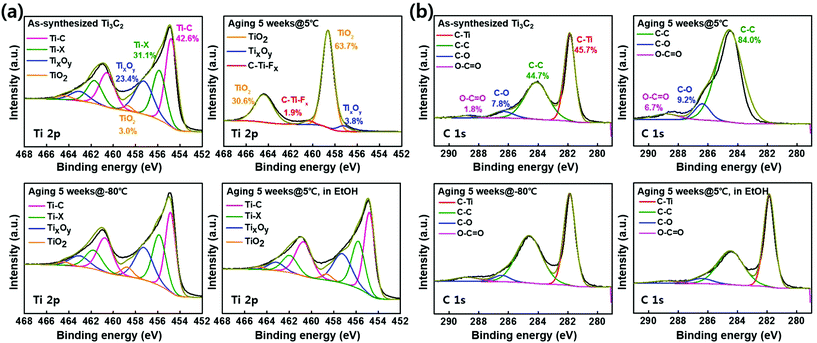
We investigated how the water and oxygen dissolved in solution influence the surface morphologies and components of the films. The Ti3C2Tx solutions stored for 5 weeks were examined by XPS to assess changes in the surface chemical bonding during aging. The Ti 2p components at 454.7, 455.8, 457.2, and 458.6 eV shown in Fig. 3a can be assigned to Ti–C, Ti–X, TixOy, and TiO2, respectively.24–26 These XPS data show that the freshly prepared Ti3C2Tx barely shows the TiO2 peak but the Ti3C2Tx solution stored at 5 °C produces a remarkable TiO2 peak. That is, Ti–C bonding of 42.6% was not detected after 5 weeks and TiO2 is quantitatively confirmed to increase from 3.0% to 94.3%. The C 1s components at 281.8, 284.1, 286.3, and 288.8 eV shown in Fig. 3b can be assigned to C–Ti, C–C, C–O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.26,27 The MXenes stored at −80 °C, 5 °C in ethanol and the as-synthesized MXene show C–Ti bonding of 45.7% approximately but MXene stored at 5 °C did not show the C–Ti bonding.
O, respectively.26,27 The MXenes stored at −80 °C, 5 °C in ethanol and the as-synthesized MXene show C–Ti bonding of 45.7% approximately but MXene stored at 5 °C did not show the C–Ti bonding.
Also, the amount of C–C bonding represents 84.0% in MXene stored at 5 °C. As the oxidation of the Ti3C2Tx sample stored in water at 5 °C progresses, the MXene flakes are converted to TiO2.28 According to the Arrhenius equation,20 the amounts of water and oxygen that come into contact with the Ti3C2Tx flakes per unit time depend on the storage temperature. At −80 °C, the amount of liquid water in the solution with access to the Ti3C2Tx per unit time is small and the diffusion rate of oxygen is low.29 Whereas, Ti3C2Tx flakes of D@5 could more frequently contact with water or dissolved oxygen than those stored at −80 °C, resulting in the oxidation of the flakes. Therefore, the storage of Ti3C2Tx solutions at extremely low temperatures can ensure reproducibility for maintaining the characteristics of the flakes. In addition, deconvoluted O 1s XPS data and integrated XPS data according to temperature conditions are also shown as insets in Fig. S2 and S3.†
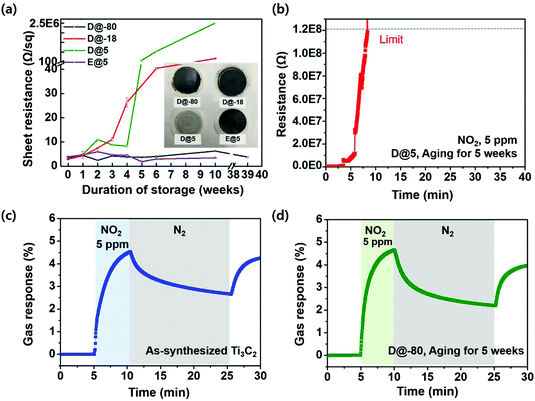
To probe the longevity of materials over time, D@-80, D@-18 and D@5 samples were fabricated into a film to measure the sheet resistances and perform gas sensor tests. Our experimental results for the sheet resistances are shown in Fig. 4a and Fig. S4.† As mentioned above, the sheet resistance of the film prepared from a D@5 solution for 10 weeks increased to approximately 2.5 × 106 Ω sq−1; the resistance ratio (R/R0) reached 3 × 105 after 10 weeks. Typically, Ti3C2Tx rapidly oxidizes under an atmosphere with abundant moisture and oxygen. As MXenes are typically well-dispersed in water, the functional groups on the surface of Ti3C2Tx react with water to form TiO2 products, and the water molecules trapped between the Ti3C2Tx thin films can accelerate the oxidation of the MXene.
On the other hand, the sheet resistance of Ti3C2Tx solutions stored at −80 °C was almost constant even after storage for up to 39 weeks, displaying the importance of storing the materials in a controlled environment. Thus, the oxidation rate of a Ti3C2Tx solution over time can be reduced, even though the solution is stored in air, by using a cryogenic storage method, which eliminates the effect of the liquid water. Interestingly, at a temperature of −18 °C, the sheet resistance increased from 2.9 Ω sq−1 to approximately 225 Ω sq−1 after the storage for 10 weeks, while the sheet resistance is kept constant for 39 weeks at −80 °C. The reason for the dramatic difference in the storage at −80 °C and −18 °C can be interpreted that the lower the temperature, the slower the oxidation reaction occurs (lower diffusion rates of oxidants).29 Besides, the lower the environmental temperature, the faster the freezing rate, and generally, the more the crystal disorder becomes. Therefore, we believe that it is hard for the oxidants to penetrate into the Ti3C2Tx MXene in the disordered crystals (−80 °C).30–34
To further investigate the influence of water, additional experiments were conducted on dispersions of Ti3C2Tx in an anhydrous organic solvent. The reason why Ti3C2Tx stored in EtOH can maintain sheet resistance for 10 weeks is because the amount of water in anhydrous organic solvent is negligible and the oxidation factor does not affect MXene (Fig. 4a, Fig. S5 and S6†). Another reason for the constant sheet resistance after the storage at 5 °C is the reduction in the contact area exposed to the environment, as particles were aggregated in ethanol. To extend our insights into the influence of water on the Ti3C2Tx flakes, the sheet resistances of the MXene thin films were measured during storage in various environments, as shown in Fig. S7 and S8.†
The MXene thin films were stored in four different storage environments, with different levels of oxygen and moisture, and the variations with time in the sheet resistance were measured. In this process, some samples were stored in an 80 °C oven to activate the air and others were placed in a wet environment to determine the effects of moisture. The RH values of the humid environment and the dry environment were approximately 95% and 40%, respectively. The humidity (r.h.) was measured by using a commercial sensor with a nominal detection range of 20–99% r.h. and an uncertainty of 0.1% r.h. The Ti3C2 MXene films are typically kept at room temperature (RT) and a humidity of 40% (h40%). However, the sheet resistance of Ti3C2Tx film D, stored for 5 weeks at RT–h40%, is approximately 10 times higher than the original resistance. Thus, these storage conditions are not appropriate for the preservation of electrical performance. In contrast, film B was stored at the same temperature as film D, but the water content was very high (h95%), so less than 5 days were required for the sheet resistance to increase tenfold. The sheet resistance of film B increased up to 8 × 104 in approximately 4 weeks. It is obvious that the sheet resistance undergoes a significant change at the high temperature from the comparison of the results for films A and C. The resistance of film A, which was stored at 80 °C-h95%, the highest temperature and humidity tested in this study, reached 2 × 105 in one day, which is a dramatic increase. Film C was stored at a high temperature but very low humidity, which results in a relatively slow increase in resistance. The films stored in a glovebox are completely protected from oxygen and moisture, so oxidation is unlikely.
To further examine the stability of Ti3C2Tx MXene samples stored under various conditions, we measured their gas sensing responses. To measure the resistance signal of each synthesized Ti3C2Tx sensor, a constant bias was applied to the two-probe-resistor-type sensor, and the changes in the electrical resistance of the sensor upon exposure to analytes were monitored and recorded as sensing signals. The sensors were simultaneously loaded into a home-made gas sensing chamber, and the sensing signals of each channel were measured with a multi-channel sensing system.35 The gas delivery system, which was fabricated in-house, is shown in detail in Fig. S9.† The gas sensing response was defined as the relative change in the channel resistance compared to the baseline resistance (100% × (R − Rb)/Rb). Sensing devices were fabricated by using the as-prepared D@-80, D@5 and E@5 Ti3C2Tx aged for 5 weeks. 5 ppm of NO2 gas was introduced into a N2-based environment; NO2 was used for comparison as it is easily detected by most gas sensing channel materials, including MXene.
D@5 samples aged for 5 weeks did not exhibit a meaningful gas response behavior since the sample resistance was higher than the sensing range (>120 M Ohm) (Fig. 4b). As shown in Fig. 4c, the gas response of the as-synthesized MXene toward 5 ppm NO2 was approximately 4.5%. The gas responses of the MXenes stored for 5 weeks in water vary significantly with the storage temperature. The D@-80 sample for 5 weeks was found to exhibit a gas response of approximately 4.5%, which is similar to that of the as-synthesized Ti3C2Tx MXene, as shown in Fig. 4d. The response speeds and recovery behaviors of these two samples are also similar. Since surface functionality and electrical conductivity both influence the gas response of the films, such results imply that both properties should be well-preserved when MXene is stored at −80 °C. These results show that a low storage temperature is crucial to the preservation of the original surface/electrical properties of MXene. For Ti3C2Tx dispersed in an anhydrous organic solvent (ethanol), a valid gas response toward NO2 was observed, but with a lower value (0.63%) than that of the as-prepared Ti3C2Tx MXene (Fig. S10†). We assume that this lower value is due to the adsorption of the organic content onto the Ti3C2Tx flake surface, which inhibits gas adsorption, or due to the inhibition of the diffusion of gas molecules by the intercalated organic molecules. Compared to dispersions in water, titanium carbides MXenes do not disperse in ethanol also, and the gas response may be affected by the aggregation of Ti3C2Tx MXene in ethanol.
Conclusions
In summary, although Ti3C2Tx MXene has outstanding electrical properties, the use of Ti3C2Tx is difficult because of its oxidation rate. In the attempt to solve these problems, we have identified the temperature and exposure to water molecules as the most important elements of oxidation. We have studied the dominant factors influencing the oxidation of Ti3C2Tx MXene flakes, and have presented guidelines for their storage to maintain the intrinsic properties of the as-prepared MXene. The oxidation stability of the Ti3C2Tx flakes is dramatically improved in the systems where the influence of water molecules and the temperature was well-controlled, even in the presence of oxygen-containing air (up to 39 weeks). With their superior performance and long lifetimes, our Ti3C2Tx MXenes and the storage system will have a broad range of applications in research into MXenes.Experimental sections
List of materials
Ti powders (Yee Young Cerachem, 99.5%, 1–2 μm), TiC powders (Yee Young Cerachem, 99.5%, <45 μm), Al powders (Yee Young Cerachem, 99%, 35–45 μm), LiF (Aldrich, 300 mesh), hydrochloric acid (Daejung, 35%), ethyl alcohol, pure (Aldrich, ≥99.5%), Anodisc inorganic filter membrane (AAO, pore diameter 0.2 μm, Whatman), and polycarbonate membrane (PC, 0.1 μm pore size, Whatman) were used.Preparation of Ti3C2Tx
Ti3AlC2 was prepared by ball-milling the TiC, Ti, and Al powders in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 18 h with zirconia balls. The mixture was prepared under a pressure of 7 MPa for 30 s to create a 2 g pellet (see Fig. S11†). The pellets were heated to 1450 °C for 3 h under argon. The compact pellet was then crushed by using a mortar and pestle. Roughly, 2 g of each powder was sieved with a 74 μm mesh to prepare a fine powder. 2 g of lithium fluoride (LiF) was dissolved in 20 mL of 9 M LiF/HCl, then 2 g of the fine powder (≤74 μm) was slowly poured into the solution over a few minutes. This solution was stirred continuously (350 rpm) for 24 h at room temperature. The resulting Ti3C2Tx product was shaken by using a shaker (FINEPCR, Multi shaker 3D-300) for 1 h and centrifuged at 5000 rpm for 10 min. Ti3C2Tx solution was washed using deionized water until the pH ≈ 7. To obtain multilayered Ti3C2Tx, two grams of MXene were added to 100 mL of deionized water, then the solution was shaken for 1 h with a shaker again. The resulting Ti3C2Tx product was centrifuged at 5000 rpm for 5 min and this process was once again carried out with the obtained suspension.
1 for 18 h with zirconia balls. The mixture was prepared under a pressure of 7 MPa for 30 s to create a 2 g pellet (see Fig. S11†). The pellets were heated to 1450 °C for 3 h under argon. The compact pellet was then crushed by using a mortar and pestle. Roughly, 2 g of each powder was sieved with a 74 μm mesh to prepare a fine powder. 2 g of lithium fluoride (LiF) was dissolved in 20 mL of 9 M LiF/HCl, then 2 g of the fine powder (≤74 μm) was slowly poured into the solution over a few minutes. This solution was stirred continuously (350 rpm) for 24 h at room temperature. The resulting Ti3C2Tx product was shaken by using a shaker (FINEPCR, Multi shaker 3D-300) for 1 h and centrifuged at 5000 rpm for 10 min. Ti3C2Tx solution was washed using deionized water until the pH ≈ 7. To obtain multilayered Ti3C2Tx, two grams of MXene were added to 100 mL of deionized water, then the solution was shaken for 1 h with a shaker again. The resulting Ti3C2Tx product was centrifuged at 5000 rpm for 5 min and this process was once again carried out with the obtained suspension.
Fabrication of the Ti3C2Tx MXene film
To confirm the sheet resistance of a Ti3C2Tx film, the films were fabricated via the vacuum filtration process of 1.5 mL of Ti3C2Tx solutions (0.2 mg ml−1) on a PC (polycarbonate, 0.1 μm pore size) filter membrane. Then, the sheet resistances of films prepared from the solutions stored at each temperature were measured every 7 days.Ti3C2Tx film fabrication and transfer onto a sensor device
To fabricate sensor devices using the Ti3C2Tx samples, 70 nm thick Au electrodes with a pre-deposited 5 nm thick adhesion layer of Cr, a spacing of 100 μm, and a width of 100 μm were deposited with e-beam evaporation by using a customized mask on SiO2/Si substrates. Thin films were prepared from the Ti3C2Tx solutions by performing vacuum filtrations through porous Anodisc inorganic filter membranes (AAO). The filtered Ti3C2Tx films on AAO membranes were placed in a vacuum oven to dry at 60 °C for 1 hour, and then floated on 2 M NaOH solution. The Ti3C2Tx membrane floats free on the NaOH solution because the AAO film gradually dissolves in the NaOH solution. We transferred the prepared Ti3C2Tx membranes onto SiO2 wafers, after washing with DI water. The MXenes on the SiO2 wafers were dried in a vacuum oven at 60 °C for 1 h.1Gas sensor device fabrication and measurements
The sensing devices were mounted in a sensing chamber designed to measure resistance signals by using a data acquisition module (Agilent 34970A). NO2 gas was passed through the sensing chamber. A gas delivery system designed in-house was used to control the gas flow into the sensing chamber to measure the sensors’ responses to the analytes. The serial dilution system used to vary the analyte concentration in the range of 0.125 to 1000 ppm consisted of a mass flow controller (MFC, Brooks 5850E), Teflon tubing (PFA, 1/8′′), Lok-type fittings, and a system of valves. N2 was used as the reference gas, and the total flow rate for the reference gas and tested analytes was maintained at 400 sccm. The injection times of the analytes and N2 were 5 min and 15 min, respectively. The dimensions of the sealed gas sensing chamber were approximately 10 cm (width), 5 cm (length), and 8 mm (height).Characterization of the products
A scanning electron microscope (SEM, FEI, Sirion) and a transmission electron microscope (TEM, JEOL Ltd, JEM-3011HR) were used to obtain high-magnification images of the surfaces of the as-synthesized and oxidized Ti3C2Tx. Raman spectroscopy (NOST, FEX) was used to confirm the production of Ti3C2Tx. Energy-dispersive X-ray spectroscopy (EDS, JEM-ARM200F) images were obtained to assess changes in the surfaces. Dynamic light scattering (DLS, Malvern, Zetasizer Nano ZS) was used to determine the sizes of the flakes. X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, K-Alpha) was used to characterize the chemical compositions of the flakes. A four-point probe station (AIT, CMT-100MP) was used to determine the sheet resistances of the flakes.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Natalia Noriega (Drexel University) for helpful comments on the manuscript. This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (NRF-2015K1A4A3047100, NRF-2015M3A7B6027973, and NRF-2015M3A7B7046618). This work was supported by the Open Innovation Program funded by NNFC (PCOI1801M004).Notes and references
- S. J. Kim, H.-J. Koh, C. E. Ren, O. Kwon, K. Maleski, S.-Y. Cho, B. Anasori, C.-K. Kim, Y.-K. Choi, J. Kim, Y. Gogotsi and H.-T. Jung, ACS Nano, 2018, 12, 986–993 CrossRef CAS PubMed.
- E. Lee, A. VahidMohammadi, B. C. Prorok, Y. S. Yoon, M. Beidaghi and D.-J. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 37184–37190 CrossRef CAS PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- B. Mendoza-Sánchez and Y. Gogotsi, Adv. Mater., 2016, 28, 6104–6135 CrossRef PubMed.
- Q. Wang, J. Yan and Z. Fan, Energy Environ. Sci., 2016, 9, 729–762 RSC.
- A. D. Dillon, M. J. Ghidiu, A. L. Krick, J. Griggs, S. J. May, Y. Gogotsi, M. W. Barsoum and A. T. Fafarman, Adv. Funct. Mater., 2016, 26, 4162–4168 CrossRef CAS.
- C. Zhang and V. Nicolosi, Energy Storage Mater., 2019, 16, 102–125 CrossRef.
- Z. W. Seh, K. D. Fredrickson, B. Anasori, J. Kibsgaard, A. L. Strickler, M. R. Lukatskaya, Y. Gogotsi, T. F. Jaramillo and A. Vojvodic, ACS Energy Lett., 2016, 1, 589–594 CrossRef CAS.
- T. Y. Ma, J. L. Cao, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2016, 55, 1138–1142 CrossRef CAS PubMed.
- F. J. Robertson and J. Wu, J. Am. Chem. Soc., 2012, 134, 2775–2780 CrossRef CAS PubMed.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78 CAS.
- M. Khazaei, M. Arai, T. Sasaki, M. Estili and Y. Sakka, Phys. Chem. Chem. Phys., 2014, 16, 7841–7849 RSC.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Chem. Mater., 2017, 29, 7633–7644 CrossRef CAS.
- Q. Tao, M. Dahlqvist, J. Lu, S. Kota, R. Meshkian, J. Halim, J. Palisaitis, L. Hultman, M. W. Barsoum, P. O. Å. Persson and J. Rosen, Nat. Commun., 2017, 8, 14949 CrossRef PubMed.
- J. Zhou, X. Zha, X. Zhou, F. Chen, G. Gao, S. Wang, C. Shen, T. Chen, C. Zhi, P. Eklund, S. Du, J. Xue, W. Shi, Z. Chai and Q. Huang, ACS Nano, 2017, 11, 3841–3850 CrossRef CAS PubMed.
- S. Huang and V. N. Mochalin, Inorg. Chem., 2019, 58, 1958–1966 CrossRef CAS PubMed.
- T. Habib, X. Zhao, S. A. Shah, Y. Chen, W. Sun, H. An, J. L. Lutkenhaus, M. Radovic and M. J. Green, NPJ 2D Mater. Appl., 2019, 3, 8 CrossRef.
- K. Maleski, V. N. Mochalin and Y. Gogotsi, Chem. Mater., 2017, 29, 1632–1640 CrossRef CAS.
- C. J. Zhang, S. Pinilla, N. McEvoy, C. P. Cullen, B. Anasori, E. Long, S.-H. Park, A. Seral-Ascaso, A. Shmeliov, D. Krishnan, C. Morant, X. Liu, G. S. Duesberg, Y. Gogotsi and V. Nicolosi, Chem. Mater., 2017, 29, 4848–4856 CrossRef CAS.
- B. Y. Liaw, E. P. Roth, R. G. Jungst, G. Nagasubramanian, H. L. Case and D. H. Doughty, J. Power Sources, 2003, 119–121, 874–886 CrossRef CAS.
- T. Hu, J. Wang, H. Zhang, Z. Li, M. Hu and X. Wang, Phys. Chem. Chem. Phys., 2015, 17, 9997–10003 RSC.
- J. Zhu, Y. Tang, C. Yang, F. Wang and M. Cao, J. Electrochem. Soc., 2016, 163, A785–A791 CrossRef CAS.
- Q. Yang, Z. Xu, B. Fang, T. Huang, S. Cai, H. Chen, Y. Liu, K. Gopalsamy, W. Gao and C. Gao, J. Mater. Chem. A, 2017, 5, 22113–22119 RSC.
- J. Halim, K. M. Cook, M. Naguib, P. Eklund, Y. Gogotsi, J. Rosen and M. W. Barsoum, Appl. Surf. Sci., 2016, 362, 406–417 CrossRef CAS.
- J. Halim, M. R. Lukatskaya, K. M. Cook, J. Lu, C. R. Smith, L.-Å. Näslund, S. J. May, L. Hultman, Y. Gogotsi, P. Eklund and M. W. Barsoum, Chem. Mater., 2014, 26, 2374–2381 CrossRef CAS PubMed.
- V. Schier, H.-J. Michel and J. Halbritter, Fresenius’ J. Anal. Chem., 1993, 346, 227–232 CrossRef CAS.
- S. Myhra, J. A. A. Crossley and M. W. Barsoum, J. Phys. Chem. Solids, 2001, 62, 811–817 CrossRef CAS.
- O. Mashtalir, K. M. Cook, V. N. Mochalin, M. Crowe, M. W. Barsoum and Y. Gogotsi, J. Mater. Chem. A, 2014, 2, 14334–14338 RSC.
- T. R. Marrero and E. A. Mason, J. Phys. Chem. Ref. Data, 1972, 1, 3–118 CrossRef CAS.
- A. V. Narlikar, Frontiers in Superconducting Materials, Springer, 2005, pp. 365–391 Search PubMed.
- W. F. Kuhs, C. Sippel, A. Falenty and T. C. Hansen, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 21259–21264 CrossRef CAS PubMed.
- J. Durão and L. Gales, Materials, 2012, 5, 1593–1601 CrossRef.
- T. Ikeda-Fukazawa, K. Kawamura and T. Hondoh, Mol. Simul., 2004, 30, 973–979 CrossRef CAS.
- T. Ikeda-Fukazawa, S. Horikawa, T. Hondoh and K. Kawamura, J. Chem. Phys., 2002, 117, 3886–3896 CrossRef CAS.
- S.-Y. Cho, Y. Lee, H.-J. Koh, H. Jung, J.-S. Kim, H.-W. Yoo, J. Kim and H.-T. Jung, Adv. Mater., 2016, 28, 7020–7028 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: (1) Optical images of MAX powder and pellets, (2) SEM images of Ti3C2 MXene films dispersed in ethanol and DI water, (3) sheet resistances of Ti3C2 MXenes dispersed in ethanol stored under various conditions, (4) normalized sheet resistances of Ti3C2 MXenes stored under various conditions, (5) (a) normalized resistances of various Ti3C2Tx MXene films obtained with vacuum filtration after storage for up to 8 weeks at various temperatures and humidity. (b) Sequential SEM images of film B, which was stored at room temperature (25 °C) and a humidity of 95%. (6) Schematic diagram of the gas delivery system. (7) Gas sensor responses of Ti3C2Tx flakes stored at 5 °C in an anhydrous organic solvent. See DOI: 10.1039/c9nr00084d |
| ‡ Present address: Materials Architecturing Research Center, Korea Institute of Science and Technology (KIST), Seoul 02792, South Korea. |
| § Present address: Department of Nano-structured Materials Research, National Nano Fab Center (NNFC), Daejeon 34141, Korea. |
| This journal is © The Royal Society of Chemistry 2019 |