Metal-free sulfonyl radical-initiated cascade cyclization to access sulfonated indolo[1,2-a]quinolines†
Kai
Sun
 a,
Xiao-Lan
Chen
a,
Xiao-Lan
Chen
 *a,
Yin-Li
Zhang
a,
Kai
Li
*a,
Yin-Li
Zhang
a,
Kai
Li
 *a,
Xian-Qiang
Huang
b,
Yu-Yu
Peng
c,
Ling-Bo
Qu
a and
Bing
Yu
*a,
Xian-Qiang
Huang
b,
Yu-Yu
Peng
c,
Ling-Bo
Qu
a and
Bing
Yu
 *a
*a
aCollege of Chemistry, Zhengzhou University, Zhengzhou 450001, China. E-mail: chenxl@zzu.edu.cn; likai@zzu.edu.cn; bingyu@zzu.edu.cn
bSchool of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong 252059, China
cHunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China
First published on 20th September 2019
Abstract
A metal-free cascade reaction was developed for the synthesis of indolo[1,2-a]quinoline derivatives from arylsulfonyl hydrazides and 1-(2-(arylethynyl)phenyl)indoles in the presence of TBAI/TBHP. Impressively, these products exhibit excellent fluorescence properties, which is promising for cell imaging.
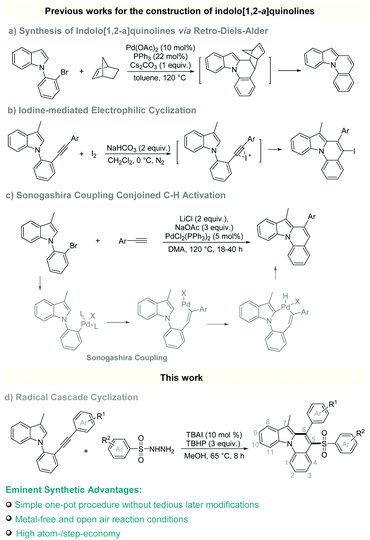
N-Heterocycles are structural elements of natural products, drugs and functional materials.1 Among them, indolo[1,2-a]quinolines have unique nitrogen-containing tetracyclic scaffolds which are widely spread in many bioactive pharmaceuticals and organic semiconductors.2 However, only limited synthetic methods toward indolo[1,2-a]quinolines have been reported.3 For example, in 2007, Lautens et al. first developed an elegant Pd-catalyzed retro-Diels–Alder strategy to access indolo[1,2-a]quinoline (Scheme 1a).3f In 2011, Verma and co-workers developed an iodine-mediated electrophilic cyclization to access iodo-substituted indolo[1,2-a]quinolines (Scheme 1b).3d One year later in 2012, Verma's group further developed a Pd-catalyzed Sonogashira coupling conjoined C–H activation strategy for the preparation of indolo[1,2-a]quinolines (Scheme 1c).3c Despite these significant advances, the development of straightforward and efficient synthetic methodologies for the synthesis of diverse functionalized indolo[1,2-a]quinolines, especially those that cannot be directly prepared via the previous reported strategies, has been enthusiastically pursued and highly desired.
Sulfones not only are versatile synthetic intermediates, but also exhibit a wide range of physical, chemical and biological activities.4 The preparation of sulfonated N-heterocycles has been one of the most challenging tasks which have gained increasing attention in recent years.5 Over the past decade, radical cascade cyclization reactions have emerged as an enabling platform to access a variety of sulfonyl-substituted heterocycles and carbocycles via sulfonyl radical-initiated cascade cyclization reactions.6 Those cascade cyclization reactions were able to incorporate the biologically valuable sulfonyl group into the cyclic ring construction within a one-step reaction, showing remarkable atom-/step-economy.7 However, it is especially worth mentioning here that the practical and efficient strategy for incorporating sulfonyl groups into indolo[1,2-a]quinolines has not been well established. In radical cascade cyclization reactions, one of the most prominent research objectives is developing new radical partners.8 As part of our continuing efforts in the development of convenient radical-initiated reactions,9 we herein disclose a novel and efficient sulfonyl radical-initiated cascade cyclization strategy, by which, a wide range of indolo[1,2-a]quinolines with an arylsulfonyl group attached at the 5-position and an aryl group at the 6-position were prepared via reaction of 1-(2-(arylethynyl)phenyl)indoles (1) with arylsulfonyl hydrazides (2) in the presence of TBAI/TBHP under mild reaction conditions (Scheme 1d). To the best of our knowledge, this is the first example to construct indolo[1,2-a]quinolines via radical cascade cyclization reactions. This method features metal-free and mild conditions, providing a novel and efficient procedure to access indolo[1,2-a]quinolines.
We initiated the study by establishing optimal experimental conditions using the model reaction of 3-methyl-1-(2-(phenylethynyl)phenyl)indole (1a) with TsNHNH2 (2a), as summarized in Table S1 (ESI†). After extensive experimentation, the optimized reaction conditions were established as follows: 1a (0.5 mmol), 2a (1 mmol), TBAI (10 mol%) and TBHP (3 equiv.) were mixed in MeOH at 65 °C for 8 h.
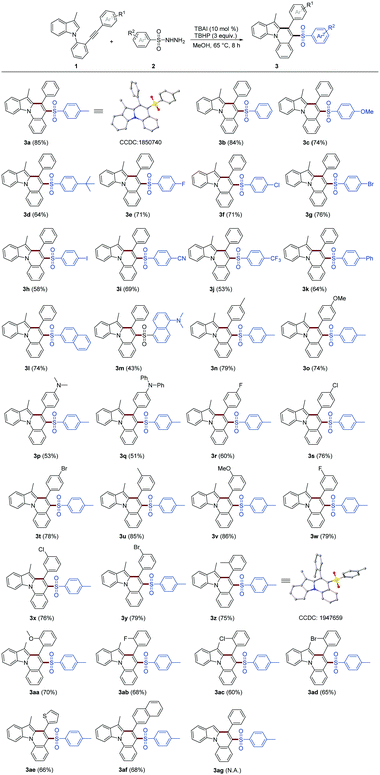
With the optimized reaction conditions established, the substrate scope was then explored by examining various 1-(2-(arylethynyl)phenyl)indoles (1) and sulfonyl hydrazides (2), as illustrated in Table 1. As can be seen, a group of phenylsulfonyl hydrazides bearing electron-withdrawing groups (–F, –Cl, –Br, –CF3, –CN) and electron-donating substituents (–Me, –OMe, –N(Me)2, –tBu, –Ph) at the para-position of the phenyl group, reacted smoothly with 1a, affording the corresponding products 3a–k in moderate to excellent yields (53–85%). No obvious electronic effects were observed in those cases (3a–k). Further screening indicated that two naphthalene sulfonylhydrazides were also good at reacting with 1a, rendering 3l–m in moderate to good yields. Besides that, various 1-(2-(phenylethynyl)phenyl)indoles (1) bearing different substituents on Ar1, including electron-withdrawing groups (–F, –Cl, –Br) and electron-donating substituents (–Me, –OMe, –N(Me)2, –N(Ph)2), were also chosen to react with TsNHNH2 (2a), giving target 3n–3ad in moderate to excellent yields. No obvious electronic effects were observed from those cases (3n–3ad) as well. In addition, two starting reactants (1) containing thiophene and naphthalene rings (Ar1) could react with TsNHNH2 smoothly, leading to the formation of 3ae–3af in satisfactory yields, respectively. Finally, the substrate without a methyl group at the 3-position of the indole failed to afford the desired product 3ag but produced a complex mixture. All the newly synthesized sulfonated indolo[1,2-a]quinolines are new compounds, and the structures of 3a and 3z were confirmed by X-ray crystallography (hydrogen atoms have been omitted for clarity).
| a Reaction conditions: 1 (0.5 mmol), 2 (1 mmol), TBAI (10 mol%), TBHP (3 equiv.), MeOH (10 mL) at 65 °C for 8 h. TBHP = tert-butyl hydroperoxide (70% aqueous solution). Isolated yields are given. N.A. = not analysed. |
|---|
|
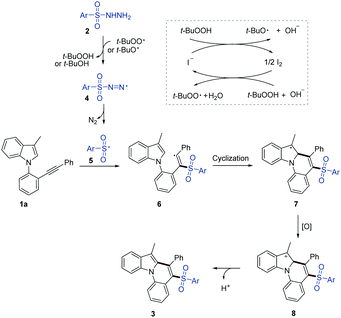
A plausible mechanism is proposed as shown in Scheme 2. Initially, TBHP reacts with the iodide anion from TBAI, generating t-BuO˙ as well as t-BuOO˙ radicals.10 Then, successive H-abstraction of sulfonyl hydrazide 2 by the resultant radicals affords sulfonyldiazene radical 4, which subsequently yields sulfonyl radical 5 with the release of N2. Then, the regioselective addition of sulfonyl radical 5 to the carbon–carbon triple bond of 1a forms alkenyl radical 6, which subsequently undergoes an intramolecular cyclization giving radical 7. Then, 7 is quickly oxidized to carbocation intermediate 8. Finally, a rapid deprotonation of carbocation 8 regenerated the aromatic ring to give the final product 3.
Additional control experiments were then carried out, giving substantial support to the proposed reaction mechanism. As can be seen from Scheme S1 (ESI†), when the model reaction was performed in the presence of (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl (TEMPO) or 2,6-di-tert-butyl-4-methylphenol (BHT), two widely used radical scavengers, no or little desired product 3 was obtained, suggesting that the reaction might experience a radical process. In particular, when the model reaction was performed in the presence of BHT (Scheme S1b, ESI†), we successfully isolated product 9, evidencing that tosyl radical (Ts˙) was indeed produced from TsNHNH2 and then trapped by BHT.
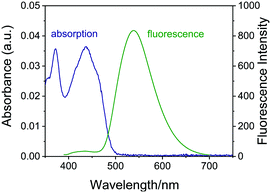
The large π-conjugated systems give the potential for these synthetic compounds to possess good fluorescence properties. Therefore, compound 3z was selected to carefully investigate the photo-physical properties. UV/vis and fluorescence spectra were thoroughly recorded in CH2Cl2 at room temperature. As can be seen in Fig. 1, a strong absorption for compound 3z at 300–450 nm and excellent fluorescence at 525 nm can be observed. There is almost no overlap between the fluorescence spectrum and absorption spectrum, exhibiting large Stokes shifts.
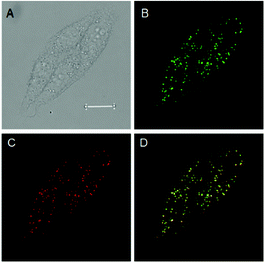
Lipid droplets (LDs) play important roles in a number of physiological processes, such as the construction and maintenance of membranes, regulations of the storage and metabolism of neutral lipids, signal transduction and protein degradation etc.11 Therefore, the specific imaging of LDs has attracted widespread attention in recent years. In light of the excellent fluorescence property of 3z, further attempts were carried out to examine its fluorescence activity in living cells. When fluorophores are used for living cells imaging, cytotoxicity must be controlled. The cytotoxicity of 3z to HepG-2 cells was initially examined using a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. The results showed even when HepG-2 cells were treated with 3z at 30 μmol L−1, the cell viability was still higher than 80%, demonstrating the low toxicity of 3z to living cells (Fig. S1, ESI†). Afterward, HepG-2 cells were co-incubated with 3z (5 μmol L−1) and the commercially available lipid probe Nile Red (2 μmol L−1) for 30 minutes at 37 °C. As can be seen in Fig. 2, compound 3z showed a group of clearly distinguishable green fluorescence dots within the cells under excitation at 405 nm (Fig. 2B), while Nile Red showed a group of similarly distributed red fluorescence dots under excitation at 488 nm (Fig. 2C). The merged image (Fig. 2D) revealed that the fluorescence signals from 3z overlap nicely with those from Nile Red. The Pearson correlation coefficient value of 3z and Nile Red in the image was determined as 0.92, showing that 3z is well qualified as a LDs-targeted fluorescence probe.
In conclusion, we have developed a novel and efficient sulfonyl radical-initiated cascade cyclization strategy, by which a wide range of indolo[1,2-a]quinolines with an arylsulfonyl group attached at the 5-position and an aryl group at the 6-position were prepared for the first time, via reaction of 1-(2-(arylethynyl)phenyl)indoles with arylsulfonyl hydrazides in the presence of TBAI/TBHP in MeOH at 65 °C for 8 h. Impressively, the synthetic compounds exhibit excellent fluorescence properties, and cell imaging experiments were conducted to show the application in cell organelle imaging. Further research on the fluorescence properties and applications of these synthetic compounds is currently ongoing in our laboratory.
We acknowledge the financial support from National Natural Science Foundation of China (21501010, 21501150, 21971224), and Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation (2019CL03).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) K. Sun, Z. Shi, Z. Liu, B. Luan, J. Zhu and Y. Xue, Org. Lett., 2018, 20, 6687 CrossRef CAS PubMed; (b) G.-P. Yang, S.-X. Shang, B. Yu and C.-W. Hu, Inorg. Chem. Front., 2018, 5, 2472 RSC; (c) K. Sun, Y.-F. Si, X.-L. Chen, Q.-Y. Lv, N. Jiang, S.-S. Wang, Y.-Y. Peng, L.-B. Qu and B. Yu, Adv. Synth. Catal., 2019 DOI:10.1002/adsc.201900691; (d) L.-Y. Xie, S. Peng, F. Liu, Y.-F. Liu, M. Sun, Z.-L. Tang, S. Jiang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 7193 CrossRef CAS; (e) L.-Y. Xie, S. Peng, T.-G. Fan, Y.-F. Liu, M. Sun, L.-L. Jiang, X.-X. Wang, Z. Cao and W.-M. He, Sci. China: Chem., 2019, 62, 460 CrossRef CAS; (f) Y. Peng, C.-T. Feng, Y.-Q. Li, F.-X. Chen and K. Xu, Org. Biomol. Chem., 2019, 17, 6570 RSC; (g) K.-J. Li, K. Xu, Y.-G. Liu, C.-C. Zeng and B.-G. Sun, Adv. Synth. Catal., 2019, 361, 1033 CrossRef CAS; (h) X.-C. Liu, K. Sun, Q.-Y. Lv, X.-L. Chen, Y.-Q. Sun, Y.-Y. Peng, L.-B. Qu and B. Yu, New J. Chem., 2019, 43, 12221 RSC; (i) C. Miao, Q. Hou, Y. Wen, F. Han, Z. Li, L. Yang and C.-G. Xia, ACS Sustainable Chem. Eng., 2019, 7, 12008 CAS; (j) X. Huang, N. Rong, P. Li, G. Shen, Q. Li, N. Xin, C. Cui, J. Cui, B. Yang, D. Li, C. Zhao, J. Dou and B. Wang, Org. Lett., 2018, 20, 3332 CrossRef CAS PubMed; (k) L. Peng, Z. Hu, Z. Tang, Y. Jiao and X. Xu, Chin. Chem. Lett., 2019, 30, 1481 CrossRef CAS; (l) D.-Q. Dong, W.-J. Chen, Y. Yang, X. Gao and Z.-L. Wang, ChemistrySelect, 2019, 4, 2480 CrossRef CAS; (m) L.-Y. Xie, L.-L. Jiang, J.-X. Tan, Y. Wang, X.-Q. Xu, B. Zhang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 14153 CrossRef CAS; (n) H. Xu, B. Zhou, P. Zhou, J. Zhou, Y. Shen, F.-C. Yu and L.-L. Lu, Chem. Commun., 2016, 52, 8002 RSC.
- (a) R. Liu, Q. Wang, Y. Wei and M. Shi, Chem. Commun., 2018, 54, 1225 RSC; (b) A. K. Verma, T. Kesharwani, J. Singh, V. Tandon and R. C. Larock, Angew. Chem., Int. Ed., 2009, 48, 1138 CrossRef PubMed; (c) E. Ahmed, A. L. Briseno, Y. Xia and S. A. Jenekhe, J. Am. Chem. Soc., 2008, 130, 1118 CrossRef CAS PubMed.
- (a) X. Liu, X. Li, H. Liu, Q. Guo, J. Lan, R. Wang and J. You, Org. Lett., 2015, 17, 2936 CrossRef CAS PubMed; (b) J. Gao, Y. Shao, J. Zhu, J. Zhu, H. Mao, X. Wang and X. Lv, J. Org. Chem., 2014, 79, 9000 CrossRef CAS PubMed; (c) S. P. Shukla, R. K. Tiwari and A. K. Verma, J. Org. Chem., 2012, 77, 10382 CrossRef CAS; (d) A. K. Verma, S. P. Shukla, J. Singh and V. Rustagi, J. Org. Chem., 2011, 76, 5670 CrossRef CAS PubMed; (e) J. Panteleev, K. Geyer, A. Aguilar-Aguilar, L. Wang and M. Lautens, Org. Lett., 2010, 12, 5092 CrossRef CAS PubMed; (f) D. G. Hulcoop and M. Lautens, Org. Lett., 2007, 9, 1761 CrossRef CAS PubMed.
- (a) Y. Xia, X. Chen, L. Qu, K. Sun, X. Xia, W. Fu, X. Chen, Y. Yang, Y. Zhao and C. Li, Asian J. Org. Chem., 2016, 5, 878 CrossRef CAS; (b) G. Qiu, K. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561 RSC; (c) K. Hofman, N.-W. Liu and G. Manolikakes, Chem. – Eur. J., 2018, 24, 11852 CrossRef CAS; (d) F.-L. Yang and S.-K. Tian, Tetrahedron Lett., 2017, 58, 487 CrossRef CAS; (e) L.-Y. Xie, Y.-J. Li, J. Qu, Y. Duan, J. Hu, K.-J. Liu, Z. Cao and W.-M. He, Green Chem., 2017, 19, 5642 RSC; (f) L.-Y. Xie, S. Peng, F. Liu, G.-R. Chen, W. Xia, X. Yu, W.-F. Li, Z. Cao and W.-M. He, Org. Chem. Front., 2018, 5, 2604 RSC; (g) L.-Y. Xie, S. Peng, J.-X. Tan, R.-X. Sun, X. Yu, N.-N. Dai, Z.-L. Tang, X. Xu and W.-M. He, ACS Sustainable Chem. Eng., 2018, 6, 16976 CrossRef CAS; (h) L.-Y. Xie, T.-G. Fang, J.-X. Tan, B. Zhang, Z. Cao, L.-H. Yang and W.-M. He, Green Chem., 2019, 21, 3858 RSC.
- (a) P. Bao, L. Wang, Q. Liu, D. Yang, H. Wang, X. Zhao, H. Yue and W. Wei, Tetrahedron Lett., 2019, 60, 214 CrossRef CAS; (b) K. Sun, X.-L. Chen, X. Li, L.-B. Qu, W.-Z. Bi, X. Chen, H.-L. Ma, S.-T. Zhang, B.-W. Han, Y.-F. Zhao and C.-J. Li, Chem. Commun., 2015, 51, 12111 RSC; (c) W.-K. Fu, K. Sun, C. Qu, X.-L. Chen, L.-B. Qu, W.-Z. Bi and Y.-F. Zhao, Asian J. Org. Chem., 2017, 6, 492 CrossRef CAS; (d) S. Peng, Y.-X. Song, J.-Y. He, S.-S. Tang, J.-X. Tan, Z. Cao, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019 DOI:10.1016/j.cclet.2019.08.002; (e) G. Li, Q. Yan, X. Gong, X. Dou and D. Yang, ACS Sustainable Chem. Eng., 2019, 7, 14009 CrossRef CAS; (f) M. Sun, J. Jiang, J. Chen, Q. Yang and X. Yu, Tetrahedron, 2019, 75, 130456 CrossRef.
- (a) Y. Zhang, K. Sun, Q. Lv, X. Chen, L. Qu and B. Yu, Chin. Chem. Lett., 2019, 30, 1361 CrossRef CAS; (b) M. H. Muhammad, X.-L. Chen, B. Yu, L.-B. Qu and Y.-F. Zhao, Pure Appl. Chem., 2019, 91, 33 CAS; (c) W. Wei, H. Cui, D. Yang, H. Yue, C. He, Y. Zhang and H. Wang, Green Chem., 2017, 19, 5608 RSC; (d) L. Wang, Y. Zhang, M. Zhang, P. Bao, X. Lv, H.-G. Liu, X. Zhao, J.-S. Li, Z. Luo and W. Wei, Tetrahedron Lett., 2019, 60, 1845 CrossRef CAS; (e) T. Song, H. Li, F. Wei, C.-H. Tung and Z. Xu, Tetrahedron Lett., 2019, 60, 916 CrossRef CAS; (f) H. Li, C. Shan, C.-H. Tung and Z. Xu, Chem. Sci., 2017, 8, 2610 RSC; (g) H. Li, Z. Cheng, C.-H. Tung and Z. Xu, ACS Catal., 2018, 8, 8237 CrossRef CAS; (h) B. Wang, S. Jin, S. Sun and J. Cheng, Org. Chem. Front., 2018, 5, 958 RSC.
- (a) M.-H. Huang, W.-J. Hao, G. Li, S.-J. Tu and B. Jiang, Chem. Commun., 2018, 54, 10791 RSC; (b) X. Wang, Y. Li, G. Qiu and J. Wu, Org. Chem. Front., 2018, 5, 2555 RSC; (c) W.-C. Yang, J.-G. Feng, L. Wu and Y.-Q. Zhang, Adv. Synth. Catal., 2019, 361, 1700 CrossRef CAS; (d) W. C. Yang, P. Dai, K. Luo, Y. G. Ji and L. Wu, Adv. Synth. Catal., 2017, 359, 2390 CrossRef CAS; (e) J. Zhu, W. C. Yang, X. D. Wang and L. Wu, Adv. Synth. Catal., 2018, 360, 386 CrossRef CAS.
- (a) X.-C. Liu, K. Sun, X.-L. Chen, W.-F. Wang, Y. Liu, Q.-L. Li, Y.-Y. Peng, L.-B. Qu and B. Yu, Adv. Synth. Catal., 2019, 361, 3712 CrossRef CAS; (b) Y. Liu, X.-L. Chen, K. Sun, X.-Y. Li, F.-L. Zeng, X.-C. Liu, L.-B. Qu, Y. Zhao and B. Yu, Org. Lett., 2019, 21, 4019 CrossRef CAS PubMed; (c) T.-Y. Shang, L.-H. Lu, Z. Cao, Y. Liu, W.-M. He and B. Yu, Chem. Commun., 2019, 55, 5408 RSC; (d) R. Li, X. Chen, S. Wei, K. Sun, L. Fan, Y. Liu, L. Qu, Y. Zhao and B. Yu, Adv. Synth. Catal., 2018, 360, 4807 CrossRef; (e) F.-L. Zeng, X. Chen, S.-Q. He, K. Sun, Y. Liu, R. Fu, L. Qu, Y. Zhao and B. Yu, Org. Chem. Front., 2019, 6, 1476 RSC; (f) K. Sun, S.-J. Li, X. Chen, Y. Liu, X. Huang, D. Wei, L. Qu, Y. Zhao and B. Yu, Chem. Commun., 2019, 55, 2861 RSC; (g) C. Jing, X. Chen, K. Sun, Y. Yang, T. Chen, Y. Liu, L. Qu, Y. Zhao and B. Yu, Org. Lett., 2019, 21, 486 CrossRef CAS PubMed.
- (a) H. Hu, X. Chen, K. Sun, J. Wang, Y. Liu, H. Liu, L. Fan, B. Yu, Y. Sun, L. Qu and Y. Zhao, Org. Lett., 2018, 20, 6157 CrossRef CAS PubMed; (b) H. Hu, X. Chen, K. Sun, J. Wang, Y. Liu, H. Liu, B. Yu, Y. Sun, L. Qu and Y. Zhao, Org. Chem. Front., 2018, 5, 2925 RSC; (c) Y. Liu, X.-L. Chen, F.-L. Zeng, K. Sun, C. Qu, L.-L. Fan, Z.-L. An, R. Li, C.-F. Jing, S.-K. Wei, L.-B. Qu, B. Yu, Y.-Q. Sun and Y.-F. Zhao, J. Org. Chem., 2018, 83, 11727 CrossRef CAS; (d) J. Wang, K. Sun, X. Chen, T. Chen, Y. Liu, L. Qu, Y. Zhao and B. Yu, Org. Lett., 2019, 21, 1863 CrossRef CAS PubMed.
- (a) K. Sun, X.-L. Chen, S.-J. Li, D.-H. Wei, X.-C. Liu, Y.-L. Zhang, Y. Liu, L.-L. Fan, L.-B. Qu, B. Yu, K. Li, Y.-Q. Sun and Y.-F. Zhao, J. Org. Chem., 2018, 83, 14419 CrossRef CAS PubMed; (b) X.-T. Zhu, Q.-L. Lu, X. Wang, T.-S. Zhang, W.-J. Hao, S.-J. Tu and B. Jiang, J. Org. Chem., 2018, 83, 9890 CrossRef CAS PubMed; (c) R. Fu, M.-F. Li, P. Zhou, W.-J. Hao, S.-J. Tu and B. Jiang, Adv. Synth. Catal., 2019, 361, 2280 CrossRef CAS.
- (a) M. Gao, H. Su, S. Li, Y. Lin, X. Ling, A. Qin and B. Z. Tang, Chem. Commun., 2017, 53, 921 RSC; (b) J. Yin, M. Peng, Y. Ma, R. Guo and W. Lin, Chem. Commun., 2018, 54, 12093 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1850740 and 1947659. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc06924k |
| This journal is © The Royal Society of Chemistry 2019 |