Palladium-catalyzed intermolecular dearomatic allenylation of hydrocycloalk[b]indoles with 2,3-allenyl carbonates†
Run-Duo
Gao‡
ab,
Yizhan
Zhai‡
ab,
Shu-Li
You
 *ab and
Shengming
Ma
*ab and
Shengming
Ma
 *ac
*ac
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China. E-mail: masm@sioc.ac.cn; slyou@sioc.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cDepartment of Chemistry, Fudan University, 220 Handan Lu, Shanghai 200433, P.R. China
First published on 15th March 2018
Abstract
A palladium-catalyzed intermolecular dearomatic allenylation of hydrocycloalk[b]indoles with 2,3-allenyl carbonates has been developed, providing access to functionalized allenes containing an indoline unit under optimized conditions. Both terminal and non-terminal allenes could react smoothly with an exclusive chemoselectivity and good yields by applying different reaction parameters.
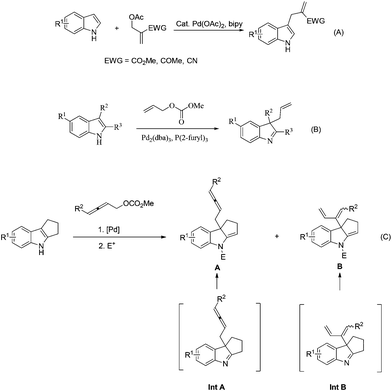
Indoles are attractive and fundamental structural motifs in numerous natural products and pharmaceuticals.1 In addition, the nucleophilicity of C-3 of indoles leads to numerous transformations.2 In 2004, Ma's group developed a palladium-catalyzed functionalization of 2-electron-deficient group-substituted acetates with indoles (Scheme 1A).2e Starting from 2008, Rawal (Scheme 1B),3a Du,3b and You3c,d reported that 2,3-disubstituted indoles could react with allylic carbonates in the presence of a palladium catalyst. On the other hand, allenes are playing an increasingly important role in organic chemistry and medicinal chemistry.4,5 Much attention has been paid to the reaction of 2,3-allenol derivatives with different nucleophiles.6–8 Herein, we wish to provide an efficient method for synthesizing functionalized allenes via the dearomatization of hydrocycloalk[b]indoles (Scheme 1C).
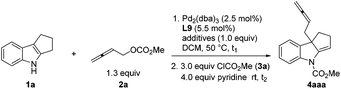
In principle, there are two types of reactivities for such a coupling reaction of 2,3-allenyl carbonates, generating two different products, allenes A and 1,3-dienes B, upon the treatment with an electrophile. We initiated our study with 1,2,3,4-tetrahydrocyclopenta[b]indole 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and buta-2,3-dienyl methyl carbonate 2a
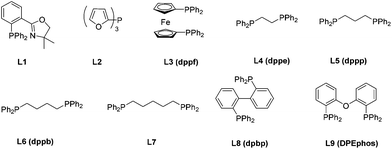
9 and buta-2,3-dienyl methyl carbonate 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10a–c followed by an immediate trapping of the possible imine product Int A or Int B with methyl chloroformate. The formation of enamine 4aaa was observed, albeit in <10% yield using P, N ligand L1 or monophosphine ligand L2. Bisphosphines with the difference of a length of carbon chain had a very limited influence on the reaction. Interestingly, decent levels of the reaction were observed by using commercially available dpbp L8 and DPEphos L9 (Table 1).
10a–c followed by an immediate trapping of the possible imine product Int A or Int B with methyl chloroformate. The formation of enamine 4aaa was observed, albeit in <10% yield using P, N ligand L1 or monophosphine ligand L2. Bisphosphines with the difference of a length of carbon chain had a very limited influence on the reaction. Interestingly, decent levels of the reaction were observed by using commercially available dpbp L8 and DPEphos L9 (Table 1).
| Entry | Ligand | t 1/t2 (h) | NMR yield of 4aaab (%) |
|---|---|---|---|
| a Reaction conditions: The reactions were carried out using 0.2 mmol of 1a, 0.26 mmol of 2a, 2.5 mol% of Pd2(dba)3, 5.5 mol% of L and 2 mL of DCM at 50 °C. After the reactions were complete, 0.8 mmol of pyridine and 0.6 mmol of ClCO2Me were added at room temperature. b Determined by using CH2Br2 as the internal standard. c 11 mol% of L was added. | |||
| 1c | L1 | 6.5/10 | 6 |
| 2c | L2 | 9.7/11.3 | 5 |
| 3 | L3 | 6.5/10 | 10 |
| 4 | L4 | 6.5/10 | NR |
| 5 | L5 | 9.7/11.3 | 8 |
| 6 | L6 | 9.7/11.3 | 26 |
| 7 | L7 | 9/13 | 8 |
| 8 | L8 | 32.7/overnight | 53 |
| 9 | L9 | 8.7/overnight | 64 |
|
|||
Further optimization of the reaction conditions indicated that increasing the amount of substrate 2a to 2.0 equiv. had no influence on the reaction. Elevating the reaction temperature even led to a worse result (entry 3, Table 2). Adding molecular sieves had a slight influence on the reaction (entries 4 and 5, Table 2). Interestingly, there is a profound base effect: with N,O-bis-(trimethylsilyl)-acetamide (BSA) as the base, the reaction afforded the best result (entries 6–9, Table 2). By reducing the concentration, the yield could be further improved (entry 10, Table 2).
| Entry | Additives | t 1/t2 (h) | Yield of 4aaab (%) |
|---|---|---|---|
| a Reaction conditions: Unless otherwise specified, the reactions were carried out using 0.2 mmol of 1a, 1.3 equiv. of 2a, 1.0 equiv. of additive, 2.5 mol% of Pd2(dba)3, and 5.5 mol% of L9 in 2.0 mL of DCM at 50 °C. After the reactions were complete, 0.8 mmol of pyridine and 0.6 mmol of ClCO2Me were added at room temperature. b The yield was determined by NMR analysis of the crude product. c The reaction was carried out with 2.0 equiv. of 2a. d The reaction was carried out at 80 °C. e 100 mg of additive was added. f The reactions were carried out using 0.2 mmol of 1a, 1.3 equiv. of 2a, 1.0 equiv. of additive, 2.5 mol% of Pd2(dba)3, and 5.5 mol% of L9 in 2.0 mL of DCM at 50 °C. After the reactions were complete, the additive was filtered from the solution. 2 mL of DCM, 0.8 mmol of pyridine and 0.6 mmol of ClCO2Me were added at room temperature. g The reaction was carried out in 4 mL of DCM. h Isolated yield. i The reaction was carried out in 1 mL of DCM. | |||
| 1 | — | 8.7/overnight | 64 |
| 2c | — | 11.5/15 | 64 |
| 3d | — | 3.5/13.5 | 56 |
| 4e,f | 3 Å MS | 8/overnight | 66 |
| 5e,f | 4 Å MS | 8/overnight | 58 |
| 6f | KOtBu | 8.5/13.5 | 13 |
| 7f | K3PO4 | 7.5/15.5 | 61 |
| 8f | MgSO4 | 9.6/12.5 | 59 |
| 9 | BSA | 6.5/15.5 | 83 |
| 10g | BSA | 10/3 | 87 (80h) |
| 11i | BSA | 6.7/16.8 | 83 |
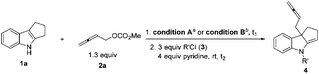
The scope for the electrophilic trapping reagent was also studied. In addition to chloroformate, different acyl chlorides such as acetyl chloride, propionyl chloride, isobutyryl chloride, chloroacetyl chloride, benzoyl chloride, cinnamoyl chloride, and benzenesulfonyl chloride may be applied (Table 3).
| Entry | R′ | Conditions (A/B) | t 1/t2 (h) | Yield of 4c (%) |
|---|---|---|---|---|
| a Conditions A: The reaction was carried out using 0.2 mmol of 1a, 1.3 equiv. of 2a, 1.0 equiv. of BSA, 2.5 mol% of Pd2(dba)3, and 5.5 mol% of L9 in 4.0 mL of DCM at 50 °C and monitored by TLC or LC-MS. After the reaction was complete, 4.0 equiv. of pyridine and 3.0 equiv. of corresponding 3 were added at room temperature. b Conditions B: The reaction was carried out using 0.2 mmol of 1a, 1.3 equiv. of 2a, 1 equiv. of BSA, 1 equiv. of Et3B, 4 mol% of Pd(acac)2, and 6 mol% of L9 in 4.0 mL of DCM at room temperature and monitored by TLC or LC-MS. After the reaction was complete, 4.0 equiv. of pyridine and 3.0 equiv. of corresponding 3 were added at room temperature. c Isolated yield. | ||||
| 1 |
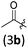
|
A | 6.5/21 | 79 (4aab) |
| 2 |
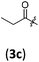
|
A | 6.3/16.8 | 86 (4aac) |
| 3 |
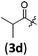
|
B | 8/3 | 91 (4aad) |
| 4 |
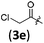
|
B | 8/3 | 88 (4aae) |
| 5 |
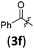
|
A | 6.4/21.3 | 63 (4aaf) |
| 6 |
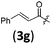
|
A | 6.3/29.8 | 58 (4aag) |
| 7 |
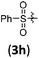
|
B | 8/3 | 58 (4aah) |
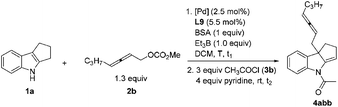
However, when non-terminal hepta-2,3-dienyl methyl carbonate 2b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10c,d was applied under conditions A, only 38% yield of 4abb was obtained (entry 1, Table 4). Thus, we commenced to optimize the reaction conditions for the substituted allenylic carbonates. With ZnCl2, no product was generated (entry 2, Table 4). There was a slight increase in the yield when 1 equiv. Et3B was added since Et3B may further activate indoles by forming a N–B bond11 (entry 3, Table 4).
10c,d was applied under conditions A, only 38% yield of 4abb was obtained (entry 1, Table 4). Thus, we commenced to optimize the reaction conditions for the substituted allenylic carbonates. With ZnCl2, no product was generated (entry 2, Table 4). There was a slight increase in the yield when 1 equiv. Et3B was added since Et3B may further activate indoles by forming a N–B bond11 (entry 3, Table 4).
| Entry | [Pd] | T (°C) | t 1/t2 (h) | Yield of 4abb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|
| a Reaction conditions: The reaction was carried out using 0.2 mmol of 1a, 1.3 equiv. of 2b, 1 equiv. of BSA, 1 equiv. of Et3B, 2.5 mol% of [Pd] and 5.5 mol% of L9 in 4.0 mL of DCM at room temperature and monitored by TLC or LC-MS. After the reaction was complete, 4 equiv. of pyridine and 3 equiv. of CH3COCl (3b) were added at room temperature. b The yield was determined by NMR analysis. c No Et3B was added. d The reaction was carried out with 2 equiv. of ZnCl2 instead of Et3B. e 0.5 equiv. of Et3B was added. f 0.1 equiv. of Et3B was added. g 4 mol% [Pd] and 6 mol% of L9 were added. | ||||
| 1c | Pd2(dba)3 | 50 | 9/12 | 38 |
| 2d | Pd2(dba)3 | 50 | 7.8/— | — |
| 3 | Pd2(dba)3 | 50 | 8/8.3 | 41 |
| 4 | Pd2(dba)3 | 45 | 9/11.2 | 45 |
| 5e | Pd2(dba)3 | 45 | 9/11.2 | 37 |
| 6f | Pd2(dba)3 | 45 | 9/11.2 | 26 |
| 7 | Pd2(dba)3 | 40 | 8/8 | 60 |
| 8 | Pd2(dba)3 | 35 | 8/11.3 | 81 |
| 9 | Pd2(dba)3 | rt | 9/11.3 | 91 |
| 10g | Pd(OAc)2 | rt | 9/10.8 | 77 |
| 11g | Pd(cod)Cl2 | rt | 9/11.3 | 68 |
| 12g | Pd(acac)2 | rt | 8/12.3 | 92 |
When we reduced the amount of Et3B, the yield decreased (entries 4–6, Table 4). To our delight, when we carried out the reaction at room temperature, 91% yield could be obtained (entry 9, Table 4). Unfortunately, the product could not be purified due to the contamination of the dba from the catalyst. Therefore other Pd catalysts were tested (entries 10–12, Table 4). Finally, Pd(acac)2, DPEphos, BSA and Et3B in DCM at room temperature were defined as the optimized reaction conditions for further study (conditions B).
With conditions A and B in hand, we began to investigate the scope of this reaction. Satisfactory yields could be observed regardless of the electronic properties on the indole moiety (entries 1–8, Table 5). It is worth noting that the MeO, F, Cl, and Br groups could also be tolerated. For allenylic carbonates, R2 may be C3H7 and C7H15, alkenyl, alkynyl and phenethyl groups (entries 9–13, Table 5).
| Entry | R1 | R2 | Conditions (A/B) | t 1/t2 (h) | Yield of 4c (%) | drd |
|---|---|---|---|---|---|---|
| a Conditions A: The reaction was carried out using 0.2 mmol of 1, 1.3 equiv. of 2, 1 equiv. of BSA, 2.5 mol% of Pd2(dba)3, and 5.5 mol% of L9 in 4.0 mL of DCM at 50 °C and monitored by TLC or LC-MS. After the reaction was complete, 4.0 equiv. of pyridine and 3.0 equiv. of CH3COCl (3b) were added at room temperature. b Conditions B: The reaction was carried out using 1.0 mmol of 1, 1.3 equiv. of 2, 1 equiv. of BSA, 1 equiv. of Et3B, 4 mol% of Pd(acac)2, and 6 mol% of L9 in 20 mL of DCM at room temperature and monitored by TLC or LC-MS. After the reaction was complete, 4.0 equiv. of pyridine and 3.0 equiv. of CH3COCl (3b) were added at room temperature. c Isolated yield. d Determined by quantitative 13C NMR experiment. | ||||||
| 1 | 7-Me (1b) | H (2a) | A | 5.6/21.8 | 83 (4bab) | — |
| 2 | 6-Me, 8-Me (1c) | H (2a) | B | 8/12.3 | 81 (4cab) | — |
| 3 | 7-MeO (1d) | H (2a) | A | 4.5/20 | 88 (4dab) | — |
| 4 | 7-Ph (1e) | H (2a) | A | 4.5/19 | 89 (4eab) | — |
| 5 | 7-F (1f) | H (2a) | A | 4.5/17.4 | 85 (4fab) | — |
| 6 | 7-Cl (1g) | H (2a) | A | 4.5/16.5 | 85 (4gab) | — |
| 7 | 7-Br (1h) | H (2a) | A | 5.3/22 | 78 (4hab) | — |
| 8 | 6-Br (1i) | H (2a) | B | 10/11.5 | 80 (4iab) | — |
| 9 | H (1a) | n-C3H7 (2b) | B | 8/13 | 68 (4abb) | 1.2/1 |
| 10 | H (1a) | n-C7H15 (2c) | B | 10/8 | 60 (4acb) | 1.4/1 |
| 11 | H (1a) |
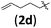
|
B | 10/13 | 48 (4adb) | 1.1/1 |
| 12 | H (1a) |
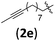
|
B | 8/11 | 68 (4aeb) | 1.2/1 |
| 13 | H (1a) |
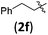
|
B | 8/9 | 78 (4afb) | 1.4/1 |
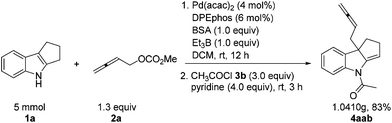
The reaction may be easily conducted on 5 mmol scale of 1a, affording 4aab in 83% yield.
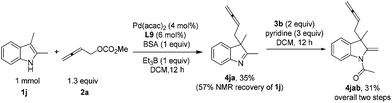
For acyclic indoles, the dearomatization reaction could also be conducted smoothly to afford indoline with an exocyclic C–C double bond 4jab (Scheme 2).
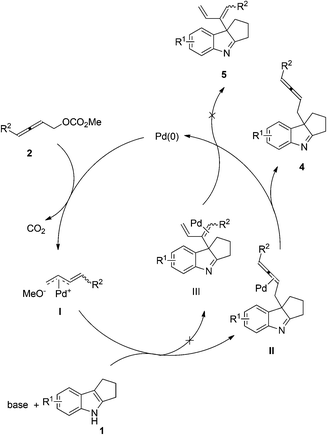
A possible mechanism is proposed. The oxidative addition of Pd(0) with 2,3-allenyl carbonates would yield, after releasing CO2, methyleneallylic palladium intermediate I, which could react with the indole to yield the product 4via intermediate II by releasing the catalytically active Pd(0) to finish the catalytic cycle. Since 1,3-diene product 5 was not detected in this reaction, the C3-carbon atom of the indole is acting as a soft nucleophile attacking the C1-atom in allenyl carbonates 2 (Scheme 3).12
In summary, a palladium-catalyzed intermolecular dearomatization of hydrocycloalk[b]indoles with 2,3-allenyl carbonates was first developed with a high chemoselectivity affording functionalized allene-substituted hydrocycloalk[b]indolines. Both mono-substituted and 1,3-disubstituted allenes could be generated smoothly with nucleophilic indoles. Further exploration of synthesizing optically active allenes with various nucleophiles is ongoing in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Basic Research Program of China (2015CB856600 for S. Ma and S.-L. You), the National Natural Science Foundation of China (21572252 for S.-L. You), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000 and QYZDY-SSW-SLH012 for S.-L. You) is greatly appreciated. We thank Penglin Wu for reproducing the results for 4fab and 4adb presented in Table 5 and 4aag presented in Table 3.Notes and references
- (a) S. Takano and K. Ogasawara, Alkaloids, 1989, 36, 225 CAS; (b) J. A. Joule and K. Mills, Heterocyclic Chemistry, Blackwell Science, Oxford, 4th edn, 2000 Search PubMed; (c) P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, Wiley, New York, 2nd edn, 2002 Search PubMed.
- For selected examples, see: (a) O. Ottoni, A. de V. F. Neder, A. K. B. Dias, R. P. A. Cruz and L. B. Aquino, Org. Lett., 2001, 3, 1005 CAS; (b) J. Zhou and Y. Tang, J. Am. Chem. Soc., 2002, 124, 9030 CrossRef CAS PubMed; (c) D. A. Evans, K. A. Scheidt, K. R. Fandrick, H. W. Lam and J. Wu, J. Am. Chem. Soc., 2003, 125, 10780 CrossRef CAS PubMed; (d) M. Bandini, A. Melloni and A. Umani-Ronchi, Org. Lett., 2004, 6, 3199 CrossRef CAS PubMed; (e) S. Ma and S. Yu, Tetrahedron Lett., 2004, 45, 8419 CrossRef CAS; (f) H. Y. Cheung, W.-Y. Yu, F. L. Lam, T.-L. Terry, Z. Zhou, T. Chan and A. S. C. Chan, Org. Lett., 2007, 9, 4295 CrossRef CAS PubMed; (g) A. B. Zaitsev, S. Gruber and P. S. Pregosin, Chem. Commun., 2007, 4692 RSC; (h) I. Usui, S. Schmidt, M. Keller and B. Breit, Org. Lett., 2008, 10, 1207 CrossRef CAS PubMed; (i) W. Liu, H. He, L.-X. Dai and S.-L. You, Org. Lett., 2008, 10, 1815 CrossRef CAS PubMed; (j) B. Sundararaju, M. Achard, B. Demerseman, L. Toupet, G. V. M. Sharma and C. Bruneau, Angew. Chem., Int. Ed., 2010, 49, 2782 CrossRef CAS PubMed; (k) Y. Tao, B. Wang, J. Zhao, Y. Song, L. Qu and J. Qu, J. Org. Chem., 2012, 77, 2942 CrossRef CAS PubMed; (l) W. Chen, Y. Gao, S. Mao, Y. Zhang, Y. Wang and Y. Wang, Org. Lett., 2012, 14, 5920 CrossRef CAS PubMed; (m) D. Das and S. Roy, Adv. Synth. Catal., 2013, 355, 1308 CrossRef CAS.
- (a) N. Kagawa, J. P. Malerich and V. H. Rawal, Org. Lett., 2008, 10, 2381 CrossRef CAS PubMed; (b) Y. Liu and H. Du, Org. Lett., 2013, 15, 740 CrossRef CAS PubMed; (c) Q.-L. Xu, L.-X. Dai and S.-L. You, Chem. Sci., 2013, 4, 97 RSC; (d) R.-D. Gao, Q.-L. Xu, B. Zhang, L.-X. Dai and S.-L. You, Chem. – Eur. J., 2016, 22, 11601 CrossRef CAS PubMed.
- For reviews, see: (a) Modern Allene Chemistry, ed. N. Krause and A. S. K. Hashmi, Wiley-VCH, Weinhein, 2005, vol. 1, 2 Search PubMed; (b) A. Hoffmann-Röder and N. Krause, Angew. Chem., Int. Ed., 2002, 41, 2933 CrossRef; (c) S. Ma, Acc. Chem. Res., 2003, 36, 701 CrossRef CAS PubMed; (d) S. Ma, Chem. Rev., 2005, 105, 2829 CrossRef PubMed; (e) M. Brasholz, H.-U. Reissig and R. Zimmer, Acc. Chem. Res., 2009, 42, 45 CrossRef CAS PubMed; (f) S. Ma, Acc. Chem. Res., 2009, 42, 1679 CrossRef CAS PubMed; (g) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954 CrossRef CAS PubMed; (h) F. Inagaki, S. Kitagaki and C. Mukai, Synlett, 2011, 594 CAS; (i) F. López and J. L. Mascareñas, Chem. – Eur. J., 2011, 17, 418 CrossRef PubMed; (j) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954 CrossRef CAS PubMed; (k) S. Yu and S. Ma, Angew. Chem., Int. Ed., 2012, 51, 3074 CrossRef CAS PubMed; (l) J. Ye and S. Ma, Acc. Chem. Res., 2014, 47, 989 CrossRef CAS PubMed.
- For some of the most recent typical examples, see: (a) T. Jiang, T. Bartholomeyzik, J. Mazuela, J. Willersinn and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2015, 54, 6024 CrossRef CAS PubMed; (b) J. Liu, Z. Han, X. Wang, Z. Wang and K. Ding, J. Am. Chem. Soc., 2015, 137, 15346 CrossRef CAS PubMed; (c) J. Dai, M. Wang, G. Chai, C. Fu and S. Ma, J. Am. Chem. Soc., 2016, 138, 2532 CrossRef CAS PubMed; (d) S. Ganss and B. Breit, Angew. Chem., Int. Ed., 2016, 55, 9738 CrossRef CAS PubMed; (e) W. Yuan, L. Song and S. Ma, Angew. Chem., Int. Ed., 2016, 55, 3140 CrossRef CAS PubMed; (f) W. Zhao and J. Montgomery, J. Am. Chem. Soc., 2016, 138, 9763 CrossRef CAS PubMed; (g) H. Zhou, Y. Wang, L. Zhang, M. Cai and S. Luo, J. Am. Chem. Soc., 2017, 139, 3631 CrossRef CAS PubMed; (h) S. Chen, X. Han, J. Wu, Q. Li, Y. Chen and H. Wang, Angew. Chem., Int. Ed., 2017, 56, 9939 CrossRef CAS PubMed; (i) Z. Yang, D. Peng, X. Du, Z. Huang and S. Ma, Org. Chem. Front., 2017, 4, 1829 RSC; (j) E. Y. Tsai, R. Y. Liu, Y. Yang and S. L. Buchwald, J. Am. Chem. Soc., 2018, 140, 2007 CrossRef CAS PubMed.
- For Pd-catalyzed such reactions generating optically active allenes with an axial chirality, see: (a) Y. Imada, K. Ueno, K. Kutsuwa and S.-I. Murahashi, Chem. Lett., 2002, 31, 140 CrossRef; (b) B. M. Trost, D. R. Fandrick and D. C. Dinh, J. Am. Chem. Soc., 2005, 127, 14186 CrossRef CAS PubMed; (c) Y. Imada, M. Nishida, K. Kutsuwa, S. Murahashi and T. Naota, Org. Lett., 2005, 7, 5837 CrossRef CAS PubMed; (d) Y. Imada, M. Nishida and T. Naota, Tetrahedron Lett., 2008, 49, 4915 CrossRef CAS; (e) T. Nemoto, M. Kanematsu, S. Tamura and Y. Hamada, Adv. Synth. Catal., 2009, 351, 1773 CrossRef CAS; (f) B. Wan, G. Jia and S. Ma, Org. Lett., 2012, 14, 46 CrossRef CAS PubMed.
- For Pd-catalyzed such reactions generating optically active allenes with a central chirality, see: (a) B. Wan and S. Ma, Angew. Chem., Int. Ed., 2013, 52, 441 CrossRef CAS PubMed; (b) Q. Li, C. Fu and S. Ma, Angew. Chem., Int. Ed., 2014, 53, 6511 CrossRef CAS PubMed.
- For Pd-catalyzed such a reaction generating optically active allenes with both an axial chirality and a central chirality, see: J. Dai, X. Duan, J. Zhou, C. Fu and S. Ma, Chin. J. Chem., 2018, 36, 387 Search PubMed.
- (a) C. M. So, C. P. Lau and F. Y. Kwong, Org. Lett., 2007, 9, 2795 CrossRef CAS PubMed; (b) S. Gore, S. Baskaran and B. König, Org. Lett., 2012, 14, 4568 CrossRef CAS PubMed.
- (a) H. Luo and S. Ma, Eur. J. Org. Chem., 2013, 3041 CrossRef CAS; (b) H. Luo, D. Ma and S. Ma, Org. Synth., 2017, 94, 153 CrossRef; (c) H. Wang, B. Beiring, D. Yu, K. D. Collins and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 12430 CrossRef CAS PubMed; (d) X. Huang, T. Cao, Y. Han, X. Jiang, W. Lin, J. Zhang and S. Ma, Chem. Commun., 2015, 51, 6956 RSC.
- A. Lin, J. Yang and M. Hashim, Org. Lett., 2013, 15, 1950 CrossRef CAS PubMed.
- J. Tsuji and T. Mandai, J. Organomet. Chem., 1993, 451, 15 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Preparation and characterisation data as well as 1H and 13C NMR spectra of all compounds. See DOI: 10.1039/c8qo00163d |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2018 |