Transition metal-free aminofluorination of β,γ-unsaturated hydrazones: base-controlled regioselective synthesis of fluorinated dihydropyrazole and tetrahydropyridazine derivatives†
Juan
Zhao
,
Min
Jiang
 * and
Jin-Tao
Liu
* and
Jin-Tao
Liu
 *
*
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: jtliu@sioc.ac.cn; jiangmin@sioc.ac.cn; Web: http://www.sioc.ac.cn Fax: +(86)-21-64166128
First published on 26th January 2018
Abstract
Various base-controlled regioselective reactions of β,γ-unsaturated hydrazones with Selectfluor were achieved under transition metal-free conditions. In the presence of K2HPO4 or NaHCO3, the intramolecular aminofluorination reaction took place readily to give the corresponding monofluoromethylated dihydropyrazoles or fluoro-tetrahydropyridazines, respectively. The possible pathways were proposed based on the experimental results.
Introduction
Selective introduction of fluorine or fluorine-containing functional groups into organic molecules is becoming increasingly prevalent with ca. 40% of agrochemicals and ca. 20% of pharmaceuticals containing at least one fluorine atom.1 Compounds containing a C–F bond frequently experience dramatic changes of their properties (i.e. hydrophobicity, solubility, and metabolic stability) compared to their corresponding C–H counterparts.2 In particular, the synthesis of functionalized fluorinated heterocycles has become a very important weapon in the armamentarium of medicinal chemistry.3Dihydropyrazoles and tetrahydropyridazines are frequently found in natural products and pharmacologically active compounds. Being privileged and valuable N–N bond-containing heterocyclic scaffolds, they also serve as versatile intermediates in organic synthesis.4 Consequently, great efforts have been devoted to the development of elegant and creative strategies to construct these nitrogen heterocycles, and most research studies are based on thermal cycloaddition reactions.5,6 Recently, a series of hydrazonyl radical-involved cyclization reactions have emerged as new powerful protocols for their assembly.7,8 In these reactions, stoichiometric oxidants were usually employed to generate the key hydrazonyl radicals.
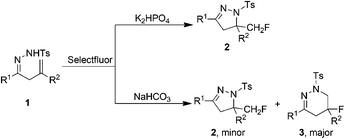
It is well known that the intramolecular aminofluorination of alkenes is a straightforward and step-economical protocol to synthesize nitrogen-containing heterocycles with vicinal amino and fluorine moieties.9 In 2009, Liu's group developed a Pd-catalyzed intramolecular aminofluorination of alkenes, which provided a variety of fluorinated piperidines.9a Later, further studies were conducted to make other types of fluorinated heterocycles.9b–i However, these elegant studies usually required transition metal catalysts and hypervalent iodine reagents.9 Recently, we reported a transition metal-free intramolecular cyclization of alkenyl oximes for the synthesis of monofluoromethylated isoxazolines.10 As part of our continuing research on the fluorination of unsaturated alkenes, we further studied the transition metal-free intramolecular cyclization/fluorination reaction of β,γ-unsaturated hydrazones. In the reaction, Selectfluor served as both oxidant and fluorine source.10,11 It was found that the reaction pathway could be controlled by different base, and the corresponding monofluoromethyl dihydropyrazoles and fluorotetrahydropyridazines were firstly obtained regioselectively in the presence of different bases (Scheme 1). The results are reported in this paper.
Results and discussion
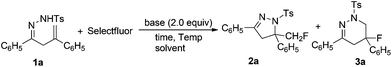
In our initial experiments, β,γ-unsaturated hydrazone 1a was chosen as the substrate. Based on our previous work,10 the reaction was carried out with 1.1 equiv. of Selectfluor and 2.0 equiv. of NaHCO3 in acetonitrile (CH3CN) at room temperature.However, only trace amount of desired product monofluoromethylated dihydropyrazole 2a was observed with a large amount of 1a being recovered (Table 1, entry 1). Increasing the reaction temperature to 50 °C improved the yield of 2a to 25% (entry 2). Surprisingly, when the reaction was carried out at above 80 °C, fluorotetrahydropyridazine 3a was formed as the major product along with a small amount of 2a. At 100 °C, the reaction could finish in only 1 hour and 3a was obtained in 54% yield (entries 3–7). Other solvents, such as dimethylformamide (DMF), ethyl acetate (EtOAc), acetone, tetrahydrofuran (THF), diethyl ether (Et2O), dichloromethane (DCM) or toluene, were not suitable for this reaction. It is worth mentioning that only 2a was formed in nitromethane (CH3NO2) (entry 8).
| Entrya |
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [F] [F] |
Base | Solvent | t/h | Temp/°C |
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/% b/% |
3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/% b/% |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), Selectfluor (0.2–0.28 mmol), base (0.4 mmol), solvent (4 mL), under a nitrogen atmosphere. b Determined by 19F NMR spectroscopy based on 1a using PhCF3 as the internal standard. | |||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
NaHCO3 | CH3CN | 12 | rt | 3 | — |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
NaHCO3 | CH3CN | 12 | 50 | 25 | — |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
NaHCO3 | CH3CN | 12 | 80 | 10 | 39 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
NaHCO3 | CH3CN | 12 | 100 | 10 | 55 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
NaHCO3 | CH3CN | 12 | 120 | 16 | 50 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
NaHCO3 | CH3CN | 4 | 100 | 12 | 52 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
NaHCO3 | CH3CN | 1 | 100 | 11 | 54 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
NaHCO3 | CH3NO2 | 1 | 100 | 42 | — |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
NaHCO3 | CH3CN | 1 | 100 | 17 | 46 |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
NaHCO3 | CH3CN | 1 | 100 | 12 | 66 |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
NaHCO3 | CH3CN | 1 | 100 | 22 | 51 |
| 12 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
— | CH3CN | 1 | 100 | 20 | — |
| 13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
Na2CO3 | CH3CN | 1 | 100 | 26 | 10 |
| 14 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
KHCO3 | CH3CN | 1 | 100 | 23 | 17 |
| 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
K2CO3 | CH3CN | 1 | 100 | 16 | 15 |
| 16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
NaOH | CH3CN | 1 | 100 | 15 | 5 |
| 17 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
Cs2CO3 | CH3CN | 1 | 100 | — | — |
| 18 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
K2HPO4·3H2O | CH3CN | 1 | 100 | 37 | — |
| 19 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
KH2PO4 | CH3CN | 1 | 100 | 60 | — |
| 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
NaH2PO4 | CH3CN | 1 | 100 | 60 | — |
| 21 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
K3PO4 | CH3CN | 1 | 100 | 24 | — |
| 22 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
Na2HPO4 | CH3CN | 1 | 100 | 64 | — |
| 23 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
K2HPO4 | CH3CN | 1 | 100 | 79 | — |
| 24 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
DABCO | CH3CN | 1 | 100 | — | — |
| 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
NEt3 | CH3CN | 1 | 100 | — | — |
The ratio of 1a and Selectfluor was then evaluated. It was found that the best result with respect to regioselectivity and the yield of 3a was obtained with 1.2 equiv. of Selectfluor (entries 9–11). Further screening of bases showed that inorganic bases such as Na2CO3, KHCO3, K2CO3, NaOH and Cs2CO3 gave both isomers with lower selectivities and yields (entries 13–17). However, it was interesting to find that some phosphates, such as K2HPO4·3H2O, KH2PO4, NaH2PO4, K3PO4, Na2HPO4 and K2HPO4 afforded 2a as the sole product (entries 18–23), and up to 79% yield of 2a was achieved in the presence of K2HPO4 (entry 23). No desired product was observed when organic bases such as DABCO and triethylamine were used (entries 24 and 25). These results indicated that the regioselectivity of this intramolecular aminofluorination reaction could be controlled by different bases: NaHCO3 helped to form fluorotetrahydropyridazine 3a as the major product, while only monofluoromethylated dihydropyrazole 2a was obtained in the presence of K2HPO4. This might be attributed to the basicity and solubility of the base. NaHCO3 (pKa = 10.25) is a medium strength base with good solubility in the reaction system, but K2HPO4 (pKa = 12.32) is poorly soluble in the reaction system.12
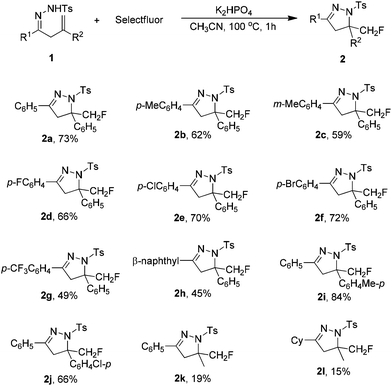
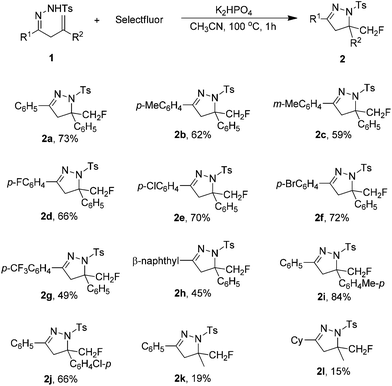
Under the optimized conditions (Table 1, entry 23), the scope of β,γ-unsaturated hydrazones 1 was first investigated in the K2HPO4-promoted reaction. As shown in Table 2, various functional groups (R1) in the hydrazone moiety were compatible with this reaction system. The substituent in the benzene ring, either electron-donating or electron-withdrawing, had a little influence on the yield of 2. For example, the reaction of 1b with a p-methylphenyl substituent gave desired product 2b in 62% yield. For hydrazones 1d–f bearing a halide substituent in the phenyl ring, the corresponding products 2d–f were obtained in 66–72% yields. Only 49% yield was achieved with hydrazone 1g containing a strong electron-withdrawing trilfuoromethyl group. Hydrazone 1h with a β-naphthyl group could also react with Selectfluor to give 2h in 45% yield. However, the reaction was complicated when R1 was an aliphatic substituent such as cyclohexyl group. Regarding the substituents in the alkene moiety (R2), it was found that hydrazones bearing an aryl group were suitable substrates for this reaction (2i and 2j), and poor results were obtained again with alkyl substitution (2k and 2l).
| a Reaction conditions: 1 (0.2 mmol), Selectfluor (0.24 mmol), K2HPO4 (0.4 mmol), CH3CN (4 mL), 100 °C, under a nitrogen atmosphere, 1 h. Isolated yields. |
|---|
|
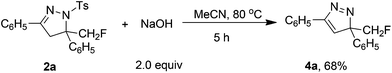
The p-toluenesulfonyl group in products 2 could be easily removed under mild conditions. The result shown in Scheme 2 indicated that compound 2a can be efficiently converted into biologically important monofluoromethyl 3H-pyrazole 4a by treatment with NaOH, and no defluorination reaction was observed in this reaction.
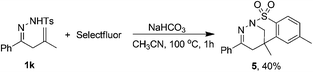
Next, we studied the scope of the NaHCO3-promoted reaction (Table 1, entry 10). According to the results in Table 3, this protocol also shows a broad substrate scope and functional group tolerance, giving fluorotetrahydropyridazines 3 as the major products. For example, a series of hydrazones bearing an aryl substituent in both hydrazone and alkene moieties reacted well with Selectfluor to give the corresponding products (3b–f and 3h–j) in moderate to good yields along with a small amount of monofluoromethylated dihydropyrazoles 2. The substitution pattern of the phenyl ring and the electronic property of the substituents had no obvious effect on the reaction. Unfortunately, hydrazone 1k containing a methyl substituent failed to give the expected cyclic product 3k, and compound 5 was obtained in 40% yield instead (Scheme 3).
| a Reaction conditions: 1 (0.2 mmol), Selectfluor (0.24 mmol), NaHCO3 (0.4 mmol), CH3CN (4 mL), 100 °C, under a nitrogen atmosphere, 1 h. Isolated yields of 3. Ratio determined by 19F NMR spectroscopy. |
|---|
|
Taking product 3a as an example, the removal of the Ts group in products 3 was also tried. Unfortunately, the treatment of 3a with NaOH resulted in the removal of both the Ts group and fluorine atom (see the ESI†). Other reagents, such as HBr/AcOH and HF/pyridine, did not give the desired deprotection product either.
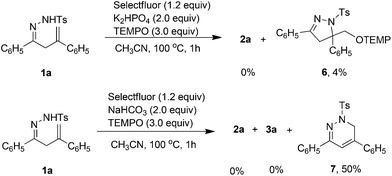
To gain additional insights into the reaction mechanism, the control experiments were conducted (Scheme 4). When 3.0 equiv. of the radical scavenger TEMPO was added to the K2HPO4-promoted system, the aminofluorination reaction was completely suppressed, and a small amount of aminooxygenation product 6 was observed in the reaction mixture. These results indicated that the fluorination might be a radical process. Similarly, the addition of TEMPO to the NaHCO3-promoted reaction also inhibited the formation of products 3a and 2a. Only 1,6-dihydropyradazine 7 was isolated in 50% yield. According to Xiao's research,13 compound 7 might be generated from a N-centered hydrazonyl radical intermediate.
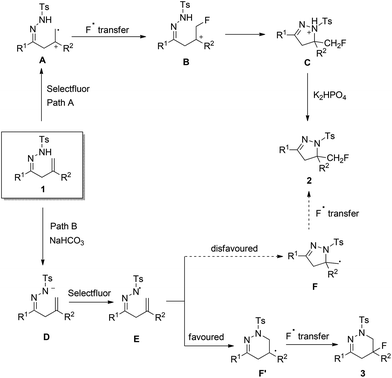
On the basis of the aforementioned mechanistic studies and related literature,10,13,14 plausible mechanisms were proposed for the base-controlled reactions. As shown in Scheme 5, the oxidation of 1 by Selectfluor might initially take place to form cation-radical intermediate A (Path A). Subsequent fluorination and cyclization of A give intermediate C, which affords product 2 by hydrogen abstraction. The existence of intermediate A and B has been confirmed through an ESI mass spectrum by Zhang et al.14 The presence of K2HPO4 may accelerate this process due to its compatible basicity. When R2 is an aryl group, intermediate A and B are more stable due to its stabilization effect and product 2 is formed in higher yield.
However, in the presence of NaHCO3, the proton atom of NHTs in hydrazones 1 may also be abstracted firstly to give anionic intermediate D, which is transformed to N-centred radical E by Selectfluor through a SET process (Path B). Then, intermediate F and F′ may be formed from E through 5-exo radical cyclization and 6-endo radical cyclization, respectively. According to the density functional theory (DFT) investigations by Xiao's group,13 the 6-endo cyclization of the N-radical was more feasible than the 5-exo process when R2 was an aryl group. In the NaHCO3-promoted reaction, both Path A and Path B may exist as competing reactions, and Path B is the dominant one which affords fluorotetrahydropyridazine 3 as the major product via intermediate F′.
Conclusions
In summary, we have successfully developed a base-controlled regioselective intramolecular aminofluorination of β,γ-unsaturated hydrazones. Using Selectfluor as the fluorine source, monofluoromethylated dihydropyrazoles and fluoro-tetrahydropyridazines were synthesized selectively in moderate to good yields in the presence of K2HPO4 or NaHCO3, respectively. Good functional group compatibility, scalability, easily available materials, and mild reaction conditions characterize this protocol. Efforts towards an asymmetric variant of this transformation are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation (No. 21572257 and 21502213) and the Science and Technology Commission of Shanghai Municipality (No. 16XD1404400) for generous financial support.Notes and references
- (a) A. M. Thayer, Chem. Eng. News, 2006, 84, 15 Search PubMed; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC.
- (a) J. T. Welch and S. Eswarakrishman, Fluorine in Bioorganic Chemistry, Wiley, New York, 1991 Search PubMed; (b) R. E. Banks, B. E. Smart and J. C. Tatlow, Organofluorine Chemistry: Principles and Commercial Applications, Plenum Press, New York, 1994 Search PubMed; (c) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed.
- (a) D. Cahard, X. Xu, S. Couve-Bonaire and X. Pannecoucke, Chem. Soc. Rev., 2010, 39, 558 RSC; (b) P. Liu, A. Sharon and C.-K. Chu, J. Fluorine Chem., 2008, 129, 743 CrossRef CAS PubMed; (c) K. B. Park, N. R. Kitteringham and P. M. O'Neill, Annu. Rev. Pharmacol. Toxicol., 2001, 41, 443 CrossRef PubMed; (d) A. V. Petrov, Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications, John Wiley & Sons, Inc., New York, NY, 2009 Search PubMed; (e) V. Gouverneur and K. Mìller, Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications, Imperial College Press, London, 2012 Search PubMed; (f) D. Barnes-Seeman, J. Beck and C. Springer, Curr. Top. Med. Chem., 2014, 14, 855 CrossRef CAS PubMed.
- For selected reviews, see: (a) L. M. Blair and J. Sperry, J. Nat. Prod., 2013, 76, 794 CrossRef CAS PubMed; (b) C. G. Wermuth, MedChemComm, 2011, 2, 935 RSC; (c) A. Marella, R. Ali, T. Alam, R. Saha, O. Tanwar, M. Akhter, M. Shaquiquzzaman and M. Mumtaz Alam, Mini-Rev. Med. Chem., 2013, 13, 921 CrossRef CAS PubMed; (d) M. Yusuf and P. Jain, Arab. J. Chem., 2014, 7, 553 CrossRef CAS; (e) J. M. Alex and R. J. Kumar, J. Enzyme Inhib. Med. Chem., 2014, 29, 427 CrossRef CAS PubMed; (f) W. Maison and C. H. Küchenthal, Synthesis, 2010, 719 CrossRef. For selected examples, see: (g) B. N. Acharya, D. Saraswat, M. Tiwari, A. K. Shrivastava, R. Ghorpade, S. Bapna and M. P. Kaushik, Eur. J. Med. Chem., 2010, 45, 430 CrossRef CAS PubMed; (h) D. Dardic, G. Lauro, G. Bifulco, P. Laboudie, P. Sakhaii, A. Bauer, A. Vilcinskas, P. E. Hammann and A. Plaza, J. Org. Chem., 2017, 82, 6032 CrossRef CAS PubMed.
- For selected examples on the synthesis of dihydropyrazoles, see: (a) J. R. Chen, W. R. Dong, M. Candy, F. F. Pan, M. Jorres and C. Bolm, J. Am. Chem. Soc., 2012, 134, 6924 CrossRef CAS PubMed; (b) M. Rueping, M. S. Maji, H. B. Kucuk and I. Atodiresei, Angew. Chem., Int. Ed., 2012, 51, 12864 CrossRef CAS PubMed; (c) X. Hong, H. B. Kücük, M. S. Maji, Y. F. Yang, M. Rueping and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 13769 CrossRef CAS PubMed; (d) C. B. Tripathi and S. Mukherjee, Org. Lett., 2014, 16, 3368 CrossRef CAS PubMed; (e) O. A. Attanasi, L. De Crescentini, G. Favi, F. Mantellini, S. Mantenuto and S. Nicolini, J. Org. Chem., 2014, 79, 8331 CrossRef CAS PubMed; (f) X. Wu, M. Wang, G. Zhang, Y. Zhao, J. Wang and H. Ge, Chem. Sci., 2015, 6, 5882 RSC; (g) D. Y. Zhang, L. Shao, J. Xu and X. P. Hu, ACS Catal., 2015, 5, 5026 CrossRef CAS; (h) J. Cheng, P. Xu, W. Li, Y. Cheng and C. Zhu, Chem. Commun., 2016, 52, 11901 RSC.
- For selected examples on the synthesis of tetrahydropyridazines, see: (a) M. C. Tong, X. Chen, J. Li, R. Huang, H. Y. Tao and C. J. Wang, Angew. Chem., Int. Ed., 2014, 53, 4680 CrossRef CAS PubMed; (b) J. Li, R. Huang, Y. K. Xing, G. Qiu, H. Y. Tao and C. J. Wang, J. Am. Chem. Soc., 2015, 137, 10124 CrossRef CAS PubMed; (c) L. Wei and C. J. Wang, Chem. Commun., 2015, 51, 15374 RSC; (d) S. M. M. Lopes, M. S. C. Henriques, J. A. Paixao and T. M. V. D. Pinho e Melo, Eur. J. Org. Chem., 2015, 6146 CrossRef CAS; (e) X. Zhong, J. Lv and S. Luo, Org. Lett., 2016, 18, 3150 CrossRef CAS PubMed; (f) L. K. B. Garve, M. Petzold, P. G. Jones and D. B. Werz, Org. Lett., 2016, 18, 564 CrossRef CAS PubMed; (g) A. M. Shelke and G. Suryavanshi, Org. Lett., 2016, 18, 3968 CrossRef CAS PubMed; (h) X. L. Yang, X. X. Peng, F. Chen and B. Han, Org. Lett., 2016, 18, 2070 CrossRef CAS PubMed; (i) Y. Deng, C. Pei, H. Arman, K. Dong, X. Xu and M. P. Doyle, Org. Lett., 2016, 18, 5884 CrossRef CAS PubMed; (j) L. Wei, Y. Zhou, Z. M. Song, H. Y. Tao, Z. Lin and C. J. Wang, Chem. – Eur. J., 2017, 23, 4995 CrossRef CAS PubMed; (k) X. R. Zhong, J. Lv and S. Z. Luo, Org. Lett., 2015, 17, 1561 CrossRef CAS PubMed.
- For selected reviews on N-radical chemistry, see: (a) S. Z. Zard, Chem. Soc. Rev., 2008, 37, 1603 RSC; (b) J. R. Chen, X. Q. Hu, L. Q. Lu and W. J. Xiao, Chem. Soc. Rev., 2016, 45, 2044 RSC; (c) T. Xiong and Q. Zhang, Chem. Soc. Rev., 2016, 45, 3069 RSC.
- (a) X. Y. Duan, N. N. Zhou, R. Fang, X. L. Yang, W. Yu and B. Han, Angew. Chem., Int. Ed., 2014, 53, 3158 CrossRef CAS PubMed; (b) X. Y. Duan, X. L. Yang, R. Fang, X. X. Peng, W. Yu and B. Han, J. Org. Chem., 2013, 78, 10692 CrossRef CAS PubMed; (c) X. Y. Duan, X. L. Yang, P. P. Jia, M. Zhang and B. Han, Org. Lett., 2015, 17, 6022 CrossRef CAS PubMed; (d) E. Brachet, L. Marzo, M. Selkti, B. König and P. Belmont, Chem. Sci., 2016, 7, 5002 RSC; (e) F. Punner, Y. Sohtome and M. Sodeoka, Chem. Commun., 2016, 52, 14093 RSC.
- (a) T. Wu, G. Yin and G. Liu, J. Am. Chem. Soc., 2009, 131, 16354 CrossRef CAS PubMed; (b) T. Xu, S. Qiu and G. Liu, Chin. J. Chem., 2011, 29, 2785 CrossRef CAS; (c) Q. Wang, W. Zhong and X. Wei, Org. Biomol. Chem., 2012, 10, 8566 RSC; (d) T. Wu, J. Cheng, P. Chen and G. Liu, Chem. Commun., 2013, 49, 8707 RSC; (e) H. Huang, T. Lacy, B. Bzachut, G. Ortiz Jr. and Q. Wang, Org. Lett., 2013, 15, 1818 CrossRef CAS PubMed; (f) W. Kong, P. Feige, T. de Haro and C. Nevado, Angew. Chem., Int. Ed., 2013, 52, 2469 CrossRef CAS PubMed; (g) Z. Li, L. Song and C. Li, J. Am. Chem. Soc., 2013, 135, 4640 CrossRef CAS PubMed; (h) D. Lu, G. Liu and C. Zhu, Org. Lett., 2014, 16, 2912 CrossRef CAS PubMed; (i) J. Cui, Q. Jia, R. Feng, S. Liu, T. He and C. Zhang, Org. Lett., 2014, 16, 1442 CrossRef CAS PubMed.
- J. Zhao, M. Jiang and J. T. Liu, Adv. Synth. Catal., 2017, 359, 1626 CrossRef CAS.
- F. Yin, Z.-T. Wang, Z.-D. Li and C.-Z. Li, J. Am. Chem. Soc., 2012, 134, 10401 CrossRef CAS PubMed.
- C. T. Kenner and K. W. Busch, Quantitative Analysis, Macmillan Publishing Co., Inc., New York, NY, 1973 Search PubMed.
- X. Q. Hu, X. T. Qi, J. R. Chen, Q. Q. Zhao, Q. Wei, Y. Lan and W. J. Xiao, Nat. Commun., 2016, 7, 11188 CrossRef PubMed.
- Q. Zhang, G. F. Zheng, Q. Zhang, Y. Li and Q. Zhang, J. Org. Chem., 2017, 82, 8258 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, spectral data and copies of spectra. See DOI: 10.1039/c7qo01105a |
| This journal is © the Partner Organisations 2018 |