A photoactivatable photochromic system serves as a self-hidden information storage material†
Yuanyuan
Li
 ab,
Kai
Li
ab,
Kai
Li
 *bc and
Ben Zhong
Tang
*c
*bc and
Ben Zhong
Tang
*c
aCollege of Chemistry, Chemical and Environmental Engineering, Henan University of Technology, Henan 450001, P. R. China
bCollege of Chemistry and Molecular Engineering, Zhengzhou University, Henan 450001, P. R. China. E-mail: likai@zzu.edu.cn
cDepartment of Chemistry and Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, The Hong Kong University of Science & Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk
First published on 15th August 2017
Abstract
A photoactivatable self-hidden information storage material was developed based on the connection of a photo-removable protecting group of o-nitrobenzyl with a rhodamine B salicylaldehyde hydrazone Zn(II) complex photochromic system. Information could be recorded on this material by UV light and read out only by blue light with a wavelength around 450 nm, which makes it safe and covert.
In recent years, there has been a growing focus on the development of new photochromic systems which could be applied in chemistry, materials science, physics and biology.1–10 Compared with classic photochromic systems such as azobenzene, spiropyran and dithienylethene,11–13 the rhodamine B salicylaldehyde hydrazone metal complex (RMC) is a more recently reported photochromic system which can be used both in solution and in a solid matrix with good fatigue resistance.14–18 Interestingly, the mechanistic study shows that the phenolic hydroxyl in the salicylaldehyde moiety is indispensable for its photochromism. If the phenolic hydroxyl is protected by an inert group, the photochromic performance would be inhibited.15 This unique property gives RMC diverse potential applications in multi-factor controlled photochromic systems. In this work, a photo-removable protecting group (PPG) of o-nitrobenzyl was connected to the phenolic hydroxyl and the very first photoactivatable self-hidden information storage material was developed.
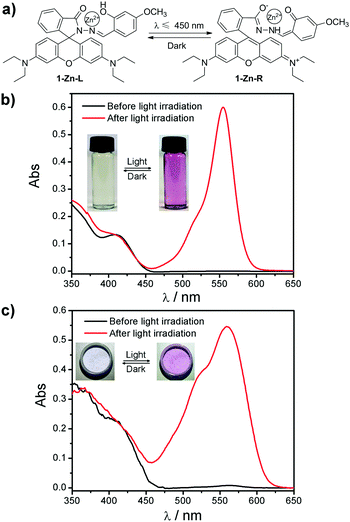
Rhodamine B 4-methoxysalicylaldehyde hydrazone (1) and its Zn(II) complex (1-Zn) were prepared according to previous reports.14 As expected, the 1-Zn complex exhibited an obvious color change from the leuco form to the red form upon light irradiation (Fig. 1). It can be seen from the absorbance spectra that the 1-Zn complex displayed no absorption band above 450 nm before light irradiation both in THF and PEG (polyethylene glycol, average molecular weight 6000) (Fig. 1b and c). However, a strong band centered at 554 nm emerged after light irradiation. The photochromic mechanism of 1-Zn could be deduced as follows referring to our previous work:15 before light irradiation, 1-Zn was in its leuco form (1-Zn-L). Light irradiation promoted the tautomerism of the salicylaldehyde hydrazone moiety in the complex from its enol form to its keto form. This change enhanced the chelation of 1 with Zn(II) and induced a spirolactam ring-opening reaction in 1-Zn to yield a rhodamine B ring-open product with a red color (1-Zn-R). This mechanism suggested that the phenolic hydroxyl in the salicylaldehyde hydrazone moiety was indispensable for the photochromism.
To determine the influence of the wavelength of the available light on the photochromism of the 1-Zn complex, different light sources were tested in this experiment. As shown in Table 1, not only the UV light but also the visible light from purple to blue (400–450 nm) could prompt the color change of 1-Zn. However, this change could not be achieved by visible light with a longer wavelength (>450 nm). These results are in accordance with the absorbance spectra of 1-Zn in Fig. 1 since before light irradiation, no absorption band above 450 nm was observed, which meant that light with a wavelength longer than 450 nm could not be absorbed by 1-Zn.
| Wavelength/nm | 365 | 405 | 425 | 450 | 475 | 500 | 600 | 650 |
|---|---|---|---|---|---|---|---|---|
| Color change of 1-Zn | Yes | Yes | Yes | Yes | No | No | No | No |
| C–O bond cleavage in 2 | Yes | Yes | No | No | No | No | No | No |
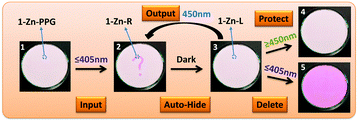
Inspired by the above results, a novel photoactivatable self-hidden information storage system was designed (1-Zn-PPG). As shown in Fig. 2, a suitable PPG, which could be activated by light with a wavelength shorter than 450 nm (e.g. 405 nm), was connected to the phenolic hydroxyl of 1-Zn to give 1-Zn-PPG. This system would be photostable under light with a wavelength longer than 405 nm but would be photochromic if the wavelength of the light was 405 nm or shorter. The information storage could be mainly divided into three steps: input, auto-hide and output. Firstly, UV light (≤405 nm) was applied to induce the photochromic reaction in a specific area of 1-Zn-PPG to record the desired information as the colored form (input). During this process, the PPG in this area would be released from the molecular matrix and leave the colored form (1-Zn-R). Secondly, the UV light was removed, followed by the transformation from the colored form (1-Zn-R) to the leuco form (1-Zn-L) and the colored information gradually faded away (auto-hide). Thirdly, blue light with a wavelength of around 450 nm was used when the recorded information needed to be displayed (output). This blue light could turn the UV-irradiated part to red again because this part existed as 1-Zn-L. The other parts remained unchanged since they were still 1-Zn-PPG, which is photostable under blue light. In the output step, blue light must be chosen because if the wavelength of the light was too long, the information-recorded area of 1-Zn molecules would be photostable (Table 1) and the information could not be revealed (protect). Also, if the wavelength was too short, all of the materials including the parts of 1-Zn-PPG would convert to the keto form (1-Zn-R) with color and the recorded information would be destroyed (delete). In short, only certain blue light with a wavelength around 450 nm could read out the information, which makes the information safe and covert.
According to the above proposed mechanism, a question arose: which PPG should be chosen? Theoretically, any PPG with a photoactivatable wavelength shorter than 450 nm can meet the requirement. However, there are some other factors which need to be considered in practical applications. On the one hand, since the range of visible light seen by human eyes is about 400–800 nm, any light with a wavelength shorter than 400 nm is usually harmful to the organism.19–21 Therefore, it is ideal to select a PPG with a photoactivatable wavelength longer than 400 nm. On the other hand, the photoactivatable wavelength should be as far away from 450 nm as possible to avoid mutual interference between the “input” and “output” processes. Given these two factors, a PPG with a photoactivatable wavelength of around 400 nm may be the best choice. Therefore, o-nitrobenzyl, which is one of the most widely used PPGs with a facile synthesis, is an ideal option.22–27 As shown in Scheme 1, the C–O bond in o-nitrobenzyl could be cleaved by UV light, producing a phenolic hydroxyl and an o-nitroso benzaldehyde. More importantly, only light with a short wavelength (≤405 nm) can prompt this reaction. Blue light or light with longer wavelengths cannot achieve this cleavage (Table 1).
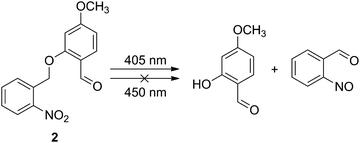 | ||
| Scheme 1 Photochemistry of 2 that results in the release of a leaving group to produce a phenolic hydroxyl group. | ||
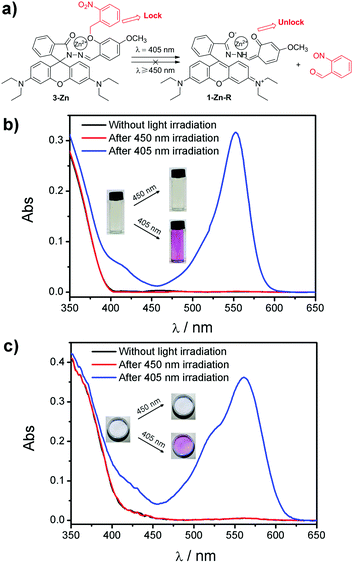
Based on these design concepts, complex 3-Zn, in which a leaving group of o-nitrobenzyl was connected to 1-Zn, was prepared. 3-Zn was facilely synthesized through a two-step reaction (see ESI†). As can be seen in Fig. 3a, the o-nitrobenzyl was treated as a “lock”, which caged the phenolic hydroxyl in the salicylaldehyde moiety. This “lock” was insensitive to light with a wavelength longer than 450 nm, ensuring that 3-Zn was photostable under long-wavelength light (≥450 nm). On the contrary, short-wavelength light (≤405 nm) could open this “lock” to yield the product of 1-Zn with a phenolic hydroxyl. The light subsequently induced the spirolactam ring-opening reaction in 1-Zn to get 1-Zn-R with a red color (Fig. 1a). When the light was removed, the red 1-Zn-R could turn back to the colorless 1-Zn-L under darkness (Fig. 1a).
The corresponding absorbance spectra changes of 3-Zn before and after 405/450 nm light irradiation in THF and PEG are shown in Fig. 3b and c. As shown, no absorption band around 554 nm is observed for 3-Zn before light irradiation both in THF and PEG. After long-wavelength light irradiation (450 nm), there was still no absorption change. Nevertheless, a strong band centered at 554 nm emerged after short-wavelength light irradiation (405 nm), which was in accordance with the appearance of the red color in the photographs. When the light was removed, the red color gradually faded away along with the absorption band at 554 nm decreasing (Fig. S1, ESI†). It was noticed that the absorbance spectra of the non-irradiated 3-Zn and dark-recovered 3-Zn (a mixture of 1-Zn-L and 2-nitrosobenzaldehyde) were very similar, which suggested that their colors were analogous to each other too, making them indistinguishable by the naked eye (Fig. S1 (inset), ESI†).
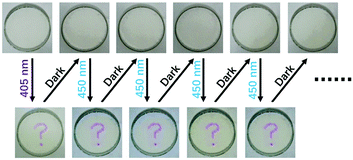
The information storage process on 3-Zn in PEG is demonstrated in Video 1 (ESI†). The information was firstly inputted by a purple-light laser pointer with a wavelength around 405 nm (the input step). After being kept in the dark for about 10 min, the information completely disappeared (the auto-hide step). Blue light with a wavelength of 450 nm was then used to irradiate this material. It can be seen that the information was read out successfully (the output step). More importantly, the information could be read out more than once (Fig. 4). This good property might be attributed to the good fatigue resistance of 1-Zn. As shown in Fig. S2 (ESI†), even though the material 1-Zn was toggled repeatedly between its leuco form and its colored form ten times, the absorbance at 554 nm was still stable without apparent degradation, indicating good fatigue resistance. Thus, the short-wavelength light irradiated part in 3-Zn, which had converted to 1-Zn, could be toggled repeatedly between its leuco form and its colored form by blue light and darkness many times.
In summary, a photoactivatable self-hidden information storage system was designed based on a rhodamine-B salicylaldehyde hydrazone metal complex photochromic system with a leaving group of o-nitrobenzyl as a “lock”. The mechanism for information storage was studied and could be divided into three steps: input, auto-hide and output. The information could be inputted into the material by UV light (≤405 nm). The information was then auto-hidden in darkness after the light was removed. The information could be read out only by light with a wavelength around 450 nm, since shorter-wavelength light would destroy the information while longer-wavelength light could not read it out. This work provides an available strategy for designing photoactivatable self-hidden information storage materials. Efforts on the development of more types of self-hidden information storage system, such as heat-activatable and electro-activatable self-hidden information storage, are in progress in our laboratories.
This research was financially supported by the National Natural Science Foundation of China (No. 51502079 and 21501150), the Scientific Research Foundation for Young Teachers of Zhengzhou University (51099075), the Fundamental Research Funds for the Henan Provincial Colleges and Universities (2015QNJH09), the Science Foundation of Henan University of Technology (2015BS004), the Research Grants Council of Hong Kong (16301614, 16305015 and N_HKUST604/14) and the Innovation and Technology Commission (ITC-CNERC14SC01).
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Bälter, S. Li, M. Morimoto, S. Tang, J. Hernando, G. Guirado, M. Irie, F. M. Raymo and J. Andréasson, Chem. Sci., 2016, 7, 5867–5871 RSC.
- M. Hammerich, C. Schütt, C. Stähler, P. Lentes, F. Röhricht, R. Höppner and R. Herges, J. Am. Chem. Soc., 2016, 138, 13111–13114 CrossRef CAS PubMed.
- M. Kathan, P. Kovaříček, C. Jurissek, A. Senf, A. Dallmann, A. F. Thünemann and S. Hecht, Angew. Chem., Int. Ed., 2016, 55, 13882–13886 CrossRef CAS PubMed.
- D. Ou, T. Yu, Z. Yang, T. Luan, Z. Mao, Y. Zhang, S. Liu, J. Xu, Z. Chi and M. R. Bryce, Chem. Sci., 2016, 7, 5302–5306 RSC.
- A. Takanabe, M. Tanaka, K. Johmoto, H. Uekusa, T. Mori, H. Koshima and T. Asahi, J. Am. Chem. Soc., 2016, 138, 15066–15077 CrossRef CAS PubMed.
- H. Wu, Y. Chen and Y. Liu, Adv. Mater., 2016, 29, 1605271 CrossRef PubMed.
- Y. Arai, S. Ito, H. Fujita, Y. Yoneda, T. Kaji, S. Takei, R. Kashihara, M. Morimoto, M. Irie and H. Miyasaka, Chem. Commun., 2017, 53, 4066–4069 RSC.
- M. Gao, H. Su, Y. Lin, X. Ling, S. Li, A. Qin and B. Z. Tang, Chem. Sci., 2017, 8, 1763–1768 RSC.
- J. Wang, Y. Gao, J. Zhang and H. Tian, J. Mater. Chem. C, 2017, 5, 4571–4577 RSC.
- L. Wang, Y. Li, X. You, K. Xu, Q. Feng, J. Wang, Y. Liu, K. Li and H. Hou, J. Mater. Chem. C, 2017, 5, 65–72 RSC.
- J. Zhang, Q. Zou and H. Tian, Adv. Mater., 2013, 25, 378–399 CrossRef CAS PubMed.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- E. Orgiu and P. Samorì, Adv. Mater., 2014, 26, 1827–1845 CrossRef CAS PubMed.
- K. Li, Y. Xiang, A. Tong and Z. Tang Ben, Sci. China: Chem., 2014, 57, 248–251 CrossRef CAS.
- K. Li, Y. Xiang, X. Wang, J. Li, R. Hu, A. Tong and B. Z. Tang, J. Am. Chem. Soc., 2014, 136, 1643–1649 CrossRef CAS PubMed.
- K. Li, Y. Li, J. Tao, L. Liu, L. Wang, H. Hou and A. Tong, Sci. Rep., 2015, 5, 14467 CrossRef CAS PubMed.
- Q. Feng, Y. Li, G. Shi, L. Wang, W. Zhang, K. Li, H. Hou and Y. Song, J. Mater. Chem. C, 2016, 4, 8552–8558 RSC.
- Y. Li, K. Li, L. Wang, Y. He, J. He, H. Hou and B. Z. Tang, J. Mater. Chem. C, 2017, 5, 7553–7560 RSC.
- X. Fang, Y. Bando, M. Liao, U. K. Gautam, C. Zhi, B. Dierre, B. Liu, T. Zhai, T. Sekiguchi and Y. Koide, Adv. Mater., 2009, 21, 2034–2039 CrossRef CAS.
- World Health Organization, Ultraviolet radiation and the INTERSUN Programme, 2010, available at http://www.who.int/uv/en/.
- H. F. Blum, Carcinogenesis by ultraviolet light, Princeton University Press, Princeton, 2015 Search PubMed.
- J. Barltrop, P. Plant and P. Schofield, Chem. Commun., 1966, 822–823 RSC.
- T. Kobayashi, T. Komatsu, M. Kamiya, C. u. Campos, M. González-Gaitán, T. Terai, K. Hanaoka, T. Nagano and Y. Urano, J. Am. Chem. Soc., 2012, 134, 11153–11160 CrossRef CAS PubMed.
- A. Rodrigues-Correia, X. M. Weyel and A. Heckel, Org. Lett., 2013, 15, 5500–5503 CrossRef CAS PubMed.
- Z. Liu, T. Liu, Q. Lin, C. Bao and L. Zhu, Chem. Commun., 2014, 50, 1256–1258 RSC.
- C. Y. Y. Yu, R. T. K. Kwok, J. Mei, Y. Hong, S. Chen, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2014, 50, 8134–8136 RSC.
- L. Peng, Y. Zheng, X. Wang, A. Tong and Y. Xiang, Chem. – Eur. J., 2015, 21, 4326–4332 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization of all compounds, selected spectra and data referred to in the paper. See DOI: 10.1039/c7qm00324b |
| This journal is © the Partner Organisations 2017 |