Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Non-cyclic formylated dipyrromethanes as phosphate anion receptors†
Murat K.
Deliomeroglu
,
Vincent M.
Lynch
and
Jonathan L.
Sessler
*
Department of Chemistry, The University of Texas at Austin, Texas 78712-1224, USA. E-mail: Sessler@cm.utexas.edu
First published on 24th February 2016
Abstract
New tetrakis- and hexakis(1H-pyrrole-2-carbaldehyde) anion receptors are described. The anion binding properties of these receptors were studied in organic media and in the solid state. The receptors displayed good affinity for the dihydrogenphosphate and pyrophosphate anions (as the tetrabutylammonium salts) in chloroform even in the presence of a polar protic solvent, methanol. Solution phase spectroscopic analyses proved consistent with the binding mode seen in single crystal structural studies of the dihydrogenphosphate and pyrophosphate complexes and provided support for the contention that these receptors undergo conformational reorganization in order to accommodate the bound oxoanions both in chloroform solution and in the solid state.
Introduction
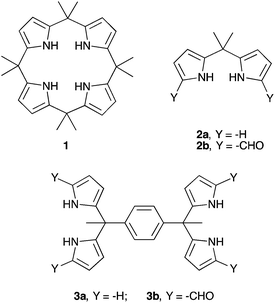
Inorganic phosphate and related anions are ubiquitous in nature. These anionic species play key roles in energy transduction and in the storage and expression of genetic information.1 Phosphates are also pervasive pollutants and may lead to eutrophication of lakes and waterways.2 The development of molecular constructs capable of binding the phosphate anion in its various protonated forms, as well as related oxoanion species is of critical interest since it may provide a first step towards solving various problems related to anion detection, extraction, and separation.3–6 In general, anions are larger than cations and therefore larger hosts are required to bind them.7 Fortunately, a number of binding motifs may be exploited to achieve anion recognition, including coulombic interactions, hydrogen bonding, halogen bonding, and anion–π interactions.8–12 Hydrogen bonding has proven particularly effective. However, certain anions, including oxoanions, such as dihydrogenphosphate (H2PO4−) and hydrogensulfate (HSO4−), are susceptible to proton transfer.13 As a result, ensuring anion binding rather than deprotonation (acid–base chemistry) has proven to be a challenging task.14,15 One hydrogen bond donor that is relatively less susceptible to deprotonation is pyrrole. An early example of a pyrrole-based system capable of binding the phosphate anion was the pentapyrrolic macrocycle, sapphyrin. This system, which is dicationic in its protonated form, proved capable of stabilizing a “sitting-atop” complex with the phosphate anion in the solid state and of binding DNA and other phosphate-containing species in solution.16 In 1996, Gale et al. reported that octamethylcalix[4]pyrrole (C[4]P; 1) may act as an anion receptor in organic media.17 Calix[4]pyrrole is a non-conjugated system long known in the literature.18 It was found to bind the fluoride and chloride anions in halogenated solvents, along with their counter cations in some cases.19,20 Halide anion recognition by C[4]P is accompanied by conformational change from a limiting 1,3-alternate conformation (the free form) to a cone conformation (halide-bonded form). This structural switching allows the formation of four NH–anion hydrogen bonds in the case of simple halide anions.17 Under most experimental conditions, C[4]P has proved less effective as a receptor for larger anions, including the dihydrogenphosphate anion. There thus remains a need for simple-to-prepare analogues of C[4]P that bind phosphate. Open-chain systems might allow this goal to be attained (Scheme 1).In the case of fluoride and chloride anion salts studied in organic media, compound 1 displayed association constants that were >2× greater than those recorded for the corresponding acyclic building block, dipyrromethane (DPM) 2a.21 This difference was ascribed to the greater pre-organization of the cyclic systems, as well as to the increased number of pyrrolic NH hydrogen bond donors provided by 1 compared to 2a. Interestingly, compound 2a displayed a higher affinity towards H2PO4− (as its tetra-n-butylammonium (TBA+) salt) than did 1 in CD2Cl2. While this apparent dichotomy was not studied in detail, it might reflect the fact that the dihydrogenphosphate anion is too large to be accommodated well within the NH-rich calix[4]pyrrole pseudo cavity. In contrast, the presumably greater flexibility of 2a might allow the stabilization of a greater number of favourable contacts with this and other oxoanions than compound 1. While untested, this hypothesis provides an incentive to prepare and study new dipyrromethane-based anion receptors. A goal of this effort would be to create phosphate anion receptors that rival C[4]P in terms of ease of preparation and which allow for good phosphate anion binding. In this study we focus on dipyrromethane (DPM) receptors that have been subject to formylation in the so-called α-pyrrolic positions.
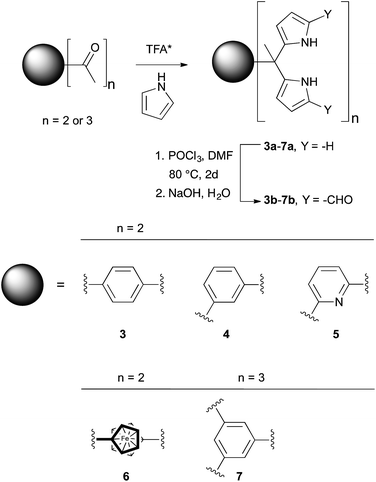
In 2003, in the context of synthetic work aimed at producing a C[4]P-texaphyrin chimera,22 the pyrrolic α-positions of 2a were subject to formylation to produce 2b. The anion binding properties of 2b with TBAF were studied by isothermal calorimetry (ITC) in acetonitrile and via X-ray crystallography in the solid state.23 On this basis, it was concluded that 2b was not an effective anion receptor. On the other hand, we recently found24 that the bisdipyyrromethane (bisDPM) 3a and its formylated derivative 3b, act as effective and somewhat selective receptors for the TBA+ salts of dihydrogenphosphate (H2PO4−) and hydrogenpyrophosphate (HP2O73−) in chloroform, as inferred from NMR spectroscopic and UV-Vis analyses. As compared to 1, the non-formylated system 3a proved to be a slightly better receptor for H2PO4− (Ka = 103vs. 102 M−1 in CHCl3 and CH2Cl2, respectively). DPM derivatives, which do not bear substituents in the pyrrolic α-positions are notoriously unstable, being prone inter alia to oxidative coupling and electrophilic aromatic substitution. On the other hand, bis-formylation yields stable systems that we believe may have a role to play as anion receptors. In preliminary work, high phosphate anion affinities were noted for the four-fold formylated derivative of 3a, receptor 3b (Ka = (8 ± 2) × 106 M−1 for 3bvs. (1.0 ± 0.1) × 103 M−1 for 3a in CHCl3; cf.Table 1).24 Presumably, this increased affinity reflects the favourable electronic changes, as well as the addition of hydrogen bond accepting sites, that result from formylation. To understand the underlying determinants and to improve on the phosphate binding affinity displayed by 3b, we have now prepared and studied several analogues bearing different spacer elements between the diformyl-DPM subunits, as well as a new hexakis(1H-pyrrole-2-carbaldehyde) receptor, 7b. Compared to 3b, receptor 7b displays a phosphate anion affinity that is enhanced by roughly 66 fold when studied under identical conditions (i.e., CHCl3 containing 3% CH3OH).
Results and discussion
Syntheses and single crystal structures
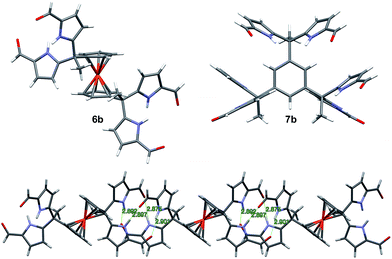
The synthesis and characterization of 3b–5b have been reported previously.24 In this paper, we present the new derivatives, 6b and 7b, obtained in moderate overall yields. The synthetic route to obtain 3b–7b is shown in Scheme 2. In the first step, commercially available diacetyl and triacetyl starting materials were converted into bisDPM 6a, and trisdipyrromethane (trisDPM) 7a, respectively, albeit in low yields (5–15%). The low yields stand in contrast to high yields observed for 3a and 5a, which precipitate from the reaction mixture and constitute the dominant reaction products.24 The unstable α-free bisDPM (6a) and trisDPM (7a) were then subject to Vilsmeier–Haack formylation to produce the stable formylated products 6b and 7b in relatively high yields (ca. 80%). Purification of 6b was carried out by recrystallization from N,N-dimethylformamide (DMF) at 80 °C, whereas 7b was purified by column chromatography over silica gel. The molecular structures were characterized by 1H and 13C NMR spectroscopy, high-resolution mass spectrometry (HRMS), and single crystal X-ray diffraction analyses.Receptor 6b co-crystallised with two molecules of DMF. The single crystal structure revealed that two diformyl-DPM units residing on opposite cyclopentadienyl (Cp) rings of the ferrocene were locked at about 90° from one another, leading to the presence of a racemic mixture of conformational isomers within the single crystal. Fig. 1 shows one of the atropisomers of 6b. It crystallizes as a one-dimensional hydrogen bond interlocked ensemble, even in the presence of DMF. The formation of these assemblies can be rationalized on a geometric basis. The bridging ferrocene adopts a conformation such that the two constituent Cp rings, each bearing a DPM substituent, are offset from one another by 90°. This allows for the formation of four intermolecular hydrogen bonds between the two self-complementary diformyl-DPM subunits, as can be seen from an inspection of Fig. 1.
Receptor 7b, on the other hand, co-crystallised with CHCl3. Each of the three diformyl-DPM units on the bridging benzene is oriented in a different direction (cf.Fig. 1). One of the three DPM subunits was found to resemble what is seen in the case of 3b24 wherein two diformyl-DPM units are seen to orient towards opposite π-faces of the intervening benzene ring. On the other hand, two of the diformyl-DPM units present in 7b adopt conformations in which the pyrrole NH groups point away from one another. As a result, 7b forms dimers stabilised by four hydrogen bonds that extend to create two-dimensional layers (cf. Fig. S2†). The assemblies formed by 6b and 7b may be viewed as being examples of Gale and co-worker's “narcissistic dimer”15 that are extended into one and two-dimensional space, respectively. The hydrogen bond interlocked aggregates seen in the solid state structures of 6b and 7b may account for the low solubility these species display in many solvents.
Anion binding studies
The anion selectivity for 3b–5b in CHCl3 was found to be H2PO4− > HP2O73− > HSO4− > C6H5CO2− ≫ NO3−, Cl− (as the commercially available TBA+ salts).24 The observed selectivity was not found to correlate with the basicity of the anions (basicity order: HP2O73− > C6H5CO2− > H2PO4− > HSO4−, NO3−, Cl−). Rather, the trend appears to correlate with an ability of the anion to interact with the receptor via hydrogen bond donation to the formyl groups (in addition to acting as a Lewis basic hydrogen bond acceptor for the pyrrolic NH protons). In fact, the H2PO4− anion, with two hydrogen bond donating sites (rather than one or none as in the other test anions), is bound with the highest relative affinity.The effect of the formyl groups in abetting dihydrogenphosphate anion binding is substantial (i.e., several orders of magnitude difference in Ka values). This was first noted for 3avs.3b in the context of our original report (vide supra).24 Further support for this conclusion came from a comparison of the α-free derivative 5a with its formylated congener 5b (cf.Table 1). Unfortunately, the other non-formylated DPM derivatives prepared in the context of this study proved too unstable to allow for analyses of their anion binding affinities.
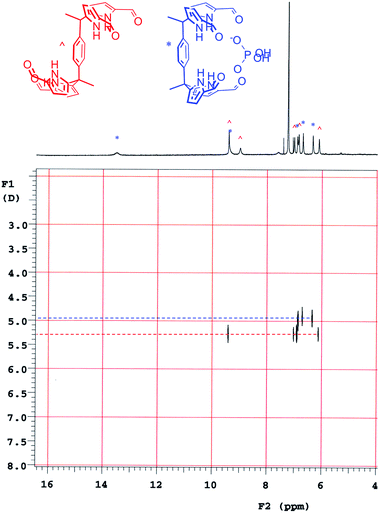
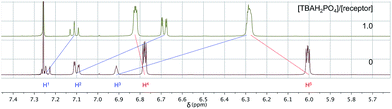
Diffusion ordered NMR spectroscopy (DOSY)25 was used to examine the interactions between anions and receptor 3b. Receptor 3b was chosen for these studies for two reasons. First, the low solubility of 3b in CHCl3 means that an equilibrium was expected to exist between species free in solution and those tied up in the solid state. Secondly, on the 1H NMR time scale, free receptor 3b and the H2PO4− bound complex (3b·H2PO4−) were subject to slow-exchange,24 allowing both species to be observed concurrently. Fig. 2 shows the DOSY spectrum of a mixture of 3b at the half-equivalence point obtained by titrating 3b with TBAH2PO4 in CDCl3. The one-dimensional 1H NMR spectrum in the horizontal axis shows two sets of receptor signals; free 3b and 3b·H2PO4−, labelled with red and blue asterisks, respectively.
The two-dimensional spectrum is characterized by the presence of two sets of receptor signals corresponding to slightly different diffusion rates. The complex 3b·H2PO4− (blue) features a lower mobility than the free receptor 3b (red). No evidence of higher order (i.e., aggregated) species is seen. Based on these observations, we conclude that in the absence of complete conversion to the bound form, samples of 3b and TBAH2PO4 consist of only two receptor species, namely the free form and the bound complex, 3b·H2PO4−. Support for the existence of two components in solution came from a UV-Vis study,24 also carried out in CHCl3. When the total concentration of 3b was held constant, isosbestic behaviour was seen upon titration with TBAH2PO4. This is as expected for a receptor solution consisting of two interconverting species.24
Mass spectrometric (MS) analyses provided evidence that receptor 3b would interact with the H2PO4− anion in the gas phase. In these studies, an equimolar mixture of 3b and TBAH2PO4 in CHCl3 was directly injected into the MS instrument without passing the species through a column. Negative ion electrospray ionization (ESI−) was used. The high-resolution MS analysis revealed two expected signals, namely, one at m/z 505.1885 corresponding to [3b-H]− and one at m/z 603.1643 ascribable to the H2PO4− complex ([3b·H2PO4−]). (cf. Fig. S4†).
Further insights into the binding modes operative in the case of the present series of receptors came from single crystal X-ray diffraction analyses.26 Diffraction-grade single crystals of the pyrophosphate complex, 3b·(TBA)2H2P2O7, were obtained by layering a solution of 3b·H2P2O72− in CH2Cl2 with n-pentane. The complex 3b·H2P2O72− in CH2Cl2 was prepared by treating 3b with (TBA)3HP2O7. This served to improve the net solubility by converting the poorly soluble receptor (vide supra) to the corresponding pyrophosphate complex. In the solid state, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
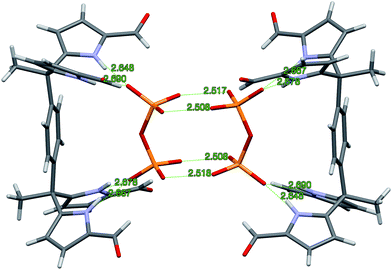
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex, [3b·H2P2O72−]2 is seen. The overall complex consists of a hydrogen-bonded dimer that lies across a crystallographic inversion centre (cf.Fig. 3).
2 complex, [3b·H2P2O72−]2 is seen. The overall complex consists of a hydrogen-bonded dimer that lies across a crystallographic inversion centre (cf.Fig. 3).
The protonation state of the bound pyrophosphate in the complex [3b·H2P2O72−]2 was deduced from the presence of two TBA+ counter cations per pyrophosphate dianion. The H2P2O72− anion lies parallel to the π-surface of the bridging benzene ring. Both diformyl-DPM arms are oriented such that four hydrogen bonds between the diformyl-DPM units in 3b and the bound H2P2O72− are favoured. The N–H⋯O distances between the NH protons of 3b and the O atoms of H2P2O72− range from 2.648 to 2.690 Å, separations that are short compared to typical N–H⋯O distances.27,28 Each bound H2P2O72− interacts with another pyrophosphate anion with the result that a hydrogen bonded dimer is formed. In the dimer, four protons are shared between eight oxygen atoms. The observed O⋯O distances of 2.508–2.518 Å are within the range expected for this type of proton bridged O–H⋯O type interaction.28–30 Because the hydrogen atoms on H2P2O72− are involved in dimer formation, the formyl groups do not participate in the hydrogen bonds that serve to bind the anion. Indeed, the formyl groups point away from the bound H2P2O72− guest.
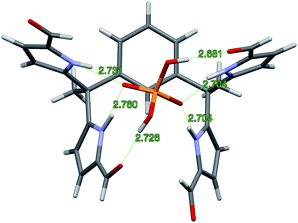
Diffraction grade single crystals of the complex 4b·TBAH2PO4 were obtained in a similar way. The resulting structure is shown in Fig. 4. There is no evidence of dimerization in this case. The lack of dimerization is ascribed to the relatively tight binding between the TBA+ counter cation and the bound dihydrogenphosphate guest, which precludes further inter-complex interactions. In the solid state, complex 4b·H2PO4− displays the same conformation for the diformyl-DPM units as observed in 3b·H2P2O72−. The four N–H⋯O distances that characterize the interaction between the NH protons of 4b and the O atoms of H2PO4− range from 2.704 to 2.760 Å. The two O–H⋯O distances associated with the hydrogen bonding between the formyl groups and the hydrogen atoms of H2PO4− are 2.681 and 2.726 Å, respectively. Overall, derivative 4b stabilizes six hydrogen bonding interactions with the H2PO4− anion. Specifically, it acts as a four-fold donor via the pyrrolic NH protons and acts as a two-fold acceptor via the two formyl moieties.
Single crystals of complex 7b·H2PO4− were obtained by using TBAH2PO4 to solubilize receptor 7b in CHCl3 (by conversion to the corresponding dihydrogenphosphate complex) and then layering with n-pentane. The resulting structure is shown in Fig. 5. Although some analogy to that of 4b·H2PO4− discussed above is seen as regard the diformyl DPM-dihydrogenphosphate interactions, in 7b·H2PO4−, the third diformyl-DPM of 7b is too distant to interact well with the bound H2PO4− anion. It thus interacts in an intermolecular sense with a dihydrogenphosphate anion bound to a second receptor. The result is a dimeric complex with overall 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry. The four pyrrole NH-to-deprotonated phosphate oxygen N–H⋯O distances range from 2.730 to 2.827 Å. The intermolecular hydrogen bond that is presumed to play a role in stabilizing the 2
2 stoichiometry. The four pyrrole NH-to-deprotonated phosphate oxygen N–H⋯O distances range from 2.730 to 2.827 Å. The intermolecular hydrogen bond that is presumed to play a role in stabilizing the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex is characterized by an O–H⋯O distance of 2.598 Å.
2 complex is characterized by an O–H⋯O distance of 2.598 Å.
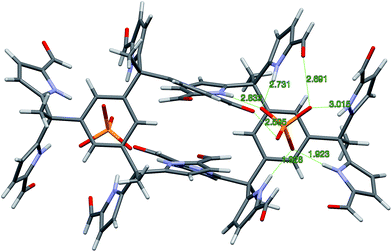 | ||
Fig. 5 Single crystal structure of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex [7b·H2PO4−]2. One TBA+ counter cation per H2PO4− and 1.5 molecules of CHCl3 per 7b have been omitted for clarity. 2 complex [7b·H2PO4−]2. One TBA+ counter cation per H2PO4− and 1.5 molecules of CHCl3 per 7b have been omitted for clarity. | ||
Based on the binding mode observed in the solid state, we considered it likely that anion–π interactions, as well as pyrrole NH–anion hydrogen bonding interactions contribute to anion binding. To explore this possibility, the effect of adding TBAH2PO4 to the most soluble receptor of the present series, namely the meta-derivative 4b, was investigated by 1H NMR spectroscopy (Fig. 6). Upon addition of one molar equivalent of TBAH2PO4 to a CDCl3 solution of 4b, discernible shifts in the proton resonances of 4b were observed (cf.Table 2). Of particular note were the 0.6 ppm upfield shifts seen for the proton signals of the linking benzene ring. Similar 1H NMR spectroscopic studies of 5b carried out in CDCl3, 7b in a more competitive solvent mixture, CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO-d6 (8
DMSO-d6 (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), likewise revealed upfield shifts in the intervening benzene resonances (cf. Fig. S8 and S10†). Such shifts are consistent with the decrease in the deshielding effect of the intervening aromatic ring current as seen in previous studies of α,α,α,α-meso-tetraaryl-C[4]P derivatives by Ballester and co-workers.31 However, as noted by a reviewer, such an observation is not sufficient to confirm or rule out the presence of an anion–π interaction. Intuitively, the interaction of anion H2PO4− with the electron-rich intervening benzene ring of 4b is expected to be repulsive, thus destabilizing the overall binding event. However, Deyà32 has suggested that the polarization of a π-electron system by an anion induces a dipole moment that may provide a contribution to the anion–receptor interaction. In the case of the present system, further studies will be necessary to determine whether anion–π interactions (if any) are playing a substantial role in mediating the observed anion recognition behaviour.
1), likewise revealed upfield shifts in the intervening benzene resonances (cf. Fig. S8 and S10†). Such shifts are consistent with the decrease in the deshielding effect of the intervening aromatic ring current as seen in previous studies of α,α,α,α-meso-tetraaryl-C[4]P derivatives by Ballester and co-workers.31 However, as noted by a reviewer, such an observation is not sufficient to confirm or rule out the presence of an anion–π interaction. Intuitively, the interaction of anion H2PO4− with the electron-rich intervening benzene ring of 4b is expected to be repulsive, thus destabilizing the overall binding event. However, Deyà32 has suggested that the polarization of a π-electron system by an anion induces a dipole moment that may provide a contribution to the anion–receptor interaction. In the case of the present system, further studies will be necessary to determine whether anion–π interactions (if any) are playing a substantial role in mediating the observed anion recognition behaviour.
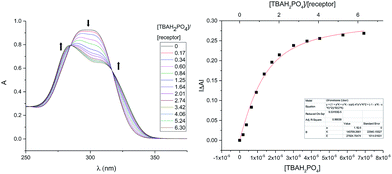
Receptor 7b proved even less soluble in neat, non-hydrogen bonding solvents, such as CHCl3, than its congeners 3b–5b. This made it impossible to carry out binding studies that were directly comparable to those reported earlier.24 However, in analogy to what was seen in the case of these other receptors, it was found that the solubility of 7b increased upon the addition of TBAH2PO4. Dilute solutions of 7b·H2PO4− in CHCl3 could thus be prepared by mixing single crystals of 7b with ca. 2.75 molar equivalents of TBAH2PO4. The UV-Vis spectrum of this solution was characterized by features similar to those seen in the reported spectrum of 3b·H2PO4− at analogous concentrations. Thus, the observed spectral features were ascribed to the complex 7b·H2PO4−. When the CHCl3 solution made up from 7b and TBAH2PO4 was titrated with CH3OH, spectral changes and isosbestic behaviour were observed. On this basis, we conclude that the H2PO4− affinity of 7b decreases in the presence of increasing quantities of CH3OH (Fig. S15†). However, it was also apparent that large quantities of methanol were required to disrupt the complex completely. It was thus decided that a solution of 3% (v/v) CH3OH in CHCl3 would provide a good balance between solubility and binding affinity and allow for quantitative analyses.
In a first set of experiments, an 11 μM solution of 7b in 3% (v/v) CH3OH in CHCl3 was prepared and then subjected to titration with TBAH2PO4 (cf.Fig. 7). The induced spectral changes were then fit to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding profile and used to calculate the binding constant corresponding to H2PO4− binding in accord with the methods described previously.24 Similar UV-Vis titrations were carried out with a series of anions in the form of their respective TBA salts. It was found that titration with either the H2PO4− or HP2O73− anions gave rise to significant spectral changes with saturation behaviour being observed in the presence of fewer than ten molar equivalents of these two anions. On the other hand, no discernable spectral changes were seen upon titration with HSO4−, Cl−, or NO3−.
1 binding profile and used to calculate the binding constant corresponding to H2PO4− binding in accord with the methods described previously.24 Similar UV-Vis titrations were carried out with a series of anions in the form of their respective TBA salts. It was found that titration with either the H2PO4− or HP2O73− anions gave rise to significant spectral changes with saturation behaviour being observed in the presence of fewer than ten molar equivalents of these two anions. On the other hand, no discernable spectral changes were seen upon titration with HSO4−, Cl−, or NO3−.
In the initial studies of 3b–5b, CHCl3 was used as the solvent. To allow for a comparison between the original tetrakis-(1H-pyrrole-2-carbaldehyde) anion receptors and the newer and hexakis analogue 7b, compound 3b was subject to titration with H2PO4− in 3% (v/v) CH3OH–CHCl3. The results from this comparison are summarised in Table 3. Briefly, 7b is characterized by a ca. 2 orders of magnitude higher dihydrogenphosphate anion affinity. This increase is in accord with the design expectations underlying 7b, namely that the presence of a third DPM subunit would force this system to adopt a preorganised conformation in a way that isn't possible for 3b.
| Anionsa | 3b | 3b | 7b |
|---|---|---|---|
| a The anions were studied as their TBA+ salts. b In CHCl3; data from ref. 24. c In CHCl3 containing 3% (v/v) CH3OH. | |||
| H2PO4− | (8 ± 2) × 106 | (2.1 ± 0.3) × 103 | (1.4 ± 0.2) × 105 |
| HP2O73− | (1.4 ± 0.1) × 105 | (3.8 ± 0.6) × 103 | (3.6 ± 0.2) × 104 |
| HSO4− | (4.1 ± 0.2) × 103 | nd | nd |
| NO3− | nd | nd | nd |
| Cl− | nd | nd | nd |
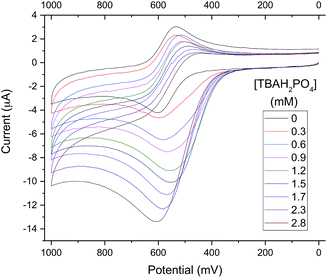
To exploit the binding characteristics of the present diformyl-DPM receptors for the purposes of anion sensing, analogue 6b was prepared. It incorporates a ferrocene spacer and was designed to perform as an electro-active analogue of the tetrakis(1H-pyrrole-2-carbaldehyde) receptors 3b–5b. This new derivative proved poorly soluble in typical organic solvents used for electrochemical studies (e.g., CH3CN, DMF, CH2Cl2). However, as above, an increase in its solubility was seen upon the addition of TBAH2PO4. This was seen as a “useful property”33 that could be used to obtain insights into the H2PO4− binding events under conditions of electrochemical analysis. It was found, for instance, that initial turbid mixtures of free receptor 6b in CH3CN containing 10% (v/v) DMF give rise to a quasi-reversible cyclic voltammogram (CV) (black line in Fig. 8). The oxidation wave for 6b becomes increasingly irreversible as the relative concentration of TBAH2PO4 increases. Eventually, the CV becomes characterized by a relatively intense anodic peak and a near-absence of a corresponding cathodic feature.
The observed increase in the anodic peak current is ascribed to the increase in the concentration of 6b (both free and complexed) that occurs as the titration runs its course. Since the 6b is not completely soluble in the solvent mixture in the absence of H2PO4−, the concentration of free 6b in the titration mixture should be constant up until the point where all traces of solid 6b are dissolved, presumably by conversion to the corresponding dihydrogenphosphate complex, 6b·H2PO4−. To the extent such a supposition is correct, it would account for the observed increase in the peak current, which could be attributed directly to the formation of the readily oxidizable species, 6b·H2PO4−. When the increase in the anodic peak current is plotted against the added H2PO4− concentration, a binding isotherm could be obtained (cf. Fig. S20†) from which an approximate H2PO4− affinity, (1.0 ± 0.1) × 103 M−1, could be calculated (cf. Table ESI-1†). The loss in reduction signal intensity is believed to reflect the strong interaction between the oxidized ferrocenium receptor (6b+) and H2PO4−.
Conclusions
In summary, we have extended the tetrakis(1H-pyrrole-2-carbaldehyde) receptor family to include a new electroactive derivative, 6b, incorporating a ferrocene linker. Also reported here is a hexaformyl trisDPM derivative, 7b. The anion binding properties of these new systems were studied by monitoring the increase in solubility electrochemically, through high-resolution mass spectrometric analyses, 1D 1H NMR and 2D DOSY spectroscopic experiments, single crystal X-ray diffraction analyses, and UV-Vis spectroscopic titrations. Conformational switching from trans-like conformations as seen in the free tetrakis- (3b,4b) and hexakis- (7b) (1H-pyrrole-2-carbaldehyde) receptors to the corresponding cis-like conformations upon interacting with phosphate derivatives was revealed by X-ray diffraction analyses in the solid state (Fig. 9). In mixed methanol–chloroform solution, the new hexakis-carbaldehyde derivative 7b was found to exhibit an affinity for H2PO4− that is roughly 100× higher than that displayed by the tetrakis analogue 3b. This increase in binding affinity is rationalized in terms of a receptor design that overcomes in part the conformational penalty that needs to be paid in order to achieve substrate binding. As such, the present findings provide experimental insights that might prove useful in the design of anion receptors based on rather simple binding motifs, such as pyrroles and dipyrromethane subunits.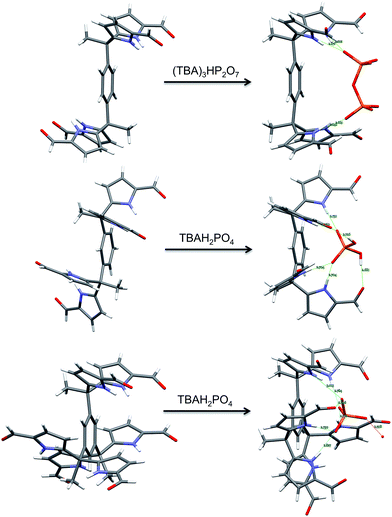 | ||
| Fig. 9 Views of the single crystal structures of 3b, 4b, and 7b in the absence and presence of bound phosphate derivatives. | ||
Acknowledgements
This work was supported by the Office of Basic Energy Sciences, U.S. Department of Energy (DOE) (Grant DE-FG02-01ER15186 to J. L. S.).Notes and references
- J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, Royal Society of Chemistry, Cambridge, 2006 Search PubMed.
- J. H. Ryther and W. M. Dunstan, Science, 1971, 171, 1008–1013 CAS.
- A. K. H. Hirsch, F. R. Fischer and F. Diederich, Angew. Chem., Int. Ed., 2007, 46, 338–352 CrossRef CAS PubMed.
- Y. Zhang, E. Desmidt, A. Van Looveren, L. Pinoy, B. Meesschaert and B. Van Der Bruggen, Environ. Sci. Technol., 2013, 47, 5888–5895 CrossRef CAS PubMed.
- E. A. Katayev, Y. A. Ustynyuk and J. L. Sessler, Coord. Chem. Rev., 2006, 250, 3004–3037 CrossRef CAS.
- A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603–6782 CrossRef CAS PubMed.
- J. W. Steed, D. R. Turner and K. Wallace, Core Concepts in Supramolecular Chemistry and Nanochemistry, John Wiley, Hoboken, NJ, 2007 Search PubMed.
- P. A. Gale, Coord. Chem. Rev., 2003, 240, 191–221 CrossRef CAS.
- S. L. Tobey and E. V. Anslyn, J. Am. Chem. Soc., 2003, 125, 14807–14815 CrossRef CAS PubMed.
- J. L. Sessler, M. J. Cyr, V. Lynch, E. McGhee and J. A. Ibers, J. Am. Chem. Soc., 1990, 112, 2810–2813 CrossRef CAS.
- G. Cavallo, P. Metrangolo, T. Pilati, G. Resnati, M. Sansotera and G. Terraneo, Chem. Soc. Rev., 2010, 39, 3772–3783 RSC.
- P. Gamez, T. J. Mooibroek, S. J. Teat and J. Reedijk, Acc. Chem. Res., 2007, 40, 435–444 CrossRef CAS PubMed.
- G. A. Jeffrey, An Introduction to Hydrogen Bonding, Oxford University Press, Oxford, 1997 Search PubMed.
- Note: Under certain conditions, deprotonation, rather than anion binding, has been observed with pyrrolic receptors.
- S. Camiolo, P. A. Gale, M. B. Hursthouse, M. E. Light and A. J. Shi, Chem. Commun., 2002, 3, 758–759 RSC.
- B. L. Iverson, K. Shreder, V. Král and J. L. Sessler, J. Am. Chem. Soc., 1993, 115, 11022–11023 CrossRef CAS.
- P. A. Gale, J. L. Sessler, V. Král and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140–5141 CrossRef CAS.
- A. Baeyer, Ber. Dtsch. Chem. Ges., 1886, 19, 2184–2185 CrossRef.
- R. Custelcean, L. H. Delmau, B. A. Moyer, J. L. Sessler, W.-S. Cho, D. Gross, G. W. Bates, S. J. Brooks, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2005, 44, 2537–2542 CrossRef CAS PubMed.
- D. E. Gross, F. P. Schmidtchen, W. Antonius, P. A. Gale, V. M. Lynch and J. L. Sessler, Chem.–Eur. J., 2008, 14, 7822–7827 CrossRef CAS PubMed.
- W.-S. Cho, PhD Dissertation, The University of Texas at Austin, 2005.
- J. L. Sessler, W.-S. Cho, S. P. Dudek, L. Hicks, V. M. Lynch and M. T. Huggins, J. Porphyrins Phthalocyanines, 2003, 07, 97–104 CrossRef CAS.
- J. L. Sessler, S. Camiolo and P. A. Gale, Coord. Chem. Rev., 2003, 240, 17–55 CrossRef CAS.
- M. K. Deliomeroglu, V. M. Lynch and J. L. Sessler, Chem. Commun., 2014, 50, 11863–11866 RSC.
- R. Neufeld and D. Stalke, Chem. Sci., 2015, 6, 3354–3364 RSC.
- R. Custelcean, Chem. Soc. Rev., 2010, 39, 3675–3685 RSC.
- N. L. Bill, D.-S. Kim, S. K. Kim, J. S. Park, V. M. Lynch, N. J. Young, B. P. Hay, Y. Yang, E. V. Anslyn and J. L. Sessler, Supramol. Chem., 2012, 24, 72–76 CrossRef CAS.
- J. Cai, B. P. Hay, N. J. Young, X. Yang and J. L. Sessler, Chem. Sci., 2013, 4, 1560–1567 RSC.
- V. Král, H. Furuta, K. Shreder, V. Lynch and J. L. Sessler, J. Am. Chem. Soc., 1996, 118, 1595–1607 CrossRef.
- M. Wei, B. Wu, L. Zhao, H. Zhang, S. Li, Y. Zhao and X.-J. Yang, Org. Biomol. Chem., 2012, 10, 8758–8761 CAS.
- G. Gil-Ramírez, E. C. Escudero-Adán, J. Benet-Buchholz and P. Ballester, Angew. Chem., Int. Ed., 2008, 47, 4114–4118 CrossRef PubMed.
- D. Quiñonero, A. Frontera and P. M. Deyà, in Anion Coordination Chemistry, ed. K. Bowman-James, A. Bianchi and E. García-Espana, Wiley-VCH Verlag & Co. KGaA, Weinheim, 2012, ch. 6, pp. 321–362 Search PubMed.
- K. A. Connors, Binding constants: The Measurement of Molecular Complex Stability, Wiley, New York, 1987 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, NMR and UV-Vis spectroscopic data, electrochemical analyses, and X-ray diffraction data. CCDC 1444557–1444561. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6sc00015k |
| This journal is © The Royal Society of Chemistry 2016 |