Self-assembled collagen-like-peptide implants as alternatives to human donor corneal transplantation†
M. Mirazul Islam‡
ab,
R. Ravichandran‡c,
D. Olsend,
M. K. Ljunggrenb,
Per Fagerholmb,
C. J. Leebc,
M. Griffith‡*ab and
J. Phopase‡c
aSwedish Medical Nanoscience Center, Dept. of Neurosciences, Karolinska Institutet, S-17177 Stockholm, Sweden. E-mail: May.Griffith@liu.se
bDept. of Clinical and Experimental Medicine, Linköping University, S-58185 Linköping, Sweden
cDept. of Physics, Chemistry and Biology (IFM), Linköping University, S-58183 Linköping, Sweden
dFibroGen Incorporated, San Francisco, CA 94158, USA
First published on 1st June 2016
Abstract
Extracellular matrix proteins like collagen promote regeneration as implants in clinical studies. However, collagens are large and unwieldy proteins, making small functional peptide analogs potentially ideal substitutes. Self-assembling collagen-like-peptides conjugated with PEG-maleimide were assembled into hydrogels. When tested pre-clinically as corneal implants in mini-pigs, they promoted cell and nerve regeneration, forming neo-corneas structurally and functionally similar to natural corneas.
Introduction
Resident stem cells capable of regeneration are present in almost every organ in the body but frequently cannot achieve the repair needed after damage by injury or disease. The limiting factor is often the extracellular matrix (ECM) surrounding the cells. During damage, the ECM is frequently replaced by scar tissue, which does not provide the required structural integrity and inhibits regeneration of functional tissue.1 The replacement of scar tissue with ECM or biomaterials that mimic its structure and function could therefore promote regeneration.1,2 Regenerating damaged tissues and organs, including corneas, could potentially mitigate the need for organ transplantation, which currently faces acute worldwide shortage and immune rejection issues.We have previously shown in human corneal transplantation clinical studies that cell-free corneal ECM mimics made from recombinant human collagen (RHC) stimulated regeneration of the human cornea, an organ that normally does not regenerate on its own.3,4 However, RHC replicates human collagen, which, like many other ECM biopolymers, is comprised of large proteins and is difficult to manipulate. Smaller units of complex proteins, particularly those capable of self-assembly have been examined as controllable ECM mimics, as they can form a wide range of structures including nanofibres.5 Collagen-like peptides (CLPs), also known as collagen-mimetic peptides (CMPs), have recently been investigated as potential alternatives to collagen, as they can self-assemble and form triple helical nanofibers like collagen.6–14 In order to stabilize the triple helices of CLPs, polymer templates that can link the three peptide chains together with sufficient flexibility to allow for proper packing of the chains with correct amino acid register have been tested.7 More recent designs have used collagen peptides as the physical crosslinks for the polymer system through triple helix formation.6,7 CLPs and CLP–polymer systems have now been tested in vitro as engineered 3D scaffolds on their own,8 conjugated to polyethylene glycol (PEG) backbones,9–11 and complexed with bioactive factors12,13 or localization agents such as gold nanoparticles.14 However, the majority of studies have been confined to in vitro testing with cells.
The trend in regenerative medicine is now towards the use of complex, naturally derived ECM macromolecules, as intact decellularised scaffolds or as processed and purified concentrated liquids or finely ground powder that are then reconstituted into hydrogels.2 Here, we report on the capacity of a CLP–PEG based hydrogel to serve as a functional analog to full-length collagen for promoting regeneration, using the corneal transplantation as a test bed.
Results and discussion
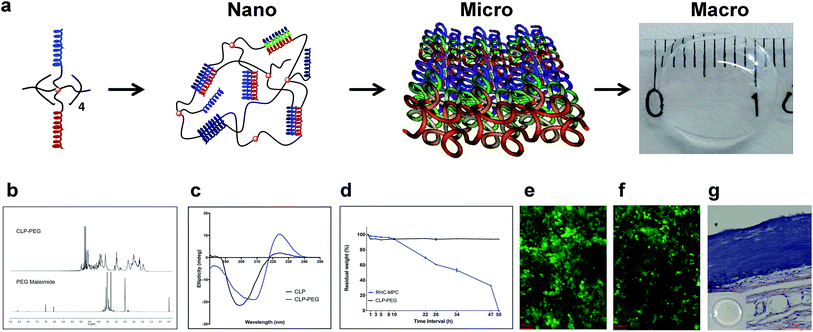
In 2011, O'Leary et al. described a 36 amino acid peptide, (Pro-Lys-Gly)4(Pro-Hyp-Gly)4(Asp-Hyp-Gly)4 that mimicked collagen fibrils.8 As described, this CLP was only able to self-assemble into a soft hydrogel, while clinical use in corneal transplantation requires implants to be sufficiently robust for surgical handling. Collagen is a large trimeric protein (approx. 300 kDa) comprising a long backbone chain (approx. 1000 amino acids per strand) that imparts high mechanical strength. Here, we modified the O'Leary CLP by the addition of two extra amino acids – a glycine spacer and a cysteine to allow covalent attachment of an 8-armed polyethylene glycol maleimide as described by Perez et al.10 The presence of a multi-arm template provided an adequate balance between rigidity and flexibility. As CLP self-assembled into triple helices, conjugation with PEG resulted in a higher order supramolecular self-assembly that was then crosslinked with N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) to form a stabilized cornea shaped and sized implant (12 mm in diameter, 500 μm thick) comprising CLP and PEG-maleimide (CLP–PEG hydrogels) (Fig. 1a). See ESI† methods online.CLP conjugation to PEG-maleimide was confirmed by 1H-NMR (Fig. 1b). Circular dichroism (CD) spectroscopy analysis of CLP–PEG showed increased triple helical propensity at 221 nm (Rpn = 0.55) compared to CLP alone (Rpn = 0.093) (Fig. 1c). The PEG template had likely enforced proximity of peptide strands for intramolecular assembly, facilitating the intermolecular, collagen-like higher order triple helical association at room temperature.
EDC-crosslinked CLP–PEG hydrogels were optically transparent, but significantly mechanically weaker than human corneas since they comprise over 90% water (human corneas only have 78% water (Table 1)). However, they were sufficiently robust to retain their shape (Fig. 1a). Furthermore, the hydrogels were stable and did not biodegrade rapidly when exposed to the artificially high concentrations of collagenase (5 U ml−1) used to simulate an accelerated biodegradation process. The CLP–PEG hydrogels showed minimal degradation in comparison to hydrogels comprising interpenetrating networks of RHC-2-methacryloyloxyethyl phosphorylcholine (RHC-MPC) implants that remained stable in the severely pathologic eyes (Fig. 1d). Human corneal epithelial cells, when seeded onto CLP–PEG implants, showed good proliferation without any cytotoxic effects (Fig. 1e) comparable to that on RHC-MPC hydrogels (Fig. 1f). This indicated good in vitro biocompatibility. Subsequent subcutaneous implantation of CLP–PEG hydrogels into the dorsum of rats for 90 days (after ethical approval from the local ethical committee, Linköpings Djurförsöksetiska Nämnd, and in compliance with the Swedish Animal Welfare Ordinance and the Animal Welfare Act) confirmed their biocompatibility in vivo (Fig. 1g) as the implants were relatively intact, and remained free of immune cells or thick fibrotic encapsulation after 90 days in vivo.
| Properties | Transmission (%) | Backscatter (%) | Tensile strength (MPa) | Elongation (%) | Modulus (MPa) | Water content (%) |
|---|---|---|---|---|---|---|
| CLP–PEG | 92.4 ± 0.95 | 0.90 ± 0.17 | 0.07 ± 0.02 | 58.30 ± 4.49 | 0.18 ± 0.06 | 91.65 ± 1.10 |
| Human cornea3 | 87.1 ± 2.0 | <3 | 3.81 ± 0.40 | — | 3–13 | 78 |
In preparation for clinical translation, CLP–PEG hydrogels were tested for potential use in transplantation as an alternative to donated human corneas, in cases where the endothelium is healthy. The cornea is the transparent front part of the eye, which acts as a lens to direct light to the back for vision. Permanent transparency loss results in blindness, which can be treated by transplantation of donor tissue. However, there is a severe worldwide donor shortage, and not all conditions are amenable to donor grafting.15
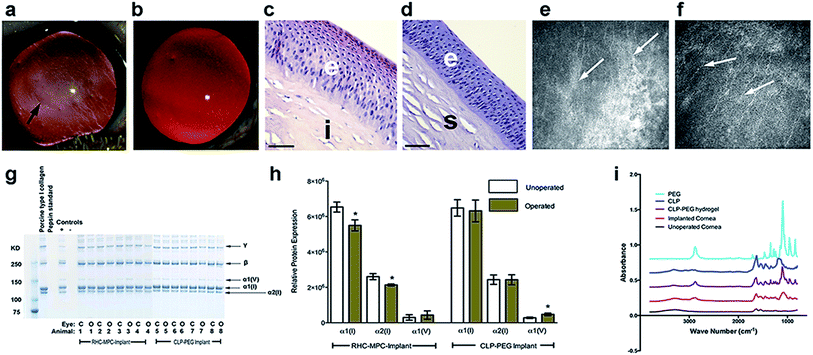
Here, hydrogel implants were molded into implants the shape and size of human corneas (500 μm thick, 12 mm diam). In compliance with the OECD Principle of Good Laboratory Practice (GLP), ENV/MC/CHEM (98) 17, 1997, and with local ethical permission from Stockholms Norra Djurförsöksetiska Nämnd, implants was grafted into the corneas of four Gottingen mini-pigs by anterior lamellar keratoplasty, after excision of the pigs own corneal tissues. At 12 months post-implantation, CLP–PEG implants remained stably incorporated and optically clear, as judged by slit lamp biomicroscopy, within the pigs' eyes (Fig. 2a), similar to the contralateral unoperated control corneas (Fig. 2b). However, mild haze was observed at the material–host interface. The optical transparency allowed for real-time imaging by in vivo confocal microscopy of corneal epithelial cells growing over the implants and in-growth of corneal stromal cells into the implants (ESI Fig. 1†). Histologically, the regenerated neo-corneas (Fig. 2c) resembled their contralateral unoperated controls (Fig. 2d). Each cell-free CLP–PEG implant had been replaced by a tissue comprising an epithelium and underlying stroma with similar morphology to the healthy control corneas. In vivo confocal microscopy also showed that corneal nerves had grown into the implants, forming a sub-epithelial nerve plexus comprising parallel nerves (Fig. 2e), resembling the pattern seen in their healthy, unoperated, contralateral counterparts (Fig. 2f). Biochemical analyses of grafted CLP–PEG implants samples showed the presence of corneal collagens type I and V, which were not present in the initial implants (Fig. 2g and h). This profile was similar to that of RHC-MPC implants that were followed longitudinally as benchmarks. More importantly, the collagen profiles were similar to those of healthy, control corneas albeit differing in the quantities of collagen. This showed that remodeling of the initial fully synthetic implant had occurred. However, FTIR spectroscopy revealed CLP and PEG profiles in the implanted area (Fig. 2i), indicating remodeling was incomplete and likely still on-going at 12 months post-operation. Overall, the implanted corneas did not differ significantly in thickness from unoperated controls. This is most likely due to the formation of neo-corneal tissue that allows for modulation of proper corneal hydration.
A range of self-assembling amphiphilic peptides, including CLPs, has now been examined as scaffolds for promoting corneal regeneration since they can form a wide range of structures including nanofibers like ECM, and they are easily tunable to support adhesion and proliferation of corneal cells.16,17 ECM-derived cell adhesion peptides containing the laminin motif, Tyr-Ile-Gly-Ser-Arg, or the fibronectin motif, Arg-Gly-Asp, have been tested as injectable scaffolds and shown to promote regeneration of the corneal stroma in rabbits.18 However, in many corneal pathologies, the tissue is scarred leading to loss of transparency and blindness. Excision of the scarred region and replacement with a robust hydrogel that could act as a corneal substitute is therefore necessary. Hence, conjugation to a polymer backbone is needed.
The most convenient method for fabrication of peptide-synthetic polymer conjugates is to couple a peptide that is functionalized (either at the termini or side chain) with a complementary functionalized synthetic polymer.6 While a number of synthetic polymers have been explored as backbones for peptides, many of the successful reported hybrids have incorporated PEG and its derivatives.6,7 CLP–PEG-maleimide hydrogels that were similar to ours, reported by Perez et al., were thermoresponsive and formed a liquid-like state close to the melting temperature of the CLPs.11 Here, a further crosslinking step using EDC and its co-reactant, N-hydroxysuccinimide (NHS) allowed us to fabricate CLP–PEG hydrogels that remained in an irreversible colloidal state. This is important as implants meant to replace pathologic tissue need to remain in place as scaffolds to permit regeneration and remodeling by incoming cells until complete formation of new tissues has occurred. The use of PEG may also serve a second function promoting immune tolerance of the implants, as conjugation of protein-based drugs has been shown to reduce immunogenicity.19
In the present study, CLP peptide conjugated with PEG-maleimide resulted in a transparent corneal implant that was sufficiently robust for transplantation. The implant showed stable and functional integration, promoting regeneration of corneal epithelial and stromal tissue and ECM components, as well as nerves in a similar manner to RHC-MPC implants that have now been clinically tested.3 Like RHC-MPC, the peptide–PEG hydrogel most likely served as a physical scaffold that provided a microenvironment that was conducive to in-growing cells to proliferate, differentiate and to synthesize ECM components, resulting in a neo-cornea. For translation into clinical application, the use of synthetic peptide analogs in place of animal extracted collagen allows for lot-to-lot homogeneity in raw materials as well as circumventing potential transmission of viruses and other pathogens. As a replacement of RHC, CLP–PEG is potentially easier to manipulate and would allow for future functionalization.
Conclusions
We have shown that self-assembling CLP–PEG successfully mimicked the function of full-length recombinant human collagen as corneal implants, promoting stable regeneration of several cell types in appropriate configurations. All regenerated neo-corneas were optically transparent, and based on early results, allowed the restoration of morphological features approximating those of natural corneas, suggesting their future applicability for clinical grafting. More extensive analyses of the implants are required to determine the extent to which these CLP–PEG implants were able to mimic hydrogels made from full-length collagen, and to elucidate possible mechanisms of collagen-induced regeneration. Furthermore, the performance of these implants in human patients remains to be determined. Nevertheless, we have demonstrated the potential utility of CLP-based hydrogels for future clinical use.Acknowledgements
This work was funded by a Vinnova Indo-Sweden grant (dnr 2013-04645), the Integrative Regenerative Medicine Centre, Linköping University (LiU) and Region Östergötland. RR was supported by a Swedish Research Council grant (dnr 621-2012-4286). The content of the manuscript is disclosed in unpublished patent applications PCT/EP2015/073010, GB1506316.7 (J Phopase, inventor; Ferentis, assignee). We thank Dr Philip Lewis, Cardiff University, UK, for his help in characterising the CLP–PEG hydrogels and Adlego AB, Sweden, for conducting the GLP mini-pig study. MG acknowledges her appointment as visiting professor at the Tokyo Dental College Ichikawa General Hospital, Japan, and affiliated researcher at Maisonneuve-Rosemont Hospital Research Center, Canada.Notes and references
- A. Atala, D. J. Irvine, M. Moses and S. Shaunak, MRS Bull., 2010, 35(8) DOI:10.1557/mrs2010.528.
- E. Jabbari, J. Leijten, Q. Xu and A. Khademhosseini, Mater. Today, 2016, 19(4), 190–196 CrossRef CAS.
- O. Buznyk, N. Pasyechnikova, M. M. Islam, S. Iakymenko, P. Fagerholm and M. Griffith, Clin. Transl. Sci., 2015, 8, 558–562 CrossRef CAS PubMed.
- P. Fagerholm, N. S. Lagali, K. Merrett, W. B. Jackson, R. Munger, Y. Liu, J. W. Polarek, M. Soderqvist and M. Griffith, Sci. Transl. Med., 2010, 2, 46ra61 Search PubMed.
- R. Ravichandran, M. Griffith and J. Phopase, J. Mater. Chem. B, 2014, 2, 8466–8478 RSC.
- T. Luo and K. L. Kiick, Eur. Polym. J., 2013, 49, 2998–3009 CrossRef CAS PubMed.
- S. M. Yu, Y. Li and D. Kim, Soft Matter, 2011, 7, 7927–7938 RSC.
- L. E. O'Leary, J. A. Fallas, E. L. Bakota, M. K. Kang and J. D. Hartgerink, Nat. Chem., 2011, 3, 821–828 CrossRef PubMed.
- P. J. Stahl, N. H. Romano, D. Wirtz and S. M. Yu, Biomacromolecules, 2010, 11, 2336–2344 CrossRef CAS PubMed.
- C. M. Perez, A. Panitch and J. Chmielewski, Macromol. Biosci., 2011, 11, 1426–1431 CrossRef PubMed.
- C. M. Rubert Perez, L. A. Rank and J. Chmielewski, Chem. Commun., 2014, 50, 8174–8176 RSC.
- A. Y. Wang, S. Leong, Y. C. Liang, R. C. C. Huang, C. S. Chen and S. M. Yu, Biomacromolecules, 2008, 9, 2929–2936 CrossRef CAS PubMed.
- P. A. Parmara, L. W. Chowa, J.-P. St-Pierrea, C.-M. Horejsa, Y. Y. Peng, J. A. Werkmeister, J. A. M. Ramshaw and M. M. Stevens, Biomaterials, 2015, 54, 213–225 CrossRef PubMed.
- X. Mo, Y. J. An, C. S. Yun and S. M. Yu, Angew. Chem., Int. Ed., 2006, 45, 2267–2270 CrossRef CAS PubMed.
- M. Griffith and D. G. Harkin, Curr. Opin. Ophthalmol., 2014, 25, 240–247 CrossRef PubMed.
- M. Miotto, R. M. Gouveia and C. J. Connon, J. Funct. Biomater., 2015, 6, 687–707 CrossRef CAS PubMed.
- M. Matsusaki, R. Amekawa, M. Matsumoto, Y. Tanaka, A. Kubota, K. Nishida and M. Akashi, Adv. Mater., 2011, 23, 2957–2961 CrossRef CAS PubMed.
- G. Uzunalli, Z. Soran, T. S. Erkal, Y. S. Dagdas, E. Dinc, A. M. Hondur, K. Bilgihan, B. Aydin, M. O. Guler and A. B. Tekinay, Acta Biomater., 2014, 10, 1156–1166 CrossRef CAS PubMed.
- J. M. Harris and R. B. Chess, Nat. Rev. Drug Discovery, 2003, 2, 214–221 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08895c |
| ‡ These authors contributed equally for experimental work. |
| This journal is © The Royal Society of Chemistry 2016 |