Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
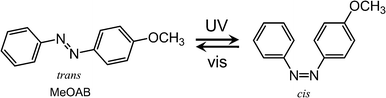
Azobenzene crystals swim on water surface triggered by light†
Y.
Norikane
*,
S.
Tanaka
and
E.
Uchida
Electronics and Photonics Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Central 5, Higashi 1-1-1, Tsukuba, Ibaraki 305-8565, Japan. E-mail: y-norikane@aist.go.jp; Fax: +81 29 861 6252; Tel: +81 29 861 4887
First published on 20th May 2016
Abstract
Crystals of 4-methoxyazobenzene move on water surface and the motion is triggered by irradiation with ultraviolet light. The propulsion is produced by dissolution of the photo-generated cis isomer, which is produced by the photoinduced crystal–liquid phase transition. A photoresponsive boat was also prepared by a filter paper adsorbed with azobenzene.
Artificial self-propelled systems have attracted significant attention recently.1–8 Molecular-sized to centimetre-sized objects have been investigated by using various sources of energy such as chemical energy, thermal gradient, electric field, electromagnetic field, and light energy. Typically, objects floating on a liquid/air interface is a key working environment for creating motion. For example, a camphor boat is a well-known self-propelled system in which dissolution of camphor molecules from the solid attached to the boat generates spontaneous motion of the boat on the water surface.9–11 Since the dissolution occurs isotropically, the direction of the motion depends on both the shape of the boat and the water channel.10,11 Once a camphor boat is placed on the water surface, it automatically starts moving on the liquid surface. Intrinsically it is difficult to trigger the action and to control the motion of the boat.
External stimuli such as light is expected to be a powerful tool and energy source for triggering the motion and controlling the direction of the self-propelling system.12–15 In particular, azobenzene has been used to generate motion in various systems.16–24 For example, it has been reported that a droplet of liquid on a solid surface modified by azobenzene moves upon irradiation with an intensity gradient.16–18 The flow of a glassy solid of azobenzene derivative has been reported on irradiation with a p-polarized laser.23,24 With regard to the molecular crystal of azobenzene, the motion of azobenzene crystals in a trimethylolpropane triacrylate solution during crystallization25 has been reported.
We are interested in photochemically-induced phase transitions between solid and liquid phases in photochromic organic compounds such as macrocyclic,26–28 rod-shaped azobenzene derivatives,29 and a spyropiran derivative.30 In the course of our study of such phase transitions, we previously found the phenomenon of continuous crawling of crystals of azobenzene derivatives (azobenzene and 3,3′-dimethylazobenzene) on a glass surface.31 This motion is produced by irradiating UV and visible light simultaneously from different directions. It is presumed that the motion is driven by crystallization and melting at the front and rear edges of the crystal, respectively, via photochemical conversion between the crystal and liquid phases induced by the isomerization of azobenzenes. This finding inspired us that this kind of phase transition possibly produces motion of objects in other media such as in a liquid. However, as yet, photo-induced motion of azobenzene crystals on a water surface is unknown.
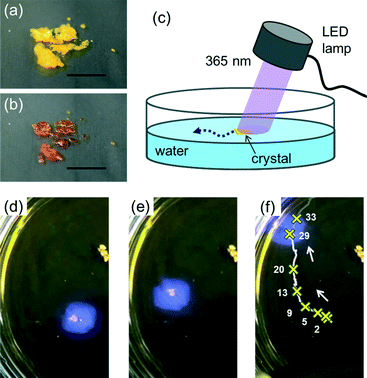
Here we report that crystals of a simple azobenzene, 4-methoxyazobenzene (MeOAB, Scheme 1) (m.p. of trans and cis isomers are 53–54 °C and 25 °C (ref. 32), respectively), move on a water–air interface. The motion is triggered by irradiation with UV light and the direction of the motion can be controlled by the orientation of the light source as illustrated in Fig. 1. In addition, a filter paper containing the azobenzene (photoresponsive boat) moved upon photoirradiation. The maximum velocity of the boat was ca. 5 cm s−1. The motion is slowed down by irradiation of visible light, which induces cis-to-trans isomerization. The phenomena is elucidated by the phase transition photoinduced crystal–liquid phase transition and dissolution of the cis-isomer into water.
We carried out the experiments using a Petri dish with a diameter of 65 mm filled with water‡ (10 mm depth). When crystals of MeOAB (originally the trans isomer) were placed on a water surface irradiated with 365 nm LED, they started moving on the surface as shown in Fig. 1d–f and ESI† Movie 1. It should be noted that the crystals do not move without UV light irradiation but the irradiation triggers the movement. Crystals moved away from the light source. Interestingly, the motion continued for a few minutes after ceasing the irradiation. This observation indicates that the motion is not caused by the heating effect. In addition, as can be seen in Fig. 1a and b, the photoirradiation to the crystal caused a crystal–liquid phase transition in concurrence with a colour change from yellow to orange. Similar crystal–liquid phase transitions have been reported in several azobenzene derivatives and it is caused by a trans-to-cis photoisomerization of azobenzene.26–29,31,33 These facts indicate that the observed motion is induced by the photochemically generated product, i.e. the cis isomer. We observed various types of motion such as spinning, splitting, winding motions as well as a linear movement (ESI† Movie 1). The velocity and the type of crystal motion seemed to depend on the original shape, irradiated portion and period. It was difficult to control the irradiation tracking the motion of the crystal, so the velocity was varied over individual experiments. As shown in Fig. 1d–f, polycrystalline powder of MeOAB exhibited a linear-like movement with a velocity of up to ca. 1 cm s−1 when irradiated with UV light at an intensity of 100 mW cm−2.
In addition, the cis-isomer of MeOAB exhibited higher solubility compared to its trans-isomer. The crystals of trans-MeOAB were hardly soluble in water. When crystals of trans-MeOAB were suspended in water and exposed to 365 nm light with stirring, the colour of the water changed from colourless to yellow (Fig. S1 and S2†). This observation is reasonable because the dipole moment of the cis isomer is generally higher than that of the initial trans isomer.34 The increase in the polarity may enhance the solubility in water. Thus we propose that the observed motion is caused by the light-induced crystal–liquid phase transition and the subsequent dissolution of the liquefied cis isomer. The light irradiation causes liquefaction of the crystal only on the irradiated portion of the crystal. Thus, anisotropic dissolution of the crystal occurs causing a concentration gradient which may lead to the propulsion.
Inspired by the motion of the camphor boat on water surface,9–11 we made an “azobenzene boat” whose motion is triggered by light. The boat was made by soaking MeOAB into a piece of filter paper. Initially, MeOAB was dissolved in ethanol and a piece of filter paper was dipped in the solution. Then the paper was removed from the solution and dried under vacuum for 1 h. The boat was placed on a water surface and irradiated with UV light. The detail of the preparation of the boat is shown in the ESI.†
When the boat was placed on a water surface, motion was not observed. The boat started moving on the water surface when it was irradiated by UV light (Fig. 2 and ESI† Movie 2). It is noteworthy that the motion is directional when the boat was exposed partially to the aft of the boat. In this case the velocity was about 1 cm s−1. Acceleration can be achieved by continuous irradiation of the boat. In addition, we were able to spin the boat when the light spot was positioned on an edge of the boat (ESI† Movie 3). Here the propulsion is caused by dissolution of the photo-generated cis isomer. Actually, the colour of the water became yellowish orange after vigorous irradiation experiments. However, we observed motion and we did not observe deterioration in the photoresponse for 10 minutes which is a period of time typical for our experiments.
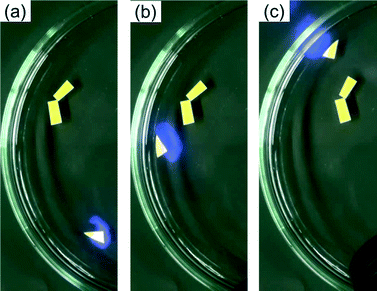 | ||
| Fig. 2 (a–c) Snapshots of the motion (every 2 seconds) of the filter paper containing MeOAZ on the water surface triggered with UV light irradiation (ca. 100 mW cm−2). | ||
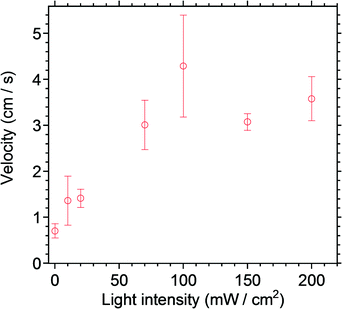
To find a condition for the motion of the boat, the effects of the concentration of the solution as well as the light intensity were investigated. Since it was difficult to precisely irradiate a boat moving on a water surface, the irradiation was carried out before placing the boat on water. A triangle-shaped boat prepared as described above and ESI† was put on a lab bench and exposed partially to the aft of the boat for 1 minute. Then the boat was placed on a water surface and the motion was recorded. A typical experiment is shown in the ESI† Video 4. Fig. 3 shows the light-intensity dependence of the motion of the boat. The velocity seems to be proportional to the light intensity up to 100 mW cm−2, and it is saturated around this point, indicating that conversion to the cis isomer is complete. A velocity of 3–4 cm s−1 can be achieved.
On the other hand, the effect of the concentration of MeOAZ was investigated. The concentration was varied from 0–100 mg ml−1 for the ethanolic solution used for dipping the filter paper. The boat containing different amounts of MeOAZ was irradiated before placing on a water surface. The boat was then placed on a water surface and the motion was recorded. The result is shown in Fig. S4.† It shows that a concentration of 20 mg ml−1 is the lower threshold for motion. Also, the velocity is saturated at around 80 mg ml−1.
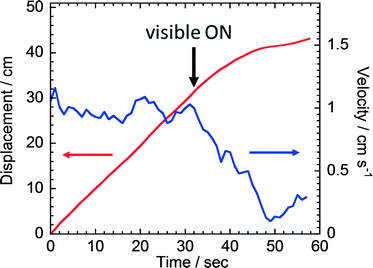
In the above experiments, the motion of the crystals or the boat continued even after the UV irradiation ceased. So we attempted to decelerate the moving boat. A pre-irradiated boat was placed on a water surface exhibiting motion. Then the moving boat was exposed to a visible light (450 nm, 50 mW cm−2) and the noticeable deceleration was observed (Fig. 4 and ESI† Movie 5). It took ca. 20 seconds to stop the boat which was initially moving at 1 cm s−1. This observation indicates that the amount of fuel (the cis-isomer) was decreased by the cis-to-trans photoisomerization caused by the visible light irradiation.
Conclusions
We demonstrated a photoinduced motion of crystals of a simple azobenzene (4-methoxyazobenzene) on a water surface. The motion is triggered by irradiation with ultraviolet light. The direction of motion can be controlled by the orientation of the light. Inspired by the camphor boat, a photoresponsive boat was prepared by soaking filter paper with the azobenzene. The maximum velocity of the boat was ca. 5 cm s−1, while the irradiation of the visible light slowed the motion. The propulsion is produced by dissolution of the photo-generated cis isomer, which is produced by the photoinduced crystal–liquid phase transition. The photoinduced solid–liquid phase transition via photochromic reaction can produce intriguing phenomena such as the motion of objects. The fuel of the boat is stored in an inert state (solid state) and light can activate/deactivate the fuel.Acknowledgements
This work was supported in part by JSPS/MEXT KAKENHI (26288096) and The Canon Foundation.Notes and references
- J. Wang, Nanomachines, Wiley-VCH Verlag GmbH & Co. KGaA., Weinheim, Germany, 2013 Search PubMed.
- G. Zhao and M. Pumera, Chem. – Asian J., 2012, 7, 1994–2002 CrossRef CAS PubMed.
- Y. Mei, A. a Solovev, S. Sanchez and O. G. Schmidt, Chem. Soc. Rev., 2011, 40, 2109–2119 RSC.
- M. Pumera, Nanoscale, 2010, 2, 1643–1649 RSC.
- S. Sánchez and M. Pumera, Chem. – Asian J., 2009, 4, 1402–1410 CrossRef PubMed.
- J. Wang, ACS Nano, 2009, 3, 4–9 CrossRef CAS PubMed.
- W. F. Paxton, S. Sundararajan, T. E. Mallouk and A. Sen, Angew. Chem., Int. Ed., 2006, 45, 5420–5429 CrossRef CAS PubMed.
- W. F. Paxton, A. Sen and T. E. Mallouk, Chem. – Eur. J., 2005, 11, 6462–6470 CrossRef CAS PubMed.
- Y. Karasawa, S. Oshima, T. Nomoto, T. Toyota and M. Fujinami, Chem. Lett., 2014, 43, 1002–1004 CrossRef CAS.
- S. Nakata, M. Hata, Y. S. Ikura, E. Heisler, A. Awazu, H. Kitahata and H. Nishimori, J. Phys. Chem. C, 2013, 117, 24490–24495 CAS.
- Y. S. Ikura, E. Heisler, A. Awazu, H. Nishimori and S. Nakata, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 88, 012911 CrossRef PubMed.
- D. Okawa, S. J. Pastine, A. Zettl and J. M. J. Fréchet, J. Am. Chem. Soc., 2009, 131, 5396–5398 CrossRef CAS PubMed.
- J. Berna, D. Leigh, M. Lubomska, S. Mendoza, E. Perez, P. Rudolf, G. Teobaldi and F. Zerbetto, Nat. Mater., 2005, 4, 704–710 CrossRef CAS PubMed.
- R. Eelkema, M. M. Pollard, J. Vicario, N. Katsonis, B. S. Ramon, C. W. M. Bastiaansen, D. J. Broer and B. L. Feringa, Nature, 2006, 440, 163 CrossRef CAS PubMed.
- M. Xiao, C. Jiang and F. Shi, NPG Asia Mater., 2014, 6, e128 CrossRef CAS.
- K. Ichimura, S.-K. Oh and M. Nakagawa, Science, 2000, 288, 1624–1626 CrossRef CAS PubMed.
- S. Oh, M. Nakagawa and K. Ichimura, J. Mater. Chem., 2002, 12, 2262–2269 RSC.
- K. Seki and M. Tachiya, J. Phys.: Condens. Matter, 2005, 17, S4229–S4237 CrossRef CAS.
- A. Diguet, R. Guillermic, N. Magome, A. Saint-Jalmes, Y. Chen, K. Yoshikawa and D. Baigl, Angew. Chem., Int. Ed., 2009, 48, 9281–9284 CrossRef CAS PubMed.
- A. Kausar, H. Nagano, T. Ogata, T. Nonaka and S. Kurihara, Angew. Chem., Int. Ed., 2009, 48, 2144–2147 CrossRef CAS PubMed.
- J. Abid, M. Frigoli, R. Pansu and J. Szeftel, Langmuir, 2011, 27, 7967–7971 CrossRef CAS PubMed.
- T. Yamamoto and M. Yoshida, Appl. Phys. Express, 2012, 5, 101701 CrossRef.
- H. Nakano and M. Suzuki, J. Mater. Chem., 2012, 22, 3702–3704 RSC.
- M. Suzuki and H. Nakano, J. Photopolym. Sci. Technol., 2012, 25, 159–160 CrossRef CAS.
- K. Milam, G. O'Malley, N. Kim, D. Golovaty and T. Kyu, J. Phys. Chem. B, 2010, 114, 7791–7796 CrossRef CAS PubMed.
- Y. Norikane, Y. Hirai and M. Yoshida, Chem. Commun., 2011, 47, 1770–1772 RSC.
- E. Uchida, K. Sakaki, Y. Nakamura, R. Azumi, Y. Hirai, H. Akiyama, M. Yoshida and Y. Norikane, Chem. – Eur. J., 2013, 19, 17391–17397 CrossRef CAS PubMed.
- M. Hoshino, E. Uchida, Y. Norikane, R. Azumi, S. Nozawa, A. Tomita, T. Sato, S. Adachi and S. Koshihara, J. Am. Chem. Soc., 2014, 136, 9158–9164 CrossRef CAS PubMed.
- Y. Norikane, E. Uchida, S. Tanaka, K. Fujiwara, E. Koyama, R. Azumi, H. Akiyama, H. Kihara and M. Yoshida, Org. Lett., 2014, 16, 5012–5015 CrossRef CAS PubMed.
- E. Uchida, R. Azumi and Y. Norikane, Chem. Lett., 2014, 43, 1619–1621 CrossRef CAS.
- E. Uchida, R. Azumi and Y. Norikane, Nat. Commun., 2015, 6, 7310 CrossRef CAS PubMed.
- R. Le Fèvre and J. Northcott, J. Chem. Soc., 1953, 867–870 RSC.
- Y. Okui and M. Han, Chem. Commun., 2012, 48, 11763–11765 RSC.
- E. Merino and M. Ribagorda, Beilstein J. Org. Chem., 2012, 8, 1071–1090 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ce00738d |
| ‡ We used three different types of water: ultrapure water (Milli-Q water), water purified by a reverse osmosis membrane (RO water), and tap water (Tsukuba, Japan). However, there is no dependence of the quality of water on the motion. In experiments shown here, we used RO water. |
| This journal is © The Royal Society of Chemistry 2016 |