 Open Access Article
Open Access ArticleNaturally-derived and bioinspired materials
Jennie B.
Leach
*a and
Molly S.
Shoichet
b
aDepartment of Chemical, Biochemical & Environmental Engineering, UMBC, 1000 Hilltop Circle, Baltimore, MD 21250, USA. E-mail: jleach@umbc.edu
bDepartment of Chemical Engineering & Applied Chemistry, Institute of Biomaterials & Biomedical Engineering, Department of Chemistry, Donnelly Centre, University of Toronto, 160 College Street, Toronto, ON M5S3E1, Canada
Introduction
Cell behavior and tissue function are influenced by the composition and architecture of their surroundings. These cues can drive normal processes during, for example, development, wound healing and homeostasis, or promote harmful processes including tumor growth and metastasis, fibrosis, disease and degeneration. Recent advances in materials synthesis, processing and characterization as well as our ever-improving knowledge of the biological bases for physiological and pathological processes set the stage for new technologies to better recapitulate the complex in vivo scenario. Materials, which are either naturally-derived, and are inherently useful for biomedical applications or created by biology-inspired means, are poised to play key roles in recapitulating the in vivo environment and ultimately achieve more effective therapeutics and diagnostics.The articles found in this themed issue focusing on naturally-derived and bioinspired materials report novel aspects in the field that fall into one or more of the following sources of biological inspiration: composition, structure, physicochemical properties and bioactive factor presentation (Fig. 1). The composition of a material may consist of naturally-derived proteins (e.g., fibrin and fibronectin), polysaccharides (e.g., chitosan and dextran) or peptides. The structure of the material can be used to control how a cell perceives its surroundings; for example, fibers or patterned shapes can influence cell shape. More generally, two-dimensional (2D) surfaces provide a polarized presentation of matrix vs. soluble cues whereas three-dimensional (3D) matrices that encapsulate cells may better represent the in vivo scenario where cells are surrounded by matrix and soluble cues. The physicochemical properties of a material, including mechanical properties, porosity, hydrophilicity/hydrophobicity and electrical conductivity, can also influence cell behavior through means such as mechanotransduction, transport of bioactive molecules, protein–protein interactions and electrical stimulation, respectively. Finally, bioactive factors can be selectively presented to cells in a variety of ways, including control of their transport (sequestration, release and diffusion), reaction kinetics (rates of consumption, generation, degradation) and availability of sites for cell receptor binding and adhesion. Each of these four classes of bioinspiration and related articles are highlighted briefly below.
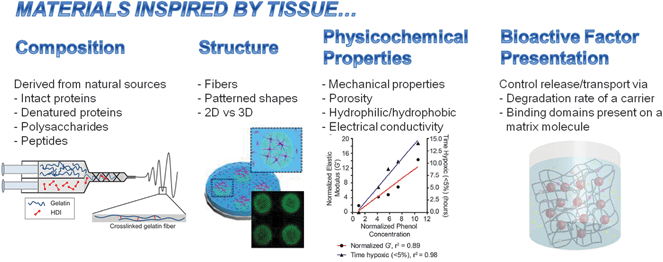 | ||
| Fig. 1 Overview of articles in this issue. Representative examples shown in images (left to right) from Cosgriff-Hernandez (DOI: 10.1039/c5tb00937e), Kilian (DOI: 10.1039/c5tb01294e), Gerecht (DOI: 10.1039/c5tb01038a), Stabenfeldt (DOI: 10.1039/c5tb00935a) and co-workers. | ||
Bioinspired by tissue composition
Materials with unique biological functions can be derived from natural sources and applied as whole molecules or as fragments that are selected for their unusual bioactivity (Table 1). Works by Stegemann (DOI: 10.1039/c5tb00934k) and Kilian (DOI: 10.1039/c5tb01294e) and their co-workers chose naturally-derived materials for their inherent biochemical properties. Stegemann et al. selected chondroitin sulfate, chitosan and agarose to synthesize microbeads capable of encapsulating cells for cartilage repair applications. They recognized that the composition of the cellular microenvironment is critical to promoting differentiation to chondrocytes from mesenchymal stem cells. With this in mind, they selected chondroitin sulfate, a major component of cartilage, and also a component that can form polyelectrolyte complexes with chitosan. Together with cells, these complexes were encapsulated in agarose microbeads, resulting in high cell viability and increased synthesis of cartilage extracellular matrix (ECM) components vs. agarose alone. Kilian et al. selected the naturally-derived materials fibronectin and Matrigel™ to manufacture micropatterned areas of blood vessel growth. They conjugated fibronectin to polyethylene glycol (PEG) diacrylate to yield a photopolymerizable composite with tunable physical properties and an inherent ability to promote blood vessel growth. They patterned these materials into islands (∼1 mm or less in size) with encapsulated mesenchymal stem cells and covered the islands with Matrigel™ containing endothelial cells; the close proximity of the two cell types and the use of biologically-active engineered materials allowed selective areas of endothelial cell tube formation, which is an initial step in blood vessel growth.| Material | Source | Articles |
|---|---|---|
| Abbreviations: extracellular matrix, ECM. | ||
| Agarose | Seaweed | Stegemann (DOI: 10.1039/c5tb00934k) |
| Chitosan | Crustacean shells, fungal cell walls | Stegemann (DOI: 10.1039/c5tb00934k) |
| Chondroitin sulfate | Mammalian ECM | Stegemann (DOI: 10.1039/c5tb00934k) |
| Collagen-based peptides | Mammalian ECM | Hahn (DOI: 10.1039/c5tb00990a) |
| Dextran | Bacteria | Gerecht (DOI: 10.1039/c5tb01038a) |
| Fibrin | Mammalian blood | Stabenfeldt (DOI: 10.1039/c5tb00935a) |
| Fibrinogen | Mammalian ECM | Kilian (DOI: 10.1039/c5tb01294e) |
| Gelatin (denatured type I collagen) | Mammalian ECM | Cosgriff-Hernandez (DOI: 10.1039/c5tb00937e), Gerecht (DOI: 10.1039/c5tb01038a) |
| Matrigel | Mouse sarcoma | Kilian (DOI: 10.1039/c5tb01294e) |
For some applications, it is not preferable to apply a naturally-derived material as an intact polymer or protein. For example, Hahn et al. (DOI: 10.1039/c5tb00990a) selected a class of collagen-mimetic proteins, called Scl2-1 proteins, that contain a GXY amino acid motif that, like type I collagen, spontaneously self-assembles into stable triple helix structures. As Hahn and coworkers describe, the Scl2-1 proteins were especially chosen because, unlike collagen, their unique structure can be synthesized by recombinant expression in E. coli while retaining the ability to self-assemble. This capability provides for potentially more consistent products with less cost vs. protein derived from mammalian tissues. Also, the Scl2-1 proteins do not support cell adhesion, which may be directly favorable, for example, in vascular grafts to prevent blood clot formation. Alternatively the ability to support cell adhesion may be added selectively by conjugation with other protein-derived motifs, such as the fibronectin-derived RGD peptide.
Gelatin is denatured type I collagen, a prevalent protein in the ECM of many mammalian tissues. In this issue, both Cosgriff-Hernandez et al. (DOI: 10.1039/c5tb00937e) and Gerecht et al. (DOI: 10.1039/c5tb01038a) apply gelatin, but in unique ways. Cosgriff-Herandez and coauthors synthesized gelatin fibers (∼0.7–2.4 μm diameter) by electrospinning. They found that cross-linking during the electrospinning process enabled control over the degradation rate and morphology of the fibers, extending their stability during culture from 24 h to over 1 week. Ultimately, these materials have promise for applications such as peripheral nerve and ligament repair because these tissues derive their function in part from aligned cellular structures. Gerecht and coauthors recognized that gelatin could be cross-linked to dextran via two distinct routes, including one that utilizes transglutaminase to link primary amines present on gelatin and aminated dextran. The second cross-linking reaction consumes oxygen via a reaction mediated by laccase, which oxidizes phenol-containing groups that are conjugated to the polymers. Therefore, gel stiffness and initial depletion of oxygen from the gels could be controlled independently. These materials have unique advantages for studying processes where these two properties are known to play vital roles, such as blood vessel formation, cancer and stem cell fate.
Bioinspired by tissue structure
As already mentioned, electrospinning and micropatterning, are techniques described in this issue to mimic structures found in the body, namely fibers (Cosgriff-Hernandez et al., DOI: 10.1039/c5tb00937e as well as Willerth et al., DOI: 10.1039/c5tb00871a) and localized “microtissues” (Kilian et al., DOI: 10.1039/c5tb01294e). By guiding the alignment of tissues or by localizing cells and their secreted factors, one may coordinate cellular responses to larger sizes approaching the scale of tissues.Materials may be mechanically-stimulated by stretching to induce cells to align. Taking advantage of this idea, Wong et al. (DOI: 10.1039/c5tb01171j) created aligned sheets composed of vascular smooth muscle cells and their secreted ECM. To make the sheets, they treated the surface of an elastic membrane with a thermoresponsive polymer, poly(N-isopropylacrylamide) (p(NIPAAm)), which served as a temporary layer to support cell attachment and deposition of ECM at 37 °C, and then detachment of the intact cell sheet below the lower critical solution temperature of p(NIPAAm) at 32 °C. During culture, the elastic membrane was subjected to uniaxial cyclic strain, which induced the smooth muscle cells to align. The authors suggested that these aligned cell sheets could be stacked to assemble a 3D structure that mimics the smooth muscle cell layer of blood vessels.
Another perspective from which to consider the structure of the cellular environment is to compare 2D and 3D culture substrates. In order to directly compare these two contexts, materials that are stable over days to weeks and compatible with cell encapsulation are required. As mentioned above, Gerecht et al. (DOI: 10.1039/c5tb01038a), Stegemann et al. (DOI: 10.1039/c5tb00934k) and Kilian et al. (DOI: 10.1039/c5tb01294e) encapsulated cells within their materials. Out of these three studies, Kilian and coworkers directly compared 2D and 3D microenvironments and found that supernatants from the 3D cultures of mesenchymal stem cells were associated with ∼2× greater formation of vessel-like tubules by endothelial cells vs. those derived from 2D cultures. An article by Brey et al. (DOI: 10.1039/c5tb00952a) focused on tissue engineering methods for brown adipose tissue. They found that the 3D microenvironment and the stiffness of the encapsulating material are critical to regulating responses by the encapsulated adipose-derived stem cells that are related to brown adipose tissue function.
Bioinspired by tissue physicochemical properties
Composition and structure both influence the overall physicochemical properties of a tissue. These properties include physical or mechanical properties such as porosity and stiffness, chemical properties including hydrophilicity/hydrophobicity and electrical conductivity.The porosity or mesh size of a material is particularly important in cell encapsulation applications, such as those reported by Stegemann (DOI: 10.1039/c5tb00934k), Brey (DOI: 10.1039/c5tb00952a), Gerecht (DOI: 10.1039/c5tb01038a), Zustiak (DOI: 10.1039/c5tb01047k) and their co-workers. The material must be sufficiently cross-linked to prevent dissolution or to provide adequate strength, but the crosslink density must not be too high to prevent transport of nutrients, oxygen and waste. For some applications described herein, e.g., blood vessel growth and nerve repair, it is imperative for cells to either be able to migrate through or extend processes into the material. Moreover, the stiffness of the cellular microenvironment has recently been of great focus as a potential means to manipulate cell response while keeping other aspects of the environment (e.g., biological activity, chemical composition) constant. Material stiffness was of particular focus to the works reported by Brey, Gerecht and Zustiak and their co-workers.
The degree to which a material can interact with water, or its degree of hydrophilicity or hydrophobicity, can alter specific or non-specific interactions with proteins. For applications where the material contacts blood (e.g., engineered blood vessels, circulating contrast agents and drug delivery) it is typically favorable to reduce non-specific protein adsorption so that the material surface does not become fouled with serum proteins and eventually blood clots. In a review of iron oxide nanoparticles, Kizilel et al. (DOI: 10.1039/c5tb00931f) describe a number of methods to coat the nanoparticles with hydrophilic polymers such as PEG, chitosan and dextran, to prevent particle aggregation, improve biocompatibility with blood and prevent degradation by host immune cells.
Zustiak and co-workers (DOI: 10.1039/c5tb01047k) also describe a system that utilizes nanoparticles – PEG-based hydrogels that contain dispersed carbon nanotubes. They found that the gel helps prevent nanotube aggregation and provided cell attachment sites while also endowing the materials with greater electrical conductivity. These materials may be uniquely favorable for applications in neural tissue engineering and as electrode coatings or drug delivery depots.
Bioinspired methods to present bioactive factors
Many functions within the body are controlled by bioactive molecules such as growth factors, cytokines, chemokines and hormones. These molecules can exist within and between tissues by interacting with the ECM. By trapping or selective binding events, the ECM can sequester or release bioactive factors to control a wide variety of cellular functions including proliferation, differentiation and migration. Reports included in this issue mimic this function of the ECM by passive or active processes.A passive or non-specific way to control growth factor sequestration and release is via a hydrolytically degradable polymer, such as poly(lactic-co-glycolic acid) (PLGA) or poly(caprolactone) (PCL), which are each common suture materials with known rates of degradation and long histories of application in humans. Willerth et al. (DOI: 10.1039/c5tb00871a) report electrospun PCL fibers that release a protein, glial cell-derived neurotrophic factor (GDNF) that is critical to a number of neuronal and neural progenitor cell functions. Namely, they found that these fibers supported the extension of neuronal processes (i.e., neurites) as well as human induced pluripotent stem cell viability and differentiation into neurons.
Others consider the ECM as an active player in the sequestration, release and transport or bioactive factors. Specific binding domains, such as those in heparin, bind to classes of growth factors to prevent or slow down their transport and degradation. Upon release of a cellular cue, such as a matrix-degrading enzyme, the growth factor is released and may come in contact with cells to provide an “on-demand” response. With both passive and active processes in mind, Stabenfeldt and co-workers (DOI: 10.1039/c5tb00935a) synthesized PLGA nanoparticles that release stromal cell-derived factor 1α (SDF-1α) and embedded these particles within a fibrin gel to provide a second means to control release. The SDF-1α has a quick initial release (i.e., “burst release”) that is common in PLGA particles. To slow down the initial release and keep SDF-1α concentrations within the therapeutic window, fibrin was selected because it contains a heparin-binding domain that would link to SDF-1α via heparin. The released SDF-1α was found to promote neural progenitor cell chemotaxis at similar levels to native SDF-1α; as a mediator of endogenous regenerative response, this SDF-1α delivery depot may find advantage in neural repair applications in the central nervous system.
Concluding comment
This themed issue provides highlights from the broad field of naturally-derived and bioinspired materials, providing insight into the intersection of interdisciplinary fields – materials chemistry and engineering, biological sciences and surgical applications. As new tools are invented and knowledge gained, we will be able to better emulate tissues in development, injury and disease, and consequently provide better solutions to challenging medical problems.Acknowledgements
The authors would like to thank funding support from the National Science Foundation (CBET 1447057; JL), UMBC (JL), the Natural Sciences and Engineering Research Council of Canada (NSERC, MSS) and the Canadian Institutes of Health Research (CIHR, MSS). MSS is especially grateful to JBL for her excellent work on this special issue.| This journal is © The Royal Society of Chemistry 2015 |


