Isoreticular synthesis of 2D MOFs with rotating aryl rings†
Brianna C.
Hughes
,
Christopher R.
Murdock
and
David M.
Jenkins
*
Department of Chemistry, University of Tennessee, Knoxville, Tennessee 37996-1600, USA. E-mail: jenkins@ion.chem.utk.edu
First published on 21st September 2015
Abstract
A series of isoreticular two-dimensional metal–organic frameworks (MOFs) was synthesized with group 11 metals and semirigid ligands that bind in a syn conformation. The modular synthetic approach allows the pore to be expanded based on the aryl moiety in the center of the linker from 11.0 Å to 15.5 Å. When there is an axis of rotation, each aryl ring can act independently as a rotating gate as a function of guest molecules. For the two aryl ring system, all three states (open/open, open/closed, and closed/closed) are observed by single crystal X-ray diffraction (SCXRD). The guest dependent nature of the aryl rings makes this two-pillared topology a logical choice for breathing MOFs.
Introduction
Breathing MOFs are functional materials that combine crystallinity with softness.1,2 These materials’ softness (multiple stable states) as a function of guest has led to initial studies that focused on gas adsorption.2–4 More recent research has expanded to include the intriguing possibility that these breathing materials can act as the stationary phase in chromatographic separations.5 Denayer and De Vos have demonstrated separations utilizing MIL-53 and MIL-47 with mixtures of styrene and ethylbenzene, xylene isomers, as well as ortho-substituted aromatics.5 Nonetheless, if breathing MOFs are to be harnessed effectively as stationary phases for separations, rational synthetic strategies that lead to a multitude of new breathing MOFs must be achieved. Therefore, breathing MOFs, like static MOFs, should be synthesized using the principles of isoreticular design.Breathing occurs at the “kneecap” of the material, which is the flexing point, and the “kneecap” is normally at the metal–ligand interface (as in MIL-53).2,6 As rigid linkers are used for the synthesis of these flexible MOFs, isoreticular design principles have led to various functionalized versions of the original rigid ligand and their subsequent adsorption studies.7,8 For example, MIL-53 has been synthesized with halides, hydroxides, amine, methyl and nitro functional groups which has led to changes in CO2 adsorption behavior.7 Although researchers are making progress designing new breathing MOF systems,4,9 employment of rigid linkers often makes it difficult, and current work in the field is frequently limited to the isostructural synthesis of a few parent MOFs (for example MIL-53, MIL-88, and MIL-47).7,8
To skirt this limitation, researchers have proposed putting the hinge solely on the ligand itself. Ligand-based breathing can be accomplished via a double-hinged screw mechanism or through employing a “gate” on an anisotropic MOF. The gate effect allows for the linker to rotate based on the guest present, thus opening or closing the pore. Gate-based breathing offers the chance for isoreticular design since additional gates can be added to an otherwise fixed design.
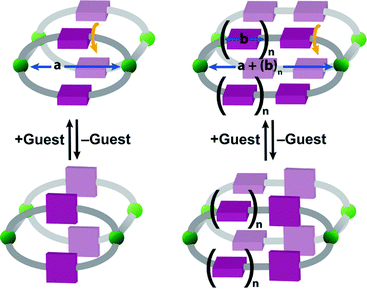
Consider the case of an isostructural 2-pillared 2D MOF system with multiple aryl rings (Fig. 1). As the number of aryl rings (n) increase, not only will the pore size broaden (a + (b)n), but the permutations for rotated rings will also proliferate. When n is 0, there are only two options for ring position, open and closed. Assuming orientation does not matter, when n is 1, three combinations are possible: open/open, open/closed, and closed/closed. With these expanded aryl ring systems, the complexity for both synthesis and characterization clearly increases as n increases.
Our previous research showcased a 2D framework wherein a phenyl ring moiety on the linker rotates and blocks the pore as a function of guest.10 The backbone was comprised of a bidentate semirigid linker in a syn conformation with a phenyl ring subunit that rotated between parallel and perpendicular positions as a function of guest solvent. An isoreticular approach to synthesizing gate-based breathing MOFs would involve increasing the size of the aryl moiety between the triazoles. If the aryl moiety has a linear axis of rotation, then the MOFs should possess gate-based breathing.
Initial studies by Férey on the effects of adding flexibility to ligands determined that, as flexible ligands do not impose strong geometric constraints, isoreticular principles cannot be applied.11 Even if the ligand core remained rigid, the introduction of some peripheral flexibility would drastically change the predicted structure.11 In this manuscript, we have expanded upon our original research and have synthesized a series of 2D sheet MOFs with group 11 metals and semirigid ligands that are arranged in a syn conformation. The preferential syn conformation has allowed for a modular synthetic approach and allows additional aryl rings to be added to the linker, providing the first isoreticular set of gate-based breathing MOFs. With widths ranging from 11.0 Å to 15.5 Å, single crystal X-ray diffraction studies show that, not only can semirigid ligands be employed, but all combinations of open and closed ring positions are possible.
Results and discussion
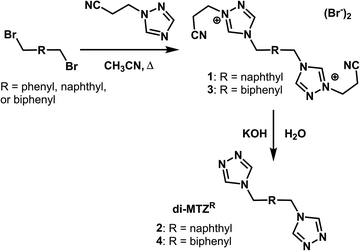
We have previously reported the synthesis of the double-hinged ligand, 4,4′-(1,4-(xylene)diyl)bis(1,2,4-triazole) (di-MTZPh) and have expanded the synthetic methodology by incorporating either a naphthyl or biphenyl moiety as the central unit between the triazoles.10 Intermediate 1 was formed through addition of 2,6-bis(bromomethyl)naphthalene and 1,2,4-triazole-1-propanenitrile in 70% yield (Scheme 1). Compound 1 could be deprotected through the addition of potassium hydroxide which led to 4,4′-[naphthalene-2,6-diylbis(methylene)]bis(1,2,4-triazole), 2 (di-MTZNap), in a 94% yield. In a similar manner, the addition of 1,2,4-triazole-1-propanenitrile to 4,4′-bis(bromomethyl)-1,1′-biphenyl led to the formation of intermediate 3 in 84% yield (Scheme 1). Addition of potassium hydroxide cleaved the protecting group which generated the product, 4,4′-(4,4′-bi-tolyl)bis(1,2,4-triazole), 4 (di-MTZBiph), in 85% yield. The ligand, di-MTZBiph, was further purified via recrystallization from water.Previously reported results with di-MTZPh and 4,4′-(1,4-(trans-2-butene)diyl)bis(1,2,4-triazole) demonstrated that multiple copper MOFs can be synthesized by varying the reaction conditions, including solvent, metal-to-ligand ratio, and starting metal salt.10,12 Consequently, a combinatorial approach was employed that varied these conditions for our new ligands.
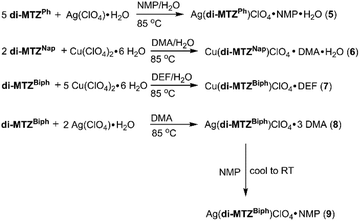
Initial reaction conditions focused on forming a silver 2D MOF with the original di-MTZPh ligand that would be complementary to the copper MOFs that we previously reported.10 The addition of five equivalents of di-MTZPh and one equivalent of silver perchlorate to a mixture of N-methyl-2-pyrrolidone (NMP) and water led to the formation of [Ag(di-MTZPh)(ClO4)]·NMP·H2O (5) (Scheme 2). Colorless needle crystals suitable for X-ray diffraction were obtained after heating the reaction mixture for 3 h at 85 °C.
We were successful in preparing 2D MOFs with the expanded ligands di-MTZNap and di-MTZBiph with copper. As with 5, excess ligand was required to form a MOF with di-MTZNap. Two equivalents of 2 plus copper perchlorate in a mixture of water and dimethyl formamide (DMA) yielded [Cu(di-MTZNap)(ClO4)]·DMA·H2O (6) (Scheme 2). The addition of one equivalent of di-MTZBiph to five equivalents of copper perchlorate to a mixture of N,N-diethylformamide (DEF) and water led to the formation of [Cu(di-MTZBiph)(ClO4)]·DEF (7). In both cases, the frameworks were collected as colorless needles after heating for 24 h at 85 °C.
Notably, when the metal was changed to silver two different MOFs could be formed. One equivalent of di-MTZBiph and two equivalents of silver perchlorate in DMA yielded [Ag(di-MTZBiph)(ClO4)]·3DMA (8) (Scheme 2). In order to obtain crystalline 8, the solution was heated for 24 h at 85 °C then pipetted to a clean vial. As the reaction mixture was cooled, colorless needles suitable for X-ray diffraction were formed. Notably, the addition of various solvents during the reactions allowed structural variation of the MOFs to form. Upon the addition of 2 mL of NMP to 8 as it was cooling, [Ag(di-MTZBiph)(ClO4)]·NMP (9) was obtained as colorless needles suitable for X-ray diffraction (Scheme 2).
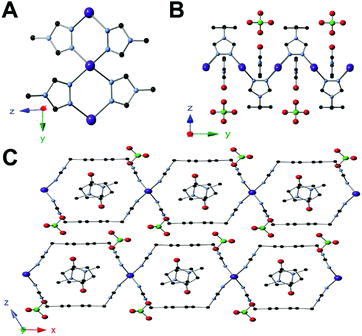
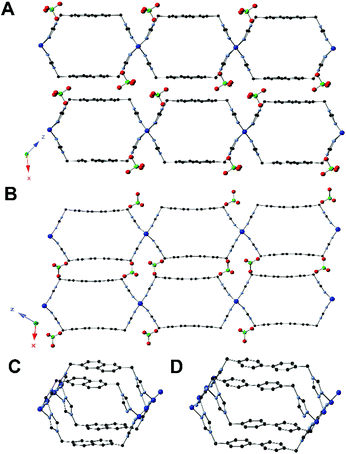
Single crystal XRD of 5 showed that each tetrahedral Ag(I) center is coordinated to four triazole ligands (Fig. 2A). Adjacent silver atoms are bridged by two triazole ligands to form an equatorial plane, which results in a linear chain of silver atoms (Fig. 2B). The semirigid ligand shows a syn conformation that forms a 2D layered structure, like those previously reported with copper and this ligand (Fig. 2C).10 This framework exhibits an open state, only one of two positions possible for a one aryl ring system. Due to the bridging triazole ligands between the silver centers in an alternating manner, the overall topology is a 2D sheet MOF. This framework incorporates guest molecules of NMP with perchlorate anions packed between the sheet layers.
The X-ray structures for MOFs 6 and 7 follow the same topology of 5, but with expanded pores, as is expected given their isoreticular design. Although the data set for the XRD of 6 was of low quality, the connectivity between the atoms could clearly be determined. Single crystal XRD of 6 revealed the same coordination with copper(I) centers bridged by the triazole ligands, resulting in a 2D MOF similar to 5 (Fig. 3A). In framework 7, the phenyl rings within the triazole linker are both parallel to one another (open/open state) which results in layers that are stacked through extensive π–π interactions (Fig. 3B). Like in 5, the perchlorate anions are found in between the 2D sheet layers of the framework. Although DEF molecules are present and confirmed through CHN analysis, the DEF molecules could not be corroborated through single crystal X-ray analysis.
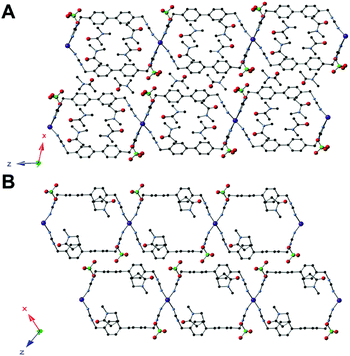
In silver MOF 8, the topology is similar to 7; however, ligand-based breathing causes a notable difference in the structure. Both the phenyl rings within the triazole linker are rotated by 35.4°, resulting in a partially perpendicular orientation (closed/closed state) compared to that of the tubes (Fig. 4A). This allows for four DMA solvent molecules to be within the pores of the extended framework, as well as additional solvent molecules between the layers of the framework. As in 7, the perchlorate anions are situated in between the 2D sheets of the framework.
The structure of 9 was topologically identical to that of 8 – consisting of Ag(I) centers bridged by two triazole ligands to form a fused-tube structure – but there was one major structural change (Fig. 4B). The orientation of one of the phenyl rings had rotated to a parallel position compared to that in 8, while the other is at 90.0° resulting in an open/closed state. This rotation allows for the migration of solvent within the pore between the rotated and non-rotated phenyl rings. Notably, 5 has the same guest solvent, but in that case the rings are parallel. The perchlorate anions have not moved with a change in guest solvent, from DMA to NMP, between 8 and 9.
A comparison of the structures of 5–9 and previously published [Cu(di-MTZPh)(ClO4)] shows that they all follow the same two-pillared topology.10 The height of the copper MOFs is always 8.9 Å, but the width varies from 11.0 Å for [Cu(di-MTZPh)(ClO4)] to 12.9 Å for 6 and 15.5 Å for 7. Since 5 and 8 are isostructural to [Cu(di-MTZPh)(ClO4)], and 7, respectively, they are almost the exact same size. These six single crystal structures for these MOFs showcase the principle of isoreticular design with semirigid ligands.
Conclusions
A series of isoreticular two-dimensional metal–organic frameworks was synthesized utilizing group 11 metals and semirigid bis(1,2,4-triazole)ligands that are arranged in a syn conformation. The modular synthetic approach allows for increasingly wide aryl moieties between the triazoles which expands the widths of the pores. In addition to the larger pores created by the biphenyl ligand, each aryl ring of the biphenyl can act independently as a rotating gate as a function of guest. Three separate X-ray structures of the MOFs showcased the three possible outcomes: two rings parallel, one ring parallel and one ring perpendicular, and two rings perpendicular. This set of MOFs is the first isoreticular set of aryl ring gate-based MOFs. The guest dependent nature of the aryl rings’ positions makes this topology a logical choice for guest selective breathing MOFs. Future research will focus on understanding the breathing motion of this isoreticular set of MOFs in the solid state.Acknowledgements
We are grateful to the State of Tennessee's Science Alliance, whose JDRD Grant provided partial support for this research.References
- G. Ferey, Chem. Soc. Rev., 2008, 37, 191 RSC; C. R. Murdock, B. C. Hughes, Z. Lu and D. M. Jenkins, Coord. Chem. Rev., 2014, 258–259, 119 CrossRef CAS PubMed; A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062 RSC.
- G. Ferey and C. Serre, Chem. Soc. Rev., 2009, 38, 1380 RSC.
- D. Banerjee, A. J. Cairns, J. Liu, R. K. Motkuri, S. K. Nune, C. A. Fernandez, R. Krishna, D. M. Strachan and P. K. Thallapally, Acc. Chem. Res., 2015, 48, 211 CrossRef CAS PubMed; N. A. Khan, Z. Hasan and S. H. Jhung, J. Hazard. Mater., 2013, 244–245, 444 CrossRef PubMed; J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; A. Schneemann, S. Henke, I. Schwedler and R. A. Fischer, ChemPhysChem, 2014, 15, 823 CrossRef PubMed; M. Taddei, F. Costantino, A. Ienco, A. Comotti, P. V. Dau and S. M. Cohen, Chem. Commun., 2013, 49, 1315 RSC; K. S. Walton, Nat. Chem., 2014, 6, 277 CrossRef PubMed; C. Wang, L. Li, J. G. Bell, X. Lv, S. Tang, X. Zhao and K. M. Thomas, Chem. Mater., 2015, 27, 1502 CrossRef; Y. Yue, J. A. Rabone, H. Liu, S. M. Mahurin, M.-R. Li, H. Wang, Z. Lu, B. Chen, J. Wang, Y. Fang and S. Dai, J. Phys. Chem. C, 2015, 119, 9442 Search PubMed.
- S. Sanda, S. Parshamoni and S. Konar, Inorg. Chem., 2013, 52, 12866 CrossRef CAS PubMed.
- L. Alaerts, C. E. A. Kirschhock, M. Maes, M. A. van der Veen, V. Finsy, A. Depla, J. A. Martens, G. V. Baron, P. A. Jacobs, J. F. M. Denayer and D. E. De Vos, Angew. Chem., Int. Ed., 2007, 46, 4293 CrossRef CAS PubMed; V. Finsy, C. E. A. Kirschhock, G. Vedts, M. Maes, L. Alaerts, D. E. De Vos, G. V. Baron and J. F. M. Denayer, Chem. – Eur. J., 2009, 15, 7724 CrossRef PubMed; V. Finsy, H. Verelst, L. Alaerts, D. De Vos, P. A. Jacobs, G. V. Baron and J. F. M. Denayer, J. Am. Chem. Soc., 2008, 130, 7110 CrossRef PubMed; M. Maes, F. Vermoortele, L. Alaerts, S. Couck, C. E. A. Kirschhock, J. F. M. Denayer and D. E. De Vos, J. Am. Chem. Soc., 2010, 132, 15277 CrossRef PubMed; M. A. Moreira, J. C. Santos, A. F. P. Ferreira, J. M. Loureiro and A. E. Rodrigues, Ind. Eng. Chem. Res., 2011, 50, 7688 CrossRef.
- K. Barthelet, J. Marrot, D. Riou and G. Férey, Angew. Chem., Int. Ed., 2002, 41, 281 CrossRef CAS; C. Serre, F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, D. Louër and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519 CrossRef PubMed.
- S. Biswas, T. Ahnfeldt and N. Stock, Inorg. Chem., 2011, 50, 9518 CrossRef CAS PubMed; S. Couck, J. F. M. Denayer, G. V. Baron, T. Rémy, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2009, 131, 6326 CrossRef PubMed.
- S. Biswas, D. E. P. Vanpoucke, T. Verstraelen, M. Vandichel, S. Couck, K. Leus, Y.-Y. Liu, M. Waroquier, V. Van Speybroeck, J. F. M. Denayer and P. Van Der Voort, J. Phys. Chem. C, 2013, 117, 22784 Search PubMed; S. Surble, C. Serre, C. Mellot-Draznieks, F. Millange and G. Ferey, Chem. Commun., 2006, 284 RSC.
- H.-R. Fu and J. Zhang, Chem. – Eur. J., 2015, 21, 5700 CrossRef CAS PubMed; X. Li, Z. Yu, T. Guan, X. Li, G. Ma and X. Guo, Cryst. Growth Des., 2015, 15, 278 Search PubMed; D. L. Reger, A. Leitner, P. J. Pellechia and M. D. Smith, Inorg. Chem., 2014, 53, 9932 CrossRef PubMed; J. Xiao, Y. Wu, M. Li, B.-Y. Liu, X.-C. Huang and D. Li, Chem. – Eur. J., 2013, 19, 1891 CrossRef PubMed; D.-D. Zhou, Z.-J. Liu, C.-T. He, P.-Q. Liao, H.-L. Zhou, Z.-S. Zhong, R.-B. Lin, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Chem. Commun., 2015, 51, 12665 RSC; L. Sun, C. H. Hendon, M. A. Minier, A. Walsh and M. Dincă, J. Am. Chem. Soc., 2015, 137, 6164 CrossRef PubMed.
- C. R. Murdock, N. W. McNutt, D. J. Keffer and D. M. Jenkins, J. Am. Chem. Soc., 2014, 136, 671 CrossRef CAS PubMed.
- T. Devic, O. David, M. Valls, J. Marrot, F. Couty and G. Férey, J. Am. Chem. Soc., 2007, 129, 12614 CrossRef CAS PubMed.
- C. R. Murdock, Z. Lu and D. M. Jenkins, Inorg. Chem., 2013, 52, 2182 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of all compounds, including TGA, PXRD, and crystallographic data (CIF). CCDC 1413568–1413572. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qi00135h |
| This journal is © the Partner Organisations 2015 |