Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intermolecular reactions of gold(I)-carbenes with furans by related mechanisms†‡
David
Lebœuf§
a,
Morgane
Gaydou§
a,
Yahui
Wang
a and
Antonio M.
Echavarren
*ab
aInstitute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona, Spain. E-mail: aechavarren@iciq.es
bDepartament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/Marcel·li Domingo s/n, 43007 Tarragona, Spain
First published on 19th June 2014
Abstract
The intermolecular gold(I)-catalyzed reactions of propargyl carboxylates, 1,6-enynes, or 7-substituted 1,3,5-cycloheptatrienes with furans afford cyclopentenones, polyenes or polycyclic compounds by related mechanisms initiated by the electrophilic addition of gold(I) carbenes to furans followed by ring-opening.
Gold-catalyzed intramolecular cycloisomerization reactions of 1,n-enynes have been widely studied and applied in synthesis,1,2 following the pioneering work on similar transformations catalyzed by other late transition metals.3 However, the development of mechanistically related intermolecular cyclizations of alkynes with alkenes or other substrates has been more challenging.4
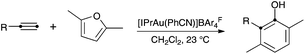
An important transformation in this area is the cyclization of alkynylfurans, which was discovered using gold5 or platinum6 catalysts. This transformation leads to substituted phenols in a rather straightforward manner. Only one example of the corresponding gold-catalyzed intermolecular reaction of a furan with an alkyne had initially been reported using the binuclear gold(I) complex [(Ph3PAu)2Cl]BF4,5f although we recently found that phenols can be obtained using air-stable [IPrAu(PhCN)]BArF4 (A) (BArF4 = 3,5-bis(trifluoromethyl)phenylborate) as the catalyst (Scheme 1).7
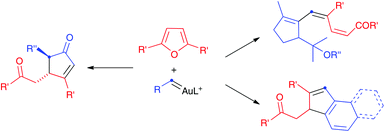
We have now found that propargyl carboxylates react differently with furans in the presence of gold(I) catalysts to give functionalized cyclopentenones or cyclopentadienyl carboxylates, which had not been reported before by related procedures (Scheme 2). Aryl gold(I) carbenes generated in enyne cyclizations1n,2,8 or by retro-Buchner reaction9,10 of 7-substituted 1,3,5-cycloheptatrienes also react with furans to give rise to polycyclic compounds.
The extent of stabilization of a carbocation by gold(I) has been the subject of discussion,11–13 although, according to theoretical calculations, a carbene-like structure is favored when gold(I) is coordinated to strongly donating ligands such as N-heterocyclic carbenes and phosphines.
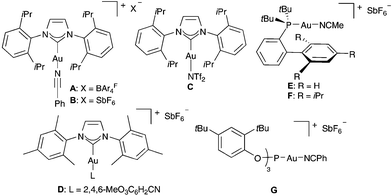
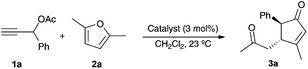
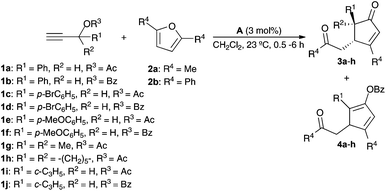
We first examined the reaction of propargylic acetate 1a with 2,5-dimethylfuran 2a in the presence of gold(I) catalysts, which in all cases gave rise diastereoselectively to cyclopentenone 3a (Table 1). The best yield of 3a was obtained using a cationic gold(I) catalyst [IPrAu(PhCN)]BArF4 (A) (Table 1, entry 1). Related IPr gold(I) complex B with hexafluoroantimonate anions gave slightly lower yield after 30 min (Table 1, entry 2), whereas neutral complex C and cationic IMes derivatives required longer reaction times (Table 1, entries 3 and 4). Phosphine and phosphite gold(I) catalysts were less reactive in this transformation (Table 1, entries 5–8). Poor results were obtained with AuCl3 or PtCl2 (Table 1, entries 9 and 10).
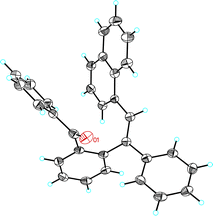
Interestingly, when the reaction of furan 2a was performed with benzoate ester 1b and catalyst A, cyclopentadienyl benzoate 4a was isolated as the major product in 63% yield (Table 2, entry 2). Similarly, while acetates 1c, 1e, 1g, and 1h gave cyclopentenones 3b–e (Table 2, entries 3, 5, 7, and 8), benzoates 1d and 1f gave cyclopentadienes 4b and 4c as the major products (Table 2, entries 4 and 6). However, 1-cyclopropylprop-2-yn-1-yl acetate (1i) and benzoate (1j) react similarly to form 3f (Table 2, entries 9 and 10). The reaction of 2,5-diphenylfuran (2b) with benzoate 1b afforded cyclopentadienyl benzoate 4d (Table 2, entry 11). The structure of 4d was confirmed by X-ray diffraction (Fig. 1).14 Cyclopentenones were obtained in lower yields (<30%) when 2-substituted furans were used as substrates.
 | ||
| Fig. 1 ORTEP plot (50% thermal ellipsoids) of the crystal structure of cyclopentadienyl benzoate 4d. | ||
| Entry | R1 | R2 | R3 | R4 | 3 (Yield %)a | 4 (Yield %)a |
|---|---|---|---|---|---|---|
a Isolated yields.
b 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 trans/cis.
c 5 1 trans/cis.
c 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 trans/cis. 1 trans/cis.
|
||||||
| 1 | Ph | H | Ac | Me | 3a (57) | — |
| 2 | Ph | H | Bz | Me | 3a (15) | 4a (63) |
| 3 | p-BrC6H4 | H | Ac | Me | 3b (65) | — |
| 4 | p-BrC6H4 | H | Bz | Me | 3b (20) | 4b (44) |
| 5 | p-MeOC6H4 | H | Ac | Me | 3c (36) | — |
| 6 | p-MeOC6H4 | H | Bz | Me | 3c (31) | 4c (48) |
| 7 | Me | Me | Ac | Me | 3d (61) | — |
| 8 | –(CH2)5– | Ac | Me | 3e (63) | — | |
| 9 | c-C3H5 | H | Ac | Me | 3f (61)b | — |
| 10 | c-C3H5 | H | Bz | Me | 3f (60)c | — |
| 11 | Ph | H | Bz | Ph | — | 4d (75) |
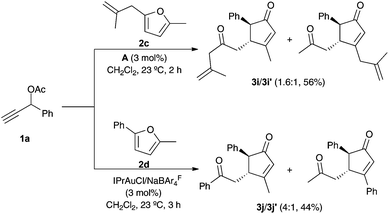
Unsymmetrically substituted furans 2c–d reacted with 1a and catalyst A to give a mixture of cyclopentenones 3i–j/3i′–j′ favoring formation of the regioisomer with the less sterically hindered group at C-3 of the cyclopentenone (Scheme 3).
We propose a mechanism for the formation of the cyclopentenones and cyclopentadienyl esters initiated by the 1,2-acyloxy migration of η2-alkyne–gold(I) complex 5,15 followed by the electrophilic trapping of the α,β-unsaturated gold(I) carbene 6 by furan 2 (Scheme 4). The resulting intermediate 7 may lead to the cyclopropanation product 8, which could open to form 9. Intermediate 9 could also be formed directly from 7 by 1,2-elimination. A Mukaiyama–Michael-type cyclization would then form 10, which leads to cyclopentenones 3 or cyclopentadienyl benzoates 4. The observed trans-stereoselectivity is presumably derived to the preferred Z-configuration of the vinyl gold(I) carbenes.16 In the case of unsymmetrically substituted furans 2c–d, the major regioisomers are formed by the attack of the less substituted site of the furan to intermediate 6.
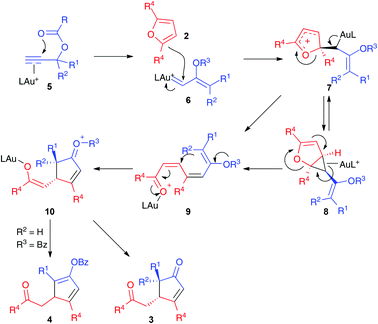 | ||
| Scheme 4 Proposed mechanism for the formation of adducts 3 and 4 by gold(I)-catalyzed reaction of propargylic carboxylates with furans. | ||
Formation of open chain products derived from intermediates similar to 9 has been reported before for Ru(II), Pt(II),17,18 and, in one case, for Au(I)-19,20 catalyzed reactions of furans. A similar reactivity was observed in the reaction of furans with gold(I) carbenes generated by the ring opening of cyclopropenes.21 It is interesting that in our case, 2,5-disubstituted furans react preferentially at C-2, instead of at C3 as it is observed with Ru(II) as a catalyst.17
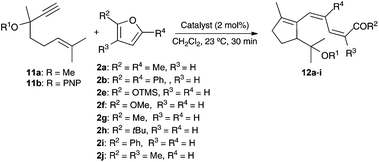
1,6-Enynes 11a–b reacted with mono- and disubstituted furans 2a–j in the presence of gold(I) catalysts to form ketones or carboxylic acid derivatives 12a–i featuring a triene moiety with a (Z,Z)-configuration (Table 3).22
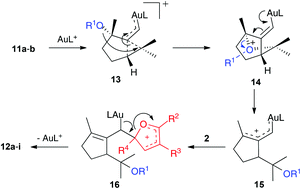
This transformation is mechanistically interesting as it features a gold(I)-catalyzed cyclization/1,5-OR migration via intermediates 13 and 14 to form α,β-unsaturated gold(I) carbenes 15,8 which react with electron-rich furans to form 16 (Scheme 5). A similar elimination to that proposed before in the elimination of 7 (Scheme 4) gives 12a or 12d after hydrolytic cleavage of the trimethylsilyl ester.
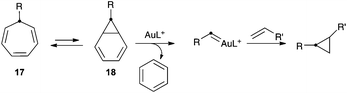
We also examined the reaction of furans with gold(I) carbenes generated by the retro-Buchner reaction of 7-substituted 1,3,5-cycloheptatrienes (17), which proceeds by retrocyclopropanation of norcaradienes 18 (Scheme 6).9 The resulting gold(I) carbenes can be trapped with alkenes to form cyclopropanes,9a,c or indenes.9b These gold(I) carbenes also react intramolecularly with arenes in Friedel–Crafts-type reactions.9b
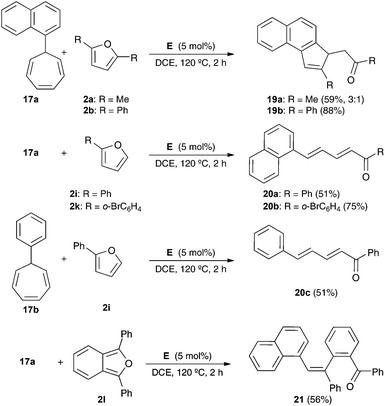
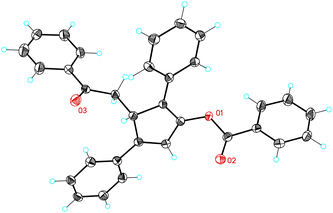
The reaction of 1-naphthyl cycloheptatriene 17a with 2,5-disubstituted furans 2a–b in the presence of catalyst E in 1,2-dichloroethane (DCE) at 120 °C gave 3H-cyclopenta[a]naphthalenes 19a–b (Scheme 7). In the former case, 19a was obtained along with a minor isomer with a tetrasubstituted double bond. The reaction of 17a–b with 2-substituted furans 2i and 2k leads to 1,5-diarylpenta-2,4-dien-1-ones 20a–c. On the other hand, the reaction of 17a with 1,3-diphenylisobenzofuran (2l) gave 21 in 56% yield. The Z configuration of 21 was determined by X-ray diffraction (Fig. 2).23
Mechanistically, the reaction of 17a–b with catalyst E leads to aryl gold(I) carbenes 22a–b,9 which react with furans by pathways similar to those observed before for other gold(I) carbenes (Scheme 8). Thus, the Friedel–Crafts-type reaction would lead to intermediates such as 23 or 25, which affords open chain derivatives 24 or 21. In the former case, the initially formed 25 affords 20a–c by Z to E isomerization or 19a–b by a Michael-type ring closing.
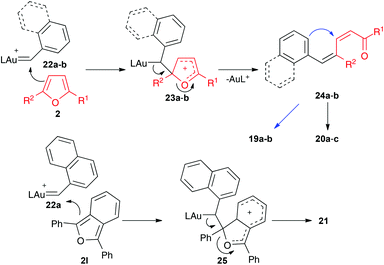 | ||
| Scheme 8 Proposed mechanism for the reaction of furans with gold(I) carbenes generated by retro-Buchner reaction. | ||
The mechanistic proposal outlined in Scheme 8 is supported by DFT calculations (M06 level, 1,2-dichloroethane, PMe3 as the phosphine ligand) (Scheme 9). Accordingly, the reaction between carbene 22b, resulting from the retro-Buchner reaction of 7-phenyl-1,3,5-cycloheptatriene (17b),9 and furan 2b through TS1 leads to intermediate 23b, which smoothly opens up to form 24b–AuL+ complex.24 The intermediate cyclopropane 25, corner-coordinated to AuL+, was also located as an intermediate, although its energy is higher than that of intermediate 23b.
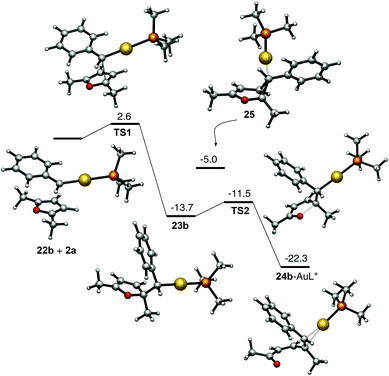 | ||
| Scheme 9 Mechanism for the reaction of carbene 22b with furan 2a based on DFT calculations. Free energies in kcal mol−1. | ||
In summary, we have found that three very different types of substrates react with furans and gold(I) catalysts under different reaction conditions by mechanistically related pathways. This is best rationalized if similar gold(I) carbenes are involved as intermediates in all these processes, which is also supported by DFT calculations. The fact that similar reactions are observed in transformations proceeding via ruthenium(II) or platinum(II) carbenes17 further supports the involvement of closely similar species using gold(I) catalysts. These reactions of furans lead to rather elaborated products from readily available substrates under mild conditions. Further applications of the trapping of reactive gold(I) carbenes with other types of nucleophiles are being explored.
We thank the European Research Council (advanced grant no. 321066), the MINECO (CTQ2010-16088/BQU), the AGAUR (2009SGR47 and Beatriu de Pinós postdoctoral fellowship to D. L.), EU Project ICT (ATMOL, contract no. FP7-270028) and the ICIQ Foundation for financial support. We also thank the ICIQ X-Ray Diffraction unit for the X-ray structures.
Notes and references
- (a) L. Zhang, J. Sun and S. A. Kozmin, Adv. Synth. Catal., 2006, 348, 2271–2296 CrossRef CAS; (b) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896–7936 CrossRef PubMed; (c) A. Fürstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410–3449 CrossRef PubMed; (d) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed; (e) Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239–3265 CrossRef CAS PubMed; (f) A. Arcadi, Chem. Rev., 2008, 108, 3266–3325 CrossRef CAS PubMed; (g) E. Jiménez-Núñez and A. M. Echavarren, Chem. Rev., 2008, 108, 3326–3350 CrossRef PubMed; (h) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351–3378 CrossRef CAS PubMed; (i) V. Michelet, P. Y. Toullec and J.-P. Genêt, Angew. Chem., Int. Ed., 2008, 47, 4268–4315 CrossRef CAS PubMed; (j) A. Fürstner, Chem. Soc. Rev., 2009, 38, 3208–3221 RSC; (k) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954–1993 CrossRef CAS PubMed; (l) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994–2009 CrossRef CAS PubMed; (m) C. Obradors and A. M. Echavarren, Chem. Comm., 2014, 50, 16–28 RSC; (n) C. Obradors and A. M. Echavarren, Acc. Chem. Res., 2014, 47, 902–912 CrossRef CAS PubMed.
- (a) C. Nieto-Oberhuber, M. P. Muñoz, E. Buñuel, C. Nevado, D. J. Cárdenas and A. M. Echavarren, Angew. Chem., Int. Ed., 2004, 43, 2402–2406 CrossRef CAS PubMed; (b) C. Nieto-Oberhuber, M. P. Muñoz, S. López, E. Jiménez-Núñez, C. Nevado, E. Herrero-Gómez, M. Raducan and A. M. Echavarren, Chem. – Eur. J., 2006, 12, 1677–1693 CrossRef CAS PubMed; (c) C. Nieto-Oberhuber, S. López, E. Jiménez-Núñez and A. M. Echavarren, Chem. – Eur. J., 2006, 12, 5916–5923 CrossRef CAS PubMed; (d) C. Ferrer, M. Raducan, C. Nevado, C. K. Claverie and A. M. Echavarren, Tetrahedron, 2007, 63, 6306–6316 CrossRef CAS PubMed; (e) N. Cabello, C. Rodríguez and A. M. Echavarren, Synlett, 2007, 1753–1758 CAS; (f) C. H. M. Amijs, V. López-Carrillo, M. Raducan, P. Pérez-Galán, C. Ferrer and A. M. Echavarren, J. Org. Chem., 2008, 73, 7721–7730 CrossRef CAS PubMed; (g) V. López-Carrillo, N. Huguet, Á. Mosquera and A. M. Echavarren, Chem. – Eur. J., 2011, 17, 10972–10978 CrossRef; (h) P. Pérez-Galán, N. J. A. Martin, A. G. Campaña, D. J. Cárdenas and A. M. Echavarren, Chem. – Asian J., 2011, 6, 482–486 CrossRef PubMed; (i) A. Escribano-Cuesta, P. Pérez-Galán, E. Herrero-Gómez, M. Sekine, A. A. C. Braga, F. Maseras and A. M. Echavarren, Org. Biomol. Chem., 2012, 10, 6105–6111 RSC.
- (a) B. M. Trost, Acc. Chem. Res., 1990, 23, 34–42 CrossRef CAS; (b) N. Chatani, K. Kataoka, S. Murai, N. Furukawa and Y. Seki, J. Am. Chem. Soc., 1998, 120, 9104–9105 CrossRef CAS; (c) N. Chatani, N. Furukawa, H. Sakurai and S. Murai, Organometallics, 1996, 15, 901–903 CrossRef CAS; (d) S. Oi, I. Tsukamoto, S. Miyano and Y. Inoue, Organometallics, 2001, 20, 3704–3709 CrossRef CAS; (e) A. Fürstner, H. Szillat and F. Stelzer, J. Am. Chem. Soc., 2000, 122, 6785–6786 CrossRef; (f) M. Méndez, M. P. Muñoz and A. M. Echavarren, J. Am. Chem. Soc., 2000, 122, 11549–11550 CrossRef; (g) E. Mainetti, V. Mouriès, L. Fensterbank, M. Malacria and J. Marco-Contelles, Angew. Chem., Int. Ed., 2002, 41, 2132–2135 CrossRef CAS.
- (a) V. López–Carrillo and A. M. Echavarren, J. Am. Chem. Soc., 2010, 132, 9292–9294 CrossRef PubMed; (b) R. B. Dateer, B. S. Shaibu and R. S. Liu, Angew. Chem., Int. Ed., 2011, 51, 113–117 CrossRef PubMed; (c) H.-S. Yeom, J. Koo, H.-S. Park, Y. Wang, Y. Liang, Z.-X. Yu and S. Shin, J. Am. Chem. Soc., 2012, 134, 208–211 CrossRef CAS PubMed; (d) S. Kramer and T. Skrydstrup, Angew. Chem., Int. Ed., 2012, 51, 4681–4684 CrossRef CAS PubMed; (e) Y. Luo, K. Ji, Y. Li and L. Zhang, J. Am. Chem. Soc., 2012, 134, 17412–17415 CrossRef CAS PubMed; (f) C. Obradors and A. M. Echavarren, Chem. – Eur. J., 2013, 19, 3547–3551 CrossRef CAS PubMed; (g) A. Homs, C. Obradors, D. Leboeuf and A. M. Echavarren, Adv. Synth. Catal., 2014, 356, 221–228 CrossRef CAS.
- (a) A. S. K. Hashmi, T. M. Frost and J. W. Bats, J. Am. Chem. Soc., 2000, 122, 11553–11554 CrossRef CAS; (b) A. S. K. Hashmi, T. M. Frost and J. W. Bats, Org. Lett., 2001, 3, 3769–3771 CrossRef CAS PubMed; (c) A. S. K. Hashmi, L. Ding, J. W. Bats, P. Fischer and W. Frey, Chem. – Eur. J., 2003, 9, 4339–4345 CrossRef CAS PubMed; (d) A. S. K. Hashmi, J. P. Weyrauch, M. Rudolph and E. Kurpejović, Angew. Chem., Int. Ed., 2004, 43, 6545–6547 CrossRef CAS PubMed; (e) A. S. K. Hashmi, M. Rudolph, J. P. Weyrauch, M. Wölfle, W. Frey and J. W. Bats, Angew. Chem., Int. Ed., 2005, 44, 2798–2801 CrossRef CAS PubMed; (f) A. S. K. Hashmi, M. C. Blanco, E. Kurpejović, W. Frey and J. W. Bats, Adv. Synth. Catal., 2006, 348, 709–713 CrossRef CAS; (g) A. S. K. Hashmi, M. Wölfle, F. Ata, M. Hamzic, R. Salathé and W. Frey, Adv. Synth. Catal., 2006, 348, 2501–2508 CrossRef CAS; (h) A. S. K. Hashmi, P. Haufe, C. Schmid, A. Rivas Nass and W. Frey, Chem. – Eur. J., 2006, 12, 5376–5382 CrossRef CAS PubMed; (i) A. S. K. Hashmi, R. Salathé and W. Frey, Chem. – Eur. J., 2006, 12, 6991–6996 CrossRef CAS PubMed; (j) A. S. K. Hashmi, M. Rudolph, H.–U. Siehl, M. Tanaka, J. W. Bats and W. Frey, Chem. – Eur. J., 2008, 14, 3703–3708 CrossRef CAS PubMed; (k) M. Rudolph, M. Q. McCreery, W. Frey and A. S. K. Hashmi, Beilstein J. Org. Chem., 2011, 7, 794–801 CrossRef CAS PubMed; (l) A. S. K. Hashmi, J. Hofmann, S. Shi, A. Schütz, M. Rudolph, C. Lothschütz, M. Wieteck, M. Bührle, M. Wölfle and F. Rominger, Chem. – Eur. J., 2013, 19, 382–389 CrossRef CAS PubMed; (m) R. Manzano, F. Rominger and A. S. K. Hashmi, Organometallics, 2013, 32, 2199–2203 CrossRef CAS.
- (a) B. Martín-Matute, D. J. Cárdenas and A. M. Echavarren, Angew. Chem., Int. Ed., 2001, 40, 4754–4757 CrossRef; (b) B. Martín-Matute, C. Nevado, D. J. Cárdenas and A. M. Echavarren, J. Am. Chem. Soc., 2003, 125, 5757–5766 CrossRef PubMed.
- N. Huguet, D. Lebœuf and A. M. Echavarren, Chem. – Eur. J., 2013, 19, 6581–6585 CrossRef CAS PubMed.
- (a) E. Jiménez-Nuñez, M. Raducan, T. Lauterbach, K. Molawi, C. R. Solorio and A. M. Echavarren, Angew. Chem., Int. Ed., 2009, 48, 6152–6155 CrossRef PubMed; (b) M. Gaydou, R. E. Miller, N. Delpont, J. Ceccon and A. M. Echavarren, Angew. Chem., Int. Ed., 2013, 52, 6396–6399 CrossRef CAS PubMed.
- (a) C. R. Solorio-Alvarado, Y. Wang and A. M. Echavarren, J. Am. Chem. Soc., 2011, 133, 11952–11955 CrossRef CAS PubMed; (b) Y. Wang, P. R. McGonigal, B. Herlé, M. Besora and A. M. Echavarren, J. Am. Chem. Soc., 2014, 136, 801–809 CrossRef CAS PubMed; (c) Y. Wang, M. E. Muratore, Z. Rong and A. M. Echavarren, Angew. Chem., Int. Ed., 2014, 53 DOI:10.1002/anie.201404029.
- The Buchner reaction to form cycloheptatrienes occurs as a side reaction in the gold(I)-catalyzed reaction between ethyl diazoacetate and arenes: (a) M. R. Fructos, T. R. Belderrain, P. de Frémont, N. M. Scott, S. P. Nolan, M. M. Díaz-Requejo and P. J. Pérez, Angew. Chem., Int. Ed., 2005, 44, 5284–5288 CrossRef CAS PubMed; (b) I. Rivilla, B. P. Gómez-Emeterio, M. R. Fructos, M. M. Díaz-Requejo and P. J. Pérez, Organometallics, 2011, 30, 2855–2860 CrossRef CAS.
- D. Benitez, N. D. Shapiro, E. Tkatchouk, Y. Wang, W. A. Goddard III and F. D. Toste, Nat. Chem., 2009, 1, 482–486 CrossRef CAS PubMed.
- (a) G. Seidel, R. Mynott and A. Fürstner, Angew. Chem., Int. Ed., 2009, 48, 2510–2513 CrossRef CAS PubMed; (b) G. Seidel and A. Fürstner, Angew. Chem., Int. Ed., 2014, 53, 4807–4811 CrossRef CAS PubMed.
- R. E. M. Brooner and R. A. Widenhoefer, Chem. Commun., 2014, 50, 2420–2423 RSC.
- X-Ray crystal structure of 4d: CCDC 1000511.
- (a) N. Marion and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2750–2752 CrossRef CAS PubMed; (b) J. Marco-Contelles and E. Soriano, Chem. – Eur. J., 2007, 13, 1350–1357 CrossRef CAS PubMed; (c) A. Correa, N. Marion, L. Fensterbank, M. Malacria, S. P. Nolan and L. Cavallo, Angew. Chem., Int. Ed., 2008, 47, 718–721 CrossRef CAS PubMed; (d) S. Wang, G. Zhang and L. Zhang, Synlett, 2010, 692–706 CAS; (e) R. K. Shiroodi and V. Gevorgyan, Chem. Soc. Rev., 2013, 42, 4991–5001 RSC.
- For lead references on the stereoselectivity in the formation of vinyl gold(I) carbenes, see: (a) L. Peng, X. Zhang, S. Zhang and J. Wang, J. Org. Chem., 2007, 72, 1192–1197 CrossRef CAS PubMed; (b) Y. Nakanishi, K. Miki and K. Ohe, Tetrahedron, 2007, 63, 12138–12148 CrossRef CAS PubMed.
- K. Miki, M. Fujita, S. Uemura and K. Ohe, Org. Lett., 2006, 8, 1741–1743 CrossRef CAS PubMed.
- The reaction between 2-methoxyfuran and 1-phenylprop-2-yn-1-yl acetate with gold(I)catalyst A leads to methyl (6-acetoxy-7-phenylhepta-2,4,6-trienoate as a mixture of stereoisomers as was originally reported using [RuCl2(CO)3]2 in ref. 16.
- B. W. Gung, L. N. Bailey and J. Wonser, Tetrahedron Lett., 2010, 51, 2251–2253 CrossRef CAS PubMed.
- For gold(I)-catalyzed [4 + 3]-cycloaddition of propargyl esters with furans: (a) W. Gung, D. T. Craft, L. N. Bailey and K. Kirschbaum, Chem. – Eur. J., 2010, 16, 639–644 CrossRef PubMed; (b) B. Gung, R. Conyers and J. Wonser, Synlett, 2013, 1238–1248 CrossRef CAS PubMed.
- M. S. Hadfield and A.-L. Lee, Chem. Commun., 2011, 47, 1333–1335 RSC.
- (a) See ESI‡ for additional results on the screening of other migrating groups and catalysts; (b) Traces of minor isomers (presumably with the E,E configuration) were also detected in the 1H NMR spectra.
- X-Ray crystal structure of 21: CCDC 1000512.
- Another pathway (exo-type) was also found with very similar energies for the first step (ΔG‡ = 2.6 kcal mol−1 (ΔG = −13.7 kcal mol−1) although the activation energy for the second step was significantly higher (ΔG‡ = 6.1 vs. 2.2 kcal mol−1).
Footnotes |
| † We dedicate this work to our colleague and friend Prof. Max Malacria on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: A text file of all computed molecule Cartesian coordinates in .xyz format. CCDC 1000511 and 1000512. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qo00130c |
| § D. L. and M. G. contributed equally to this work. |
| This journal is © the Partner Organisations 2014 |