 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nitrate anion templated assembly of a [2]rotaxane for selective nitrate recognition in aqueous solvent mixtures†
Matthew J. Langton, Lucy C. Duckworth and Paul D. Beer*
Chemistry Research Laboratory, Department of Chemistry, University of Oxford, Mansfield Road, Oxford, OX1 3TA, UK. E-mail: paul.beer@chem.ox.ac.uk; Fax: +44 (0)1865-272-690
First published on 8th August 2013
Abstract
The first nitrate anion templated assembly of an interlocked molecular architecture is demonstrated through the preparation of a [2]rotaxane. Removal of the discrete nitrate anion template from the [2]rotaxane reveals an interlocked host system capable of strong and selective recognition of nitrate, in aqueous–organic solvent mixtures, over a range of more basic mono-charged oxoanions.
The design of anion receptors capable of highly selective recognition is a key challenge for supramolecular chemistry.1 Surprisingly, little attention has been paid to the selective recognition of nitrate, despite the significant importance of this anion in environmental and medical contexts. The over use of nitrate in fertilizers has led to accumulation of the anion in water courses, leading to eutrophication (excessive plant growth) and the subsequent disruption of the aquatic ecosystem.2 Furthermore, increased nitrate levels has been implicated in the formation of carcinogenic nitrosamines and causes methemoglobinemia (blue-baby syndrome) in infants.3 With this in mind, the development of novel approaches toward selective nitrate recognition is of importance. However, the design of nitrate receptors is challenging due to a combination of high hydration energy and low basicity of the anion, which results in a low affinity for hydrogen bonds.4 The trigonal planar geometry of the anion has been exploited in a small number of tripodal, macrocyclic and cage-like host systems, which position the hydrogen bond donors in a complementary trigonal arrangement, and can recognize nitrate with modest selectivity in polar organic solvents.5 However, recognition of nitrate in aqueous media by synthetic organic host molecules remains, to the best of our knowledge, elusive and high levels of nitrate selectivity have yet to be achieved.
The exquisite anion guest selectivity displayed by nature in phosphate and sulfate binding proteins is accomplished through a three-dimensional convergent array of hydrogen bond donors and acceptors, arranged in an optimized geometry for recognition of the complementary oxoanion.6 In our group we have developed the use of discrete anion templates for the formation of interlocked molecular architectures, utilising chloride,7 bromide8 and sulfate9 as the templating anions. The resulting interlocked host molecules can encapsulate anions between the interlocked components, with a high degree of selectivity for the templating anion, through convergent hydrogen bond donors reminiscent of those found in anion binding proteins in nature.
Herein we report the first example of the use of nitrate as a template for the formation of interpenetrated and interlocked molecular architectures.10 Discrete nitrate anion templated pseudorotaxane formation of a suitably designed threading component within a macrocycle is demonstrated initially. Stoppering of the pseudorotaxane assembly led to the construction of a [2]rotaxane host system, which displays excellent selectivity for nitrate in aqueous–polar organic solvent mixtures over a range of other, more basic, mono-charged oxoanions.
Our strategy was to design a complementary threading component that contains two hydrogen bonding recognition sites for forming hydrogen bonds to two of the oxygen atoms of the nitrate anion. The remaining oxygen atom would thus be free to interact with a suitable hydrogen bonding motif that is integrated into a macrocycle component and facilitate the trigonal nitrate anion templated assembly of a rotaxane (Fig. 1).
![Schematic representation of a nitrate templated [2]rotaxane, with two hydrogen bonding recognition sites in the axle (blue and green) and one in the macrocycle (red), forming a complementary binding site for the trigonal nitrate anion between the interlocked components.](/image/article/2013/CC/c3cc45342a/c3cc45342a-f1.gif) | ||
| Fig. 1 Schematic representation of a nitrate templated [2]rotaxane, with two hydrogen bonding recognition sites in the axle (blue and green) and one in the macrocycle (red), forming a complementary binding site for the trigonal nitrate anion between the interlocked components. | ||
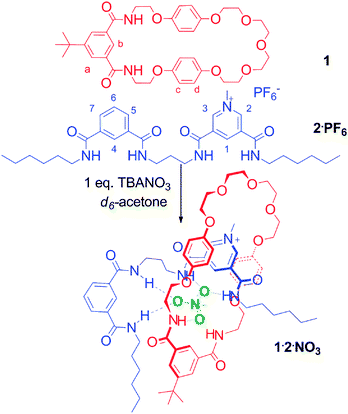
The possibility of using nitrate as a pseudorotaxane templating anion was investigated initially using the asymmetrical bidentate isophthalamide-3,5-bis-amide pyridinium containing thread 2·PF6 (Scheme 1), terminated with non-interacting hexyl chains (see ESI† for synthesis). Macrocycle 1, incorporating an isophthalamide motif to coordinate to the nitrate anion, and hydroquinone groups to provide secondary stabilization through aromatic donor–acceptor interactions with the electron deficient pyridinium moiety of the thread, was prepared according to literature procedures.11
 | ||
| Scheme 1 Assembly of nitrate templated pseudorotaxane 1·2·NO3. | ||
Initial 1H NMR pseudorotaxane assembly studies were undertaken in d6-acetone (Fig. 2). Addition of TBANO3 to a solution of macrocycle 1 led to downfield shifts of the isophthalamide amide protons and internal proton b, indicating coordination of the oxoanion within the amide binding cleft. Upon addition of one equivalent of thread 2·PF6, downfield shifts of the thread protons 1, 4 and amides were observed, which is indicative of nitrate binding, and demonstrates that both the isophthalamide and pyridinium isophthalamide moieties are involved in hydrogen bonding to the nitrate anion. Importantly, the observed upfield perturbation and increased splitting of the macrocycle hydroquinone protons c and d is characteristic of aromatic donor–acceptor interactions between the electron rich hydroquinone groups in the macrocycle and the positively charged electron deficient pyridinium group in the thread, confirming the formation of the pseudorotaxane 1·2·NO3 (Scheme 1).
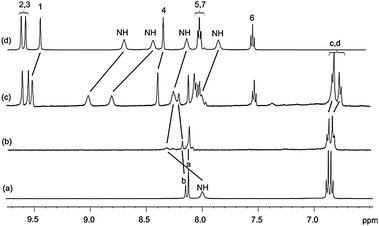 | ||
| Fig. 2 1H NMR spectra of (a) macrocycle 1, (b) macrocycle 1 + 1 equiv. TBANO3, (c) pseudorotaxane 1·2·NO3, (d) thread 2·PF6 in d6-acetone (500 MHz). For atom labels see Scheme 1. | ||
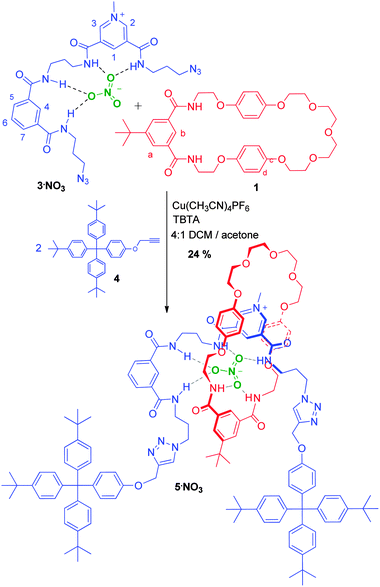
The successful formation of the nitrate templated pseudorotaxane 1·2·NO3 suggested that the synthesis of a [2]rotaxane would be possible using nitrate templation, via a stoppering strategy. To this end bis-azide functionalized axle precursor 3·NO3 was prepared (see ESI†) for a copper(I) catalysed azide–alkyne (CuAAC) click stoppering reaction with a suitable alkyne functionalized stopper. Synthesis of rotaxane 5·NO3 was achieved by mixing 1 equiv. of 3·NO3 with 1.1 equiv. of macrocycle 1 in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH2Cl2–acetone to form the initial pseudorotaxane assembly. Addition of catalytic Cu(CH3CN)4PF6 and 2.2 equiv. of stopper alkyne 4 gave a crude product whose 1H NMR spectrum revealed that the rotaxane was formed in approximately 35% yield (Scheme 2). Purification by size exclusion chromatography and silica gel chromatography gave rotaxane 5·NO3 in an isolated yield of 24%, which was fully characterized by 1H, 13C and ROESY NMR, and high resolution electrospray mass spectrometry (see ESI†). Importantly, an analogous reaction conducted in the absence of nitrate with 3·PF6 gave no evidence of rotaxane formation, which highlights the crucial templating role of the nitrate anion.12
1 CH2Cl2–acetone to form the initial pseudorotaxane assembly. Addition of catalytic Cu(CH3CN)4PF6 and 2.2 equiv. of stopper alkyne 4 gave a crude product whose 1H NMR spectrum revealed that the rotaxane was formed in approximately 35% yield (Scheme 2). Purification by size exclusion chromatography and silica gel chromatography gave rotaxane 5·NO3 in an isolated yield of 24%, which was fully characterized by 1H, 13C and ROESY NMR, and high resolution electrospray mass spectrometry (see ESI†). Importantly, an analogous reaction conducted in the absence of nitrate with 3·PF6 gave no evidence of rotaxane formation, which highlights the crucial templating role of the nitrate anion.12
 | ||
| Scheme 2 Synthesis of rotaxane 5·NO3via nitrate templation. | ||
The 1H spectra of rotaxane 5·NO3, macrocycle 1 and axle precursor 3·NO3 are compared in Fig. 3. It is noteworthy that the macrocycle hydroquinone protons c and d are split and shifted upfield, which are diagnostic of the aromatic donor–acceptor interactions between the hydroquinones in the macrocycle and the axle pyridinium motif, and confirms the interlocked nature of the rotaxane. Further evidence is obtained in the 1H NMR ROESY spectrum, in which multiple through space interactions between the macrocycle and axle are observed (see ESI†). Anion exchange to the non-coordinating hexafluorophosphate salt, in preparation for anion recognition studies, was achieved by washing a solution of the rotaxane 5·NO3 in CH2Cl2 with aqueous NH4PF6. The anion recognition properties of rotaxane 5·PF6 were investigated using 1H NMR titration experiments in a competitive aqueous–polar organic solvent mixture of 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CDCl3–CD3OD–D2O. Upon binding of nitrate the internal pyridinium axle proton 1 is strongly perturbed upfield (Δδ = 0.19 ppm after 10 equiv.) which is diagnostic of the nitrate anion binding within the rotaxane cavity. Addition of other oxoanions led to smaller perturbations of axle proton 1 (Table 1). WinEQNMR213 analysis of the titration data, monitoring proton 1, enabled the determination of 1
10 CDCl3–CD3OD–D2O. Upon binding of nitrate the internal pyridinium axle proton 1 is strongly perturbed upfield (Δδ = 0.19 ppm after 10 equiv.) which is diagnostic of the nitrate anion binding within the rotaxane cavity. Addition of other oxoanions led to smaller perturbations of axle proton 1 (Table 1). WinEQNMR213 analysis of the titration data, monitoring proton 1, enabled the determination of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric anion association constants shown in Table 1.
1 stoichiometric anion association constants shown in Table 1.
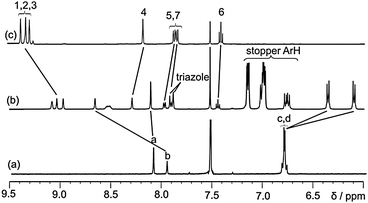 | ||
Fig. 3 1H NMR spectra of (a) macrocycle 1, (b) rotaxane 5·NO3 (c) axle precursor 3·NO3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3–CD3OD (500 MHz). For atom labels see Scheme 2. 1 CDCl3–CD3OD (500 MHz). For atom labels see Scheme 2. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CDCl3–CD3OD–D2O, free energy values and complexation induced chemical shift changes of proton 1
10 CDCl3–CD3OD–D2O, free energy values and complexation induced chemical shift changes of proton 1
| Aniona | NO3− | HCO3− | H2PO4− | AcO− | Cl− | Br− |
|---|---|---|---|---|---|---|
| T = 298 K.a Anions added as TBA salts, except for HCO3− which was added as the TEA salt.b Calculated using chemical shift data of proton 1. Errors estimated to be <10%.c Binding too weak to be quantified.d Chemical shift change of proton 1 after addition of 10 equiv. of anion. | ||||||
| Kab (M−1) | 430 | 100 | 50 | —c | 490 | —c |
| ΔG (kJ mol−1) | −15.0 | −11.4 | −9.7 | —c | −15.3 | —c |
| Δδ(1)d | 0.19 | 0.10 | 0.07 | 0.02 | 0.21 | 0.02 |
The trigonal nitrate anion was found to bind strongly within the rotaxane host's complementary tridentate hydrogen bond donor binding cavity in this competitive aqueous–polar organic solvent mixture. Impressive selectivity for nitrate was observed over a range of other more basic oxoanions: the pseudo-trigonal hydrogen carbonate anion bound considerably more weakly, and acetate and dihydrogenphosphate resulted in very weak binding, which in the case of acetate was too weak to be quantified.
The selectivity for nitrate over acetate is particularly impressive, given that acetate is 105 times more basic,14 and reflects in part the geometric complementarity of the rotaxane binding cavity for nitrate. To the best of our knowledge this is the first example of a synthetic anion receptor capable of this level of nitrate selectivity in aqueous solvent mixtures over other mono-charged oxoanions.
The spherical chloride anion, which is of comparable size to nitrate but lacks the trigonal geometric preference, was found to bind with a similar affinity to nitrate. Addition of the larger Br− anion led to very small perturbations of the internal binding cavity proton 1, and the binding could not be quantified. Analogous titrations with axle precursor 3·PF6 and NO3−, AcO− and Cl−, revealed no binding of the two oxoanions in the same solvent mixture, but significantly stronger binding of Cl− (150 M−1). This serves to further highlight the importance of the interlocked cavity in remarkably enhancing the strength of nitrate binding with respect to the non-interlocked pyridinium axle, and the crucial role the unique three-dimensional binding domain plays in achieving the shape selectivity for the trigonal nitrate anion, by encapsulating the oxoanion guest.
In summary, we have demonstrated the first example of nitrate anion templation for the formation of interlocked molecular architectures, through the preparation of a [2]rotaxane. Removal of the nitrate template affords an interlocked host system which displays unprecedented binding affinity and selectivity for nitrate over other oxoanions of significantly higher basicity, in a competitive aqueous–organic solvent mixture. The exploitation of nitrate as a templating reagent in the synthesis and development of interlocked anion receptors and sensors is continuing in our laboratories.
M.J.L. would like to thank the EPSRC for a DTA studentship.
Notes and references
- J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, RSC, Cambridge, 2006 Search PubMed.
- V. H. Smith and D. W. Schindler, Trends Ecol. Evol., 2009, 24, 201–207 CrossRef.
- R. F. Follett and J. L. Hatfield, Nitrogen in the environment sources, problems, and management, Elsevier, Amsterdam, New York, 2001 Search PubMed.
- J. W. Steed and J. L. Atwood, Supramolecular Chemistry, Wiley, Chichester, UK, 2nd edn, 2009, ch. 4, p. 224 Search PubMed.
- A. P. Bisson, V. M. Lynch, M. K. C. Monahan and E. V. Anslyn, Angew. Chem., Int. Ed., 1997, 36, 2340–2342 CrossRef CAS; K. Choi and A. D. Hamilton, J. Am. Chem. Soc., 2001, 123, 2456–2457 CrossRef; R. Herges, A. Dikmans, U. Jana, F. Köhler, P. G. Jones, I. Dix, T. Fricke and B. König, Eur. J. Org. Chem., 2002, 3004–3014 CrossRef; P. Blondeau, J. Benet-Buchholz and J. de Mendoza, New J. Chem., 2007, 31, 736–740 RSC; A. S. Singh and S. S. Sun, J. Org. Chem., 2012, 77, 1880–1890 CrossRef; J. Romański and P. J. Pitek, J. Org. Chem., 2013, 78, 4341–4347 CrossRef.
- J. W. Pflugrath and F. A. Quiocho, Nature, 1985, 314, 257–260 CrossRef CAS; F. A. Luecke and F. A. Quiocho, Nature, 1990, 347, 402–406 CrossRef.
- J. A. Wisner, P. D. Beer and M. G. B. Drew, Angew. Chem., Int. Ed., 2001, 40, 3606 CrossRef CAS; G. T. Spence and P. D. Beer, Acc. Chem. Res., 2013, 46(2), 571–586 CrossRef.
- K. M. Mullen, J. Mercurio, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2009, 48, 4781–4784 CrossRef CAS.
- B. Huang, S. M. Santos, V. Felix and P. D. Beer, Chem. Commun., 2008, 4610–4612 RSC; Y. Li, K. M. Mullen, T. D. W. Claridge, P. J. Costa, V. Felix and P. D. Beer, Chem. Commun., 2009, 7134–7136 RSC.
- Sessler et al. have reported the use of nitrate, in the form of nitric acid, as a template for macrocyclisation: J. L. Sessler, T. D. Mody and V. Lynch, Inorg. Chem., 1992, 31, 529–531 CrossRef CAS.
- M. R. Sambrook, P. D. Beer, J. A. Wisner, R. L. Paul, A. R. Cowley, F. Szemes and M. G. B. Drew, J. Am. Chem. Soc., 2005, 127, 2292–2302 CrossRef CAS.
- Analogous reactions carried out on a test scale using other anions as possible templates gave rotaxane yields determined by NMR analysis of only 5% with H2PO4− and HCO3− 15% for Cl− and no evidence of rotaxane formation was observed with the acetate anion.
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 311–312 RSC.
- C. E. Housecroft and A. G. Sharpe, Inorganic Chemistry, Pearson, Harlow, UK, 3rd edn, 2008, ch. 7, p. 181 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for synthetic procedures, additional characterisation and titration data. See DOI: 10.1039/c3cc45342a |
| This journal is © The Royal Society of Chemistry 2013 |
