Cationic tetra- and pentacoordinate complexes of nickel based on POCN- and POCOP-type pincer ligands: synthesis, characterization, and ligand exchange studies†
Abstract
Stirring acetonitrile solutions of the charge-neutral pincer complexes (POCN)NiBr (1, POCN = κP,κC,κN-{2-(i-Pr)2PO,6-CH2{c-N(CH2)5}-C6H3) and (POCOP)NiBr (2, POCOP = κP,κC,κP′-2,6-(i-Pr2OP)2C6H3) with AgSbF6 facilitates Br− abstraction to give the corresponding cationic acetonitrile adducts [(POCN)Ni(NCMe)]+, 1a, and [(POCOP)Ni(NCMe)]+, 2a. Treating 1a and 2a with pyridine (py), 2,2′-bipyridine (bipy), phenanthroline (phen), or 4,4′-bipyridine (bipy*) gave the corresponding monocationic adducts [(POCN or POCOP)Ni(ligand)]+ (ligand = py: 1b and 2b; κN,κN′-bipy: 1c and 2c; κN,κN′-phen: 1d and 2d) and the dicationic dinuclear adducts [(POCN or POCOP)2Ni2(μ-bipy*)]2+ (1e and 2e). The new adducts 1a–1d and 2b–2e have been characterized by NMR spectroscopy; complex 1e proved to be insoluble and could not be analyzed by NMR. Single crystal X-ray diffraction studies were used to establish the solid-state structures of 1a–1e, and 2b–2d. UV-vis spectra have also been recorded for the pentacoordinated complexes 1c, 1d, 2c, and 2d. Studying the equilibria that govern the displacement of halides in (pincer)NiX (X = Cl, Br) by the in-coming nucleophiles py, bipy, and phen has allowed us to determine the following order for the relative nucleophilicities of the ligands involved in the halide substitution equilibria: Cl− > Br− > phen > bipy ≫ py > MeCN. Similar Keq measurements showed that cationic species are better stabilized with the POCN platform compared to POCOP. This implies that POCN is a better net donor of electron density compared to POCOP, such that the relative Lewis acidity (electrophilicity) of the cationic fragments should follow the order [(POCOP)Ni]+ > [(POCN)Ni]+. Cyclic voltammetry measurements on the bipy adducts showed reversible, one-electron oxidation events occurring at a lower 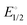 value for [(POCN)Ni(bipy)]+, implying a more electron-rich Ni(II) centre with POCN vs. POCOP. Consistent with these assertions, mixing (POCN)NiBr and [(POCOP)Ni(phen)]+ in acetonitrile gave [(POCN)Ni(phen)]+ and (POCOP)NiBr. Similarly, the Keq value of 0.19 for the equilibrium exchange (POCN)NiCl + (POCOP)NiBr ⇆ (POCN)NiBr + (POCOP)NiCl indicates that the [(POCOP)Ni]+ fragment is better stabilized by Cl−vs. Br−. These exchange reactions do not occur in THF or CH2Cl2, implying that they are driven by the nucleophilic character of the solvent.
value for [(POCN)Ni(bipy)]+, implying a more electron-rich Ni(II) centre with POCN vs. POCOP. Consistent with these assertions, mixing (POCN)NiBr and [(POCOP)Ni(phen)]+ in acetonitrile gave [(POCN)Ni(phen)]+ and (POCOP)NiBr. Similarly, the Keq value of 0.19 for the equilibrium exchange (POCN)NiCl + (POCOP)NiBr ⇆ (POCN)NiBr + (POCOP)NiCl indicates that the [(POCOP)Ni]+ fragment is better stabilized by Cl−vs. Br−. These exchange reactions do not occur in THF or CH2Cl2, implying that they are driven by the nucleophilic character of the solvent.

- This article is part of the themed collections: NJC Editorial Board web collection and Boron & Beyond - in celebration of Todd Marder


 Please wait while we load your content...
Please wait while we load your content...