Carbon nanotube cross-linked phosphorus-doped MXene for capacitive pressure microsensors†
Wenshuai
Yang‡
 a,
Shifan
Zhu‡
a,
Shifan
Zhu‡
 a,
Chenyang
Hao
a,
Tailong
Ji
b,
Yuanyuan
Liu
a,
Chenyang
Hao
a,
Tailong
Ji
b,
Yuanyuan
Liu
 *b and
Yuqiao
Wang
*b and
Yuqiao
Wang
 *abc
*abc
aResearch Center for Nano Photoelectrochemistry and Devices, School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China. E-mail: yqwang@seu.edu.cn; Fax: +862552090621; Tel: +862552090621
bSchool of Pharmaceutical and Chemical Engineering, ChengXian College, Southeast University, Nanjing 210088, China. E-mail: liuyuanyuan1985419@163.com
cYangtze River Delta Carbon Neutrality Strategy Development Institute, Southeast University, Nanjing 210096, China
First published on 2nd July 2024
Abstract
The extreme mechanical flexibility, integration and wearability of one-dimensional carbon-based cross-linked two-dimensional MXenes can exactly meet the rapid development of microelectronic technology and medical microdevices, thereby achieving miniaturization and multi-functionality. Herein, a pressure microsensor was assembled using micro-supercapacitors of phosphorus-doped MXene cross-linked by carbon nanotubes. The carbon nanotube cross-linked phosphorus-doped MXene (P-MXene/CNT) is prepared by chemical vapor deposition and electrostatic self-assembly. The interdigital electrodes are obtained through mask-assisted vacuum filtration to integrate symmetrical micro-supercapacitors using a gel electrolyte. P-doping regulates the electron distribution of the MXene and improves the specific capacity. The P-MXene is cross-linked by carbon nanotubes to alleviate the self-stacking effect for accelerated ion transport. The P-MXene/CNT based planar micro-supercapacitor shows desirable mechanical flexibility and integrability with an extraordinary area capacitance of 162.4 mF cm−2, an energy density of 32.9 μW h cm−2, and a long-term cycling stability up to 91.3% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The pressure microsensor illustrates an outstanding sensitivity toward external pressure and ensures an accurate and continuous detection of human body motions.
000 cycles. The pressure microsensor illustrates an outstanding sensitivity toward external pressure and ensures an accurate and continuous detection of human body motions.
Introduction
The rapid development of medical devices and smart wearable electronic products has stimulated the mass production of flexible, intelligent, and highly integrated micro-electronic systems.1–3 The integrated micro-electronic systems are usually composed of miniaturized energy storage devices and functional devices, where the energy storage devices provide energy to the functional devices. Micro-supercapacitors (MSCs) are favored among various energy storage devices due to their awesome performance with a long-term cycling stability, rapid charge/discharge, high power density, high-frequency response, and attractive reversibility.4–6 At present, micro-supercapacitors are generally used as energy storage devices, and also have attracted continuous attention in the field of sensing.7 In previous reports, human biosignals could be accurately detected by integrating carbon aerogels into strain/pressure sensors.8 Microporous polypyrrole/graphene foam composites were designed as a strain/pressure sensor.9 However, the large size of these devices is not conducive to attaching on human body and detecting tiny bio-signals. Therefore, micro, skin-attachable, and high-performance sensors are required to detect tiny biological signals of arterial pulse, respiration, and body movement.The popular micro-electrodes are pseudocapacitive and carbon materials, including metal oxides/sulfides (RuO2, MnO2 and MoS2),10–12 conductive polymers (polyaniline and polypyrrole),13,14 and carbon-based materials (carbon nanotubes and graphene).15,16 However, the relatively low energy density and rate capability inhibit their practical application in the field of energy storage and sensing. Transition metal carbides and nitrides Ti3C2Tx (MXenes), an emerging two-dimensional structure, have been attracting attention in energy storage and conversion due to their metallic conductivity, excellent mechanical properties, and adjustable functional groups.17–19 However, MXenes suffer from the inferior specific capacity and self-stacking of nanosheets caused by van der Waals force interaction.20 Heteroatom-doping (N, S, and P) is an efficient strategy to modulate the terminal functional groups of carbon-based materials to improve their electrochemical performance.21–23 P-doping can accelerate pseudocapacitive reaction kinetics by adjusting the proton binding energy, increasing the specific capacity of carbonized wood.24 Meanwhile, constructing a composite structure is an effective method to expand the interlayer spacing of MXenes. A variety of carbon-based materials such as carbon nanotubes (CNTs) and graphene are used with MXenes to suppress the interlayer self-stacking effect.25,26 The combination of MXenes and CNTs is an effective way to construct micro-electrodes.
Herein, CNT cross-linked P-doped MXenes (P-MXene/CNT) are prepared by chemical vapor deposition and then electrostatic self-assembly. P-doping provides abundant active adsorption sites for electrochemical reactions and improves the conductivity of MXenes. P-MXene cross-linked on CNTs inhibits the self-stacking effect of the MXene, increases the interlayer spacing of the MXene and promotes ion transport. P-MXene/CNT based MSCs exhibit excellent areal capacitance and cycle stability. After bending at different angles, the negligible attenuation of capacity verifies their good mechanical flexibility. The MSC-derived capacitive pressure micro-sensor is capable of human body motion signal detection with short response/recovery time and excellent reliability.
Results and discussion
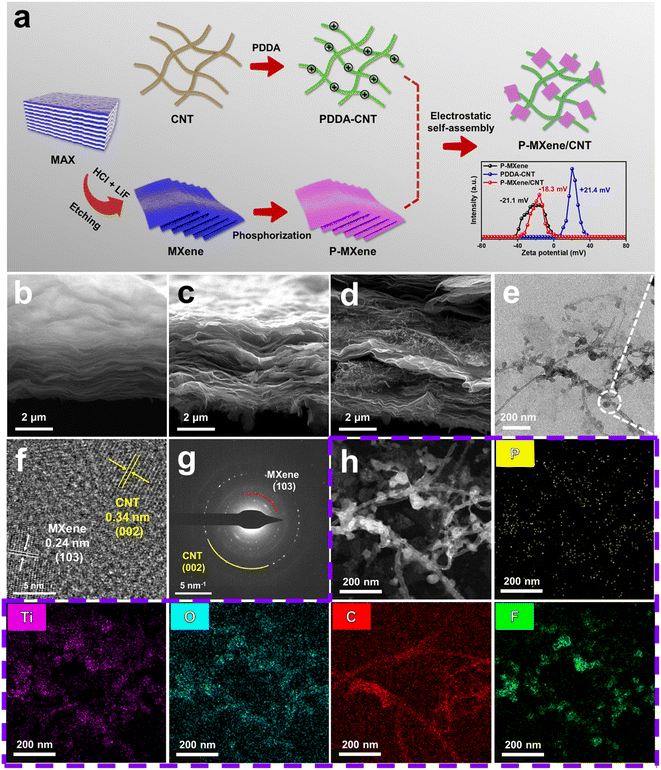
Fig. 1a illustrates the fabrication of the P-MXene/CNT through chemical vapor deposition and electrostatic self-assembly. Firstly, the MXene is obtained through selectively etching the Al layer in MAX (Ti3AlC2) using LiF/HCl. Then, MXene powder and NaH2PO2·H2O are heated at 350 °C for 2 h in a quartz tube under a N2 flow. Phosphine (PH3) can be obtained by thermal decomposition with NaH2PO2·H2O (2NaH2PO2·H2O → Na2HPO4 + 2H2O + PH3↑). The PH3 reacts with MXene powder, resulting in the doping of P atoms into the MXene. Due to the surface functional groups (e.g., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH, and –F), the as-prepared P-MXene is negatively charged with a zeta potential of −21.1 mV. When the positively charged PDDA-CNT (+21.4 mV) is added into the negatively charged P-MXene solution, P-MXene nanosheets are cross-linked on CNTs. The SEM images show that MXene and P-MXene have obvious multi-layer sheet structures (Fig. 1b and c). After electrostatic self-assembly of P-MXene and CNTs, CNTs are evenly wrapped between P-MXene sheets (Fig. 1d). The P-doping causes fluffiness of MXene nanosheets and enlarges interlayer spacing. The entanglement of MXene interlayers by CNTs inhibits the self-stacking effect and provides more active sites for charge transfer and ion diffusion. The TEM image of P-MXene/CNT shows that P-MXene is successfully cross-linked on CNTs by electrostatic self-assembly (Fig. 1e). The detected lattice distances of 0.24 and 0.34 nm can be indexed to the (103) plane of the MXene and (002) plane of CNTs, respectively (Fig. 1f). As shown in the selected area electron diffraction (SAED) patterns, the diffraction rings can be attributed to the cross-linking of the MXene on CNTs (Fig. 1g). The elemental mapping images of P-MXene/CNT and P-MXene show that Ti, O, C, F, and P are uniformly distributed, indicating that P atoms are evenly doped into the MXene (Fig. 1h, S1 and S2†).
O, –OH, and –F), the as-prepared P-MXene is negatively charged with a zeta potential of −21.1 mV. When the positively charged PDDA-CNT (+21.4 mV) is added into the negatively charged P-MXene solution, P-MXene nanosheets are cross-linked on CNTs. The SEM images show that MXene and P-MXene have obvious multi-layer sheet structures (Fig. 1b and c). After electrostatic self-assembly of P-MXene and CNTs, CNTs are evenly wrapped between P-MXene sheets (Fig. 1d). The P-doping causes fluffiness of MXene nanosheets and enlarges interlayer spacing. The entanglement of MXene interlayers by CNTs inhibits the self-stacking effect and provides more active sites for charge transfer and ion diffusion. The TEM image of P-MXene/CNT shows that P-MXene is successfully cross-linked on CNTs by electrostatic self-assembly (Fig. 1e). The detected lattice distances of 0.24 and 0.34 nm can be indexed to the (103) plane of the MXene and (002) plane of CNTs, respectively (Fig. 1f). As shown in the selected area electron diffraction (SAED) patterns, the diffraction rings can be attributed to the cross-linking of the MXene on CNTs (Fig. 1g). The elemental mapping images of P-MXene/CNT and P-MXene show that Ti, O, C, F, and P are uniformly distributed, indicating that P atoms are evenly doped into the MXene (Fig. 1h, S1 and S2†).
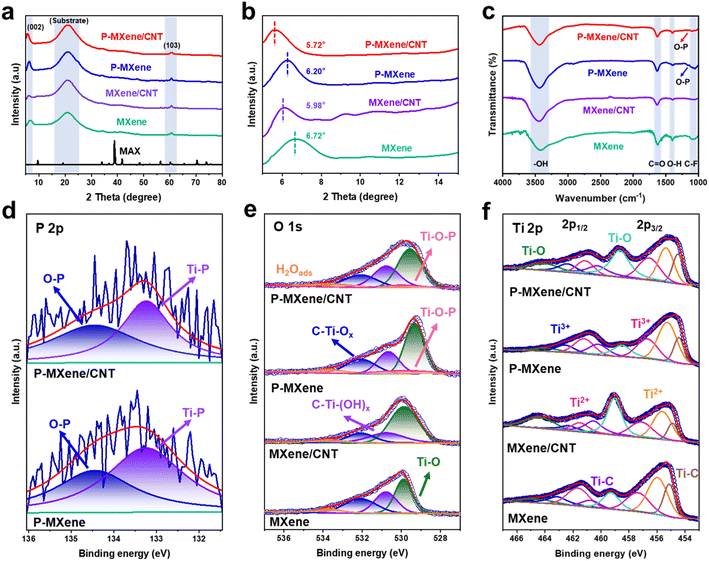
MXene, P-MXene, MXene/CNT, and P-MXene/CNT are characterized by X-ray diffraction (XRD) to analyze their structure and interlayer distance. The diffraction peak distribution of P-MXene, MXene/CNT, and P-MXene/CNT samples is basically consistent with that of MXene, indicating the well-maintained phase structure of the MXene after P-doping and cross-linking (Fig. 2a). After P-doping and combining with CNTs, the (002) peak of the original MXene moves to a low diffraction angle. The original MXene exhibits a sharp (002) diffraction peak at 6.72°, significantly higher than that of P-MXene at 6.20°, MXene/CNT at 5.98°, and P-MXene/CNT at 5.72° (Fig. 2b). According to the Bragg equation nλ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, the interlayer spacings of MXene, P-MXene, MXene/CNT, and P-MXene/CNT are calculated to be 1.31, 1.42, 1.48, and 1.54 nm, respectively. The results indicate that the interlayer spacing of the MXene can be effectively expanded by the cross-linking structure and P-doping. The MXene is cross-linked onto CNTs to construct a multi-dimensional ion transport channel and inhibit the self-stacking effect of the MXene. The introduction of P atoms with a larger radius can also enlarge the interlayer spacing, thus benefiting the ion diffusion kinetics during charge/discharge processes.
θ, the interlayer spacings of MXene, P-MXene, MXene/CNT, and P-MXene/CNT are calculated to be 1.31, 1.42, 1.48, and 1.54 nm, respectively. The results indicate that the interlayer spacing of the MXene can be effectively expanded by the cross-linking structure and P-doping. The MXene is cross-linked onto CNTs to construct a multi-dimensional ion transport channel and inhibit the self-stacking effect of the MXene. The introduction of P atoms with a larger radius can also enlarge the interlayer spacing, thus benefiting the ion diffusion kinetics during charge/discharge processes.
The MXene surfaces are terminated by –OH, –F, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups after etching and exfoliation processes (Fig. 2c). The Fourier transform infrared (FTIR) spectra exhibit a broad band around 3442 cm−1, corresponding to the –OH stretching vibration of the water molecule. The other peaks at 1620 cm−1, 1392 cm−1, and 1088 cm−1 are assigned to the C
O groups after etching and exfoliation processes (Fig. 2c). The Fourier transform infrared (FTIR) spectra exhibit a broad band around 3442 cm−1, corresponding to the –OH stretching vibration of the water molecule. The other peaks at 1620 cm−1, 1392 cm−1, and 1088 cm−1 are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH, and –F groups on the surface of the MXene, respectively. After P doping, a special peak appears at 1164 cm−1, attributed to the O–P in P-MXene/CNT and P-MXene. Further analysis of the surface structure was carried out by X-ray photoelectron spectroscopy (XPS) (Fig. S3†), corresponding to C 1s, F 1s, O 1s, P 2p, and Ti 2p peaks in P-MXene/CNT, P-MXene, MXene/CNT, and MXene. The high-resolution P 2p spectra of the P-MXene and P-MXene/CNT can be fitted into two peaks at 134.5 and 133.3 eV, assigned to O–P and Ti–P bonds, which is consistent with FTIR (Fig. 2d). The O–P and Ti–P bonds are formed by functional substitution and surface absorption, respectively.27 The O–P bond related to redox activity can provide abundant electroactive sites with high pseudocapacitance in the charge storage reaction.28 In the high-resolution O 1s spectra, the peaks at 533.7, 532.1, 530.8, 530.0, and 529.6 eV are attributed to H2Oads, C–Ti–Ox, C–Ti–(OH)x, Ti–O–P, and Ti–O, respectively (Fig. 2e). Compared with MXene and MXene/CNT, the binding energy of the C–Ti–Ox bond shifts to a higher region, 0.30 and 0.46 eV in P-MXene and P-MXene/CNT. The result suggests that P-doping regulates the surface charge distribution of the MXene and increases the valence of O element. In the high-resolution Ti 2p spectra, eight separate peaks at 463.7, 463.1, 461.7, 460.7, 459.3, 457.4, 456.0, and 455.1 eV are attributed to Ti–O 2p1/2, Ti3+ 2p1/2, Ti2+ 2p1/2, Ti–C 2p1/2, Ti–O 2p3/2, Ti3+ 2p3/2, Ti2+ 2p3/2, and Ti–C 2p3/2, respectively (Fig. 2f). The peaks of Ti–O (P-MXene and P-MXene/CNT) exhibit a noticeable shift toward a lower binding energy compared with the peaks of MXene, corresponding to the electron-donating process of P atoms. The increase of electron density at the O site is beneficial to promoting the adsorption of protons at Ti–O.
O, –OH, and –F groups on the surface of the MXene, respectively. After P doping, a special peak appears at 1164 cm−1, attributed to the O–P in P-MXene/CNT and P-MXene. Further analysis of the surface structure was carried out by X-ray photoelectron spectroscopy (XPS) (Fig. S3†), corresponding to C 1s, F 1s, O 1s, P 2p, and Ti 2p peaks in P-MXene/CNT, P-MXene, MXene/CNT, and MXene. The high-resolution P 2p spectra of the P-MXene and P-MXene/CNT can be fitted into two peaks at 134.5 and 133.3 eV, assigned to O–P and Ti–P bonds, which is consistent with FTIR (Fig. 2d). The O–P and Ti–P bonds are formed by functional substitution and surface absorption, respectively.27 The O–P bond related to redox activity can provide abundant electroactive sites with high pseudocapacitance in the charge storage reaction.28 In the high-resolution O 1s spectra, the peaks at 533.7, 532.1, 530.8, 530.0, and 529.6 eV are attributed to H2Oads, C–Ti–Ox, C–Ti–(OH)x, Ti–O–P, and Ti–O, respectively (Fig. 2e). Compared with MXene and MXene/CNT, the binding energy of the C–Ti–Ox bond shifts to a higher region, 0.30 and 0.46 eV in P-MXene and P-MXene/CNT. The result suggests that P-doping regulates the surface charge distribution of the MXene and increases the valence of O element. In the high-resolution Ti 2p spectra, eight separate peaks at 463.7, 463.1, 461.7, 460.7, 459.3, 457.4, 456.0, and 455.1 eV are attributed to Ti–O 2p1/2, Ti3+ 2p1/2, Ti2+ 2p1/2, Ti–C 2p1/2, Ti–O 2p3/2, Ti3+ 2p3/2, Ti2+ 2p3/2, and Ti–C 2p3/2, respectively (Fig. 2f). The peaks of Ti–O (P-MXene and P-MXene/CNT) exhibit a noticeable shift toward a lower binding energy compared with the peaks of MXene, corresponding to the electron-donating process of P atoms. The increase of electron density at the O site is beneficial to promoting the adsorption of protons at Ti–O.
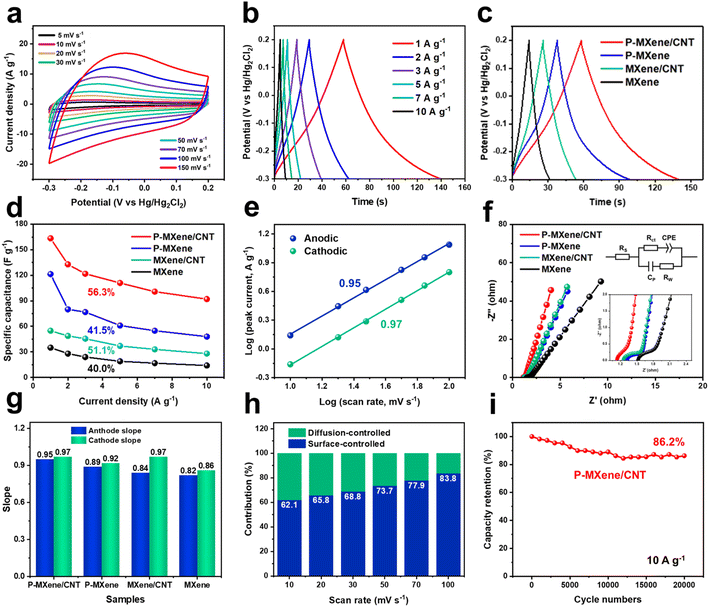
The electrochemical performances of P-MXene/CNT are evaluated using a three-electrode configuration in 1 M H2SO4. Fig. 3a and S4† show the CV curves of different scan rates in the range of −0.3 to 0.2 V. The CV curves of P-MXene/CNT have a pair of broadened redox peaks, indicating that the electrode exhibits a pseudocapacitance energy storage mechanism. The pseudocapacitance mainly originates from reversible intercalation/deintercalation of H+ along with the change of Ti oxidation state. The specific capacities of MXene-based electrodes are calculated from GCD curves at different current densities (Fig. 3b and S5†). Benefiting from P-doping and the cross-linking structure, the specific capacity of P-MXene/CNT reaches 166.1 F g−1, which is much higher than that of P-MXene (121.4 F g−1), MXene/CNT (54.8 F g−1) and MXene (35.0 F g−1) (Fig. 3c). The P-MXene/CNT exhibits an exceptional charge/discharge rate of 56.3% from 1 to 10 A g−1, indicating that P-MXene/CNT shows good rate performance (Fig. 3d). The superior rate capability of P-MXene/CNT can be attributed to the increased interlayer spacing caused by MXene cross-linked on conducting CNTs. Electrochemical impedance spectroscopy (EIS) is conducted to investigate the underlying reasons for the effective reaction behavior of the MXene. Nyquist plots are fitted with the equivalent circuit in Fig. 3e. The plots consisted of a semicircle in the high-frequency region and a straight line in the low-frequency region. In the high-frequency region, the intercept on the real-axis can represent the equivalent series resistance (Rs), and the diameter of the semicircle is correlated with the charge transfer resistance (Rct). It can be observed that in the high frequency region, the Rs of P-MXene/CNT, P-MXene, MXene/CNT and MXene electrodes are 1.13, 1.32, 1.25, and 1.52 Ω and the Rct are 0.19, 0.30, 0.35, and 0.36 Ω, respectively. The decline in Rs and Rct indicates the fast charge transfer and high reactivity. In the low frequency region, the P-MXene/CNT electrode has the largest slope, indicating a smaller Rw than MXene for accelerated ion diffusion kinetics.
The charge storage mechanism of the as-prepared electrodes can be determined from the CV curves at different scan rates. The current (i) versus scan rate (v) relationship is defined by i = avb, where a and b are adjustable values. A b-value of 0.5 indicates a diffusion-controlled process, while a b-value of 1 means a complete surface-controlled process. The anodic/cathodic b values of P-MXene/CNT, P-MXene, MXene/CNT, and MXene are calculated to be 0.95/0.97, 0.89/0.92, 0.84/0.97, and 0.82/0.86, respectively, indicating a dominant surface-controlled process of MXene-based electrodes (Fig. 3f, g and S6†). The increase of the b value proves that P-doping and cross-linking MXene on CNTs could improve the pseudocapacitance and reduce the ion transmission impedance. This is attributed to the fact that P-doping promotes the adsorption of protons. The anchoring structure exposes more active sites and accelerates the transport of ions between layers. The contribution to total capacity can be further quantified according to i = k1v + k2v1/2. k1v and k2v1/2 refer to the contribution of surface-controlled and diffusion-controlled processes and i is the response current. k1 and k2 can be estimated from the relationship between i/v1/2 and v1/2. According to the CV curve at 50 mV s−1, the surface-controlled capacitance contributions of P-MXene/CNT, P-MXene, MXene/CNT, and MXene are 73.7%, 61.9%, 61.1%, and 56.1%, respectively (Fig. S7†). The enhancement of surface-controlled capacitance also indicates the increase of pseudocapacitance, which is consistent with the anodic/cathodic b values. As shown in Fig. 3h and S8,† the capacitance contributions of as-prepared electrodes at 10–100 mV s−1 are investigated. At the high scan rate of 100 mV s−1, P-MXene/CNT makes a surface-controlled capacitance contribution of 83.9%, indicating its excellent rate performance. P-MXene/CNT exhibits a capacitance retention of 86.2% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles, showing that the P-MXene/CNT has good cycle stability (Fig. 3i).
000 charge/discharge cycles, showing that the P-MXene/CNT has good cycle stability (Fig. 3i).
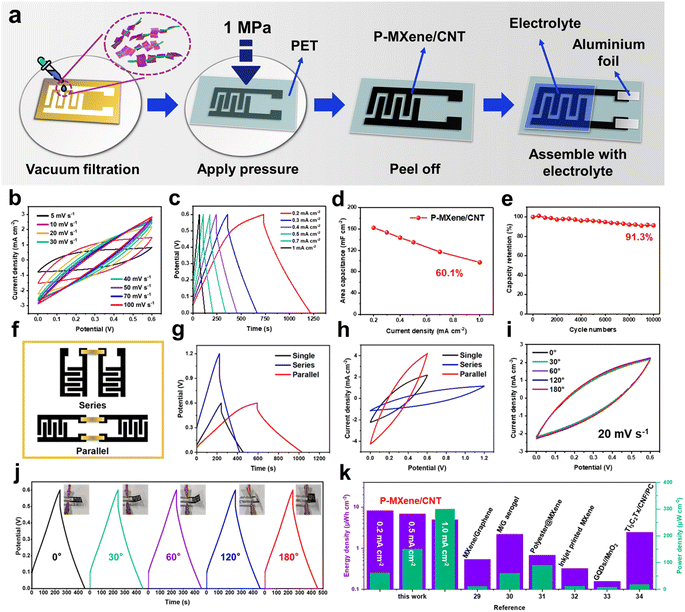
Symmetrical MSCs and capacitive pressure microsensors were prepared via mask-assisted vacuum filtration (Fig. 4a). The mask-assisted vacuum filtration strategy is commonly used to in situ fabricate microelectrodes within the interdigital pattern of the mask for MSCs.29 The size of the interdigital mask for preparing the microelectrodes is shown in Fig. S9,† its finger length and the interspace are 1 mm. Firstly, the water-based microporous membrane is covered on the vacuum filter bottle and the interdigital mask is placed on the microporous membrane. Then, 3 mL P-MXene/CNT ink (1 mg mL−1) is added dropwise to the interdigital mask under vacuum. The solid product could be directly separated from the liquid suspension by negative pressure. The interdigital electrode is obtained by the microporous membrane with a pore size of 0.22 μm. The interdigital electrode is stripped off to the polyethylene terephthalate (PET) film substrate by applying a pressure of 1 MPa. Finally, 0.5 mL H2SO4/PVA gel electrolyte is coated on the center of the interdigital electrode. The aluminum foil is connected to the pin using conductive silver paste. CV curves of PMC-MSCs show a rectangular CV shape in the range of 5 to 100 mV s−1, which may be caused by the electric double layer capacitor characteristics of the MXene electrode (Fig. 4b). The good symmetry of the GCD curves without apparent voltage drop manifests the superior reversibility in charge storage. Fig. 4c and d display the rate capability of PMC-MSCs at different current densities. When the current density is increased from 0.2 to 1 mA cm−2, the areal capacitance is decreased from 162.4 to 97.6 mF cm−2, retaining 60.1% of the capacitance, displaying high area capacitance and good rate capability of PMC-MSCs. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles, the device shows outstanding cycling stability with 91.3% capacitance retention at 1 mA cm−2 (Fig. 4e). The device is connected in parallel and in series to evaluate the feasibility of monolithic integration and the uniformity of a single device (Fig. 4f–h). Two MSCs connected in series display a multiplying voltage window, while two devices connected in parallel display twice the capacity of a single MSC. The result demonstrates that the higher operating voltage and current can be achieved through the series or parallel connection of multiple MSCs. In addition, the shape of the CV and GCD curves at the bending angles of 30°, 60°, 90°, 120°, and 180° are almost coincident, indicating the excellent flexibility of PMC-MSCs (Fig. 4i and j). The maximum energy density of PMC-MSCs reaches 32.9 μW h cm−2 at a power density of 60.02 μW cm−2, and remains at 4.88 μW h cm−2 at a high power density of 300 μW cm−2. The ultrahigh energy storage capability is superior to those of other two-dimensional planar MSCs (Fig. 4k and Table S4†).30–35
000 charge/discharge cycles, the device shows outstanding cycling stability with 91.3% capacitance retention at 1 mA cm−2 (Fig. 4e). The device is connected in parallel and in series to evaluate the feasibility of monolithic integration and the uniformity of a single device (Fig. 4f–h). Two MSCs connected in series display a multiplying voltage window, while two devices connected in parallel display twice the capacity of a single MSC. The result demonstrates that the higher operating voltage and current can be achieved through the series or parallel connection of multiple MSCs. In addition, the shape of the CV and GCD curves at the bending angles of 30°, 60°, 90°, 120°, and 180° are almost coincident, indicating the excellent flexibility of PMC-MSCs (Fig. 4i and j). The maximum energy density of PMC-MSCs reaches 32.9 μW h cm−2 at a power density of 60.02 μW cm−2, and remains at 4.88 μW h cm−2 at a high power density of 300 μW cm−2. The ultrahigh energy storage capability is superior to those of other two-dimensional planar MSCs (Fig. 4k and Table S4†).30–35
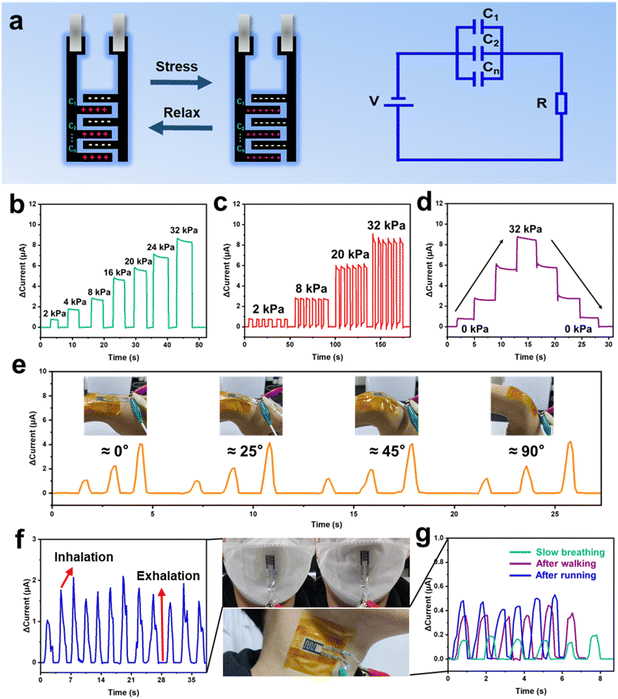
The pressure microsensor is based on the charged MSC structure, ultimately realizing dual sensing and energy storage functions in one device. After connecting PMC-MSCs to a 0.01 mV power supply, it can be utilized as a pressure sensor to detect various human motion signals. Fig. 5a shows the pressure sensing mechanism of the P-MXene/CNT capacitive microsensor, which can be attributed to the synergistic effect of capacitance and resistance variation. When the sensor is fully charged, the charges are evenly distributed at the interdigital electrode, and the electrolyte ions distributed at the electrode interface. A micro-supercapacitor unit C1 can be formed by adjacent interdigital electrodes and PVA/H2SO4 gel electrolyte. The microstructure of the P-MXene/CNT electrode is equivalent to the parallel connection of the capacitor C1, C2,… and Cn. During the pressure application process, the gel dielectric layer fills the grooves and gaps between the interdigital electrode. The interdigital electrode is in sufficient contact with the electrolyte. Under the action of the enhanced electric field inside the P-MXene/CNT electrode, more electrolyte ions are adsorbed and desorbed at the electrode/electrolyte interface. The increased capacitance produces a current response to the external pressure. Meanwhile, the layered P-MXene/CNT sheets are tightly adhered to each other under the pressure. This shortens the interlayer electron transport path and reduces the electron transport impedance, also resulting in the emergence of induced current.
Fig. 5b shows the current response effect at different pressures of 0–32 kPa. As the pressure increases, the induced current continues to increase, showing good sensitivity. The device shows a continuous and stable response current generation at pressures of 2, 8, 20, and 32 kPa, further demonstrating the excellent sensitivity and stability of the P-MXene/CNT microsensor (Fig. 5c). Fig. 5d shows the current variation when sequentially applying and unloading pressure, displaying a unique step feature, which means that the sensing process has good controllability. The pressure sensor is used for human hand wrist motion detection and signal recording to demonstrate a wearable electronic-skin sensing. The microsensor is attached on the wrist to identify different bending amplitudes (Fig. 5e). As the wrist presents different bending amplitudes, the responding current change is recorded, illustrating discernible responding current change. The 90° bending state of the wrist illustrates the strongest response due to maximum deformation of the microsensor. As shown in Fig. 5f, the microsensor can be attached to medical masks to detect human respiration. When the human body inhales, the microsensor is deformed by the mask depression, and the pressure caused by the deformation responds to the inspiratory action. The microsensor also realizes the detection of extremely small carotid artery vibration signals (Fig. 5g). With the increase of pulse vibration intensity and speed, the intensity of the response currents becomes higher and the frequency becomes faster. These results suggest that the microsensor can continuously detect human motion signals with high sensitivity.
Conclusions
In summary, we demonstrate a CNT cross-linked P-doped MXene as a high-performance electrode for pressure microsensors. The P dopant can optimize the electronic structure of the MXene to improve energy storage performance. The MXene with expanded interlayer spacing can be cross-linked by the CNT skeleton to accelerate ion transport between the layers. The flexible capacitive micro-pressure sensor demonstrates excellent sensitivity, wide pressure sensing range and fast response/recovery ability to monitor human micro-motion signals.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Joint Funds of NUAA-SEU (6907046031) and Jiangsu Province Postgraduate Scientific Research Innovation Program (KYCX23_0238). We thank the Big Data Center of Southeast University for providing the facility support for the numerical calculations in this paper.References
- J. Orangi, F. Hamade, V. A. Davis and M. Beidaghi, ACS Nano, 2020, 14, 640–650 CrossRef CAS PubMed
.
- S. Zheng, H. Wang, P. Das, Y. Zhang, Y. Cao, J. Ma, S. Liu and Z. Wu, Adv. Mater., 2021, 33, 2005449 CrossRef CAS
.
- A. Jrondi, G. Buvat, F. D. L. Pena, M. Marinova, M. Huvé, T. Brousse, P. Roussel and C. Lethien, Adv. Energy Mater., 2023, 13, 2203462 CrossRef CAS
.
- S. Zhu, Z. Xu, F. Chen, H. Tao, X. Tang and Y. Wang, J. Phys. Chem. C, 2023, 127, 21363–21373 CrossRef CAS
.
- Y. Zhu, S. Wang, Z. Wu, J. Ma, P. Das and S. Zheng, Energy Storage Mater., 2022, 51, 500–526 CrossRef
.
- S. Zheng, H. Wang, P. Das, Y. Zhang, Y. Cao, J. Ma, S. Liu and Z. Wu, Adv. Mater., 2021, 33, 2005449 CrossRef CAS
.
- Y. Zhang, L. Wang, L. Zhao, K. Wang, Y. Zheng, Z. Yuan, D. Wang, X. Fu, G. Shen and W. Han, Adv. Mater., 2021, 33, 2007890 CrossRef CAS
.
- Z. Chen, H. Zhuo, Y. Hu, H. Lai, L. Liu, L. Zhong and X. Peng, Adv. Funct. Mater., 2020, 30, 1910292 CrossRef CAS
.
- S. A. Hira, M. Yusuf, D. Annas, S. Nagappan, S. Song, S. Park and K. Park, Ind. Eng. Chem. Res., 2021, 60, 13425–13437 CrossRef CAS
.
- H. Li, X. Li, J. Liang and Y. Chen, Adv. Energy Mater., 2019, 9, 1803987 CrossRef
.
- Y. Wang, Y. Zhang, Y. Gao, G. Sheng and J. E. T. Elshofa, Nano Energy, 2020, 68, 104306 CrossRef CAS
.
- S. Kumar, A. Mukherjee, S. Telpande, A. D. Mahapatra, P. Kumarb and A. Misra, J. Mater. Chem. A, 2023, 11, 4963–4976 RSC
.
- H. Qiu, X. Qu, Y. Zhang, S. Chen and Y. Shen, Adv. Mater., 2023, 35, 230232 Search PubMed
.
- W. Tian, Y. Li, J. Zhou, T. Wang, R. Zhang, J. Cao, M. Luo, N. Li, N. Zhang, H. Gong, J. Zhang, L. Xie and B. Kong, ACS Appl. Mater. Interfaces, 2021, 13, 8285–8293 CrossRef CAS
.
- Y. Ren, F. Meng, S. Zhang, B. Ping, H. Li, B. Yin and T. Ma, Carbon Energy, 2022, 4, 446–457 CrossRef CAS
.
- T. Liu, R. Yan, H. Huang, L. Pan, X. Cao, A. DeMello and M. Niederberger, Adv. Funct. Mater., 2020, 30, 2004410 CrossRef CAS
.
- J. Chen, M. Chen, W. Zhou, X. Xu, B. Liu, W. Zhang and C. Wong, ACS Nano, 2022, 16, 2461–2470 CrossRef CAS
.
- X. Du, X. Zhang, S. Zhu, Y. Xu and Y. Wang, Sustainable Energy Fuels, 2023, 7, 4977–4983 RSC
.
- Z. He, L. Yao, W. Guo, N. Sun, F. Wang, Y. Wang, R. Wang and F. Wang, Adv. Funct. Mater., 2023, 33, 2305251 CrossRef CAS
.
- H. Zhang, Z. Wei, J. Wu, F. Cheng, Y. Ma, W. Liu, Y. Cheng, Y. Lin, N. Liu, Y. Gao and Y. Yue, Energy Storage Mater., 2022, 50, 444–453 CrossRef
.
- C. Lu, L. Yang, B. Yan, L. Sun, P. Zhang, W. Zhang and Z. Sun, Adv. Funct. Mater., 2020, 30, 2000852 CrossRef CAS
.
- P. Sun, J. Liu, Q. Liu, J. Yu, R. Chen, J. Zhu, G. Sun, Y. Li, P. Liu and J. Wang, Chem. Eng. J., 2022, 450, 138372 CrossRef CAS
.
- Y. Rao, M. Yuan, F. Luo, Z. Wang, H. Li, J. Yu and X. Chen, Carbon, 2021, 180, 56–66 CrossRef CAS
.
- F. Wang, J. Y. Cheong, Q. He, G. Duan, S. He, L. Zhang, Y. Zhao, I. Kim and S. Jiang, Chem. Eng. J., 2021, 414, 128767 CrossRef CAS
.
- J. Yan, C. E. Ren, K. Maleski, C. B. Hatter, B. Anasori, P. Urbankowski, A. Sarycheva and Y. Gogotsi, Adv. Funct. Mater., 2017, 27, 1701264 CrossRef
.
- H. Dong, J. Sun, X. Liu, X. Jiang and S. Lu, ACS Appl. Mater. Interfaces, 2022, 14, 15504–15516 CrossRef CAS PubMed
.
- C. Lu, L. Yang, B. Yan, L. Sun, P. Zhang, W. Zhang and Z. Sun, Adv. Funct. Mater., 2020, 30, 2000852 CrossRef CAS
.
- Z. Pan, L. Kang, T. Li, M. Waqar, J. Yang, Q. Gu, X. Liu, Z. Kou, Z. Wang, L. Zheng and J. Wang, ACS Nano, 2021, 15, 12975–12987 CrossRef CAS PubMed
.
- S. Zhu, Z. Xu, H. Tao, D. Yang, X. Tang and Y. Wang, Energy Adv., 2023, 2, 765–783 RSC
.
- D. Wen, G. Yinga, L. Liu, Y. Li, C. Sun, C. Hua, Y. Zhao, Z. Ji, J. Zhang and X. Wang, J. Alloys Compd., 2022, 900, 163436 CrossRef CAS
.
- Y. Yue, N. Liu, Y. Ma, S. Wang, W. Liu, C. Luo, H. Zhang, F. Cheng, J. Rao, X. Hu, J. Su and Y. Gao, ACS Nano, 2018, 12, 4224–4232 CrossRef CAS
.
- W. Shao, M. Tebyetekerwa, I. Marriam, W. Li, Y. Wu, S. Peng, S. Ramakrishna, S. Yang and M. Zhua, J. Power Sources, 2018, 396, 683–690 CrossRef CAS
.
- S. Uzun, M. Schelling, K. Hantanasirisakul, T. S. Mathis, R. Askeland, G. Dion and Y. Gogotsi, Small, 2021, 17, 2006376 CrossRef CAS
.
- W. Liu, Y. Feng, X. Yan, J. Chen and Q. Xue, Adv. Funct. Mater., 2013, 23, 4111–4122 CrossRef CAS
.
- W. Chen, D. Zhang, K. Yang, M. Luo, P. Yang and X. Zhou, Chem. Eng. J., 2021, 413, 127524 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta04029e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |