Fabrication of conducting polymer/noble metal composite nanorings and their enhanced catalytic properties†
Liu
Yang
,
Zhen
Zhang
,
Guangdi
Nie
,
Ce
Wang
and
Xiaofeng
Lu
*
Alan G. MacDiarmid Institute, Jilin University, Changchun, 130012, P. R. China. E-mail: xflu@jlu.edu.cn; Tel: +86-431-85168292
First published on 4th November 2014
Abstract
We have described a soft-template method that allows the one-pot fabrication of ring-like conducting polymer/noble metal nanocomposites. An insoluble complex of (CTA)2PdBr4 was used as a template and the composite nanorings were synthesized via the redox reaction between PdBr42− and the monomer.
Conducting polymers with nanostructures are attractive as advanced materials due to their improved performance and versatile applications.1–5 Composites of noble metals and conducting polymers exhibit many excellent properties because of the combination of different functional components.6,7 It has been reported that conducting polymers are usually employed as a supporting matrix to incorporate noble metal nanoparticles for catalytic applications.8–10 Noble metal nanoparticles supported on the conducting polymers have shown enhanced catalytic performance due to the effective surface areas and the synergistic coupling effect between the two components.11,12 For instance, Pt/poly(3,4-ethylenedioxythiophene)–poly(styrene sulfonic acid) (PEDOT–PSS) nanocomposites have exhibited an enhanced electrocatalytic activity toward the oxidation of methanol in comparison to the bulk Pt electrode, which could be attributed to the synergistic effect between Pt particles and the PEDOT–PSS matrix.12 Nanostructured conducting polymers have been fabricated by many approaches, including template-assisted,1,2 electrochemical,3 interfacial,13,14 seeding polymerization,15,16etc. The bulk approaches rely on the use of different forms of hard and soft templates. Templates can provide well-defined place for conducting polymer chains to grow into ordered nanostructures. Compared to the hard-template route, no extra processes are needed to prepare the sacrificial templates and the template-removal steps are not needed in the soft-template route. On the other hand, soft templates also provide well-defined dynamic channels to obtain nanostructured conducting polymers.17,18 Various fancy morphologies of conducting polymers fabricated through the soft-template route have been reported, such as nanoclips,19 wire/ribbon-like nanostructures,20 nanowire networks,21 nanocapsules and nanotubes,22etc. On the other hand, noble metal/conducting polymer composites could be prepared through a redox polymerization by simply mixing the metal salt and monomers of conducting polymers directly, because the standard reduction potential of some metal salts including HAuCl4, H2PtCl6, Na2PdCl4 and AgNO3 is much higher than the oxidation potential of certain conducting polymer monomers, such as aniline, pyrrole and thiophene. Various kinds of noble metal/conducting polymer composites have been fabricated through such a strategy, such as Ag/polypyrrole (PPy) nanocables,23 Au/polyaniline (PANI) nanofibers,7 Pd/PPy nanocapsules,24etc. However, there are no reports on the fabrication of ring-like conducting polymer nanostructures or conducting polymer/noble metal nanocomposites.
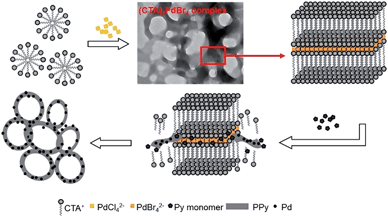
In this communication, we have described a facile soft-template approach that allows the one-pot fabrication of palladium (Pd) nanoparticles supported on conducting polymers such as PPy and PEDOT, having an unusual ring-like morphology. An insoluble complex of (CTA)2PdBr4 formed between a noble metal compound sodium tetrachloropalladate(II) (Na2PdCl4) and a cationic surfactant cetyltrimethylammonium bromide ((C16H33)N(CH3)3Br) (CTAB) with lamellar mesostructures was used as a template and the composite nanorings were synthesized via the redox reaction between PdBr42− and the monomer. Furthermore, this kind of template composed of noble metal compounds and cationic surfactants has potential for the universal fabrication of noble metal nanoparticles supported with conducting polymer nanorings.
In a typical synthesis of PPy/Pd nanorings, 0.15 mmol CTAB was dissolved in 15 mL of distilled water to form a homogeneous solution. Under stirring, 0.119 mmol of Na2PdCl4 in 1 mL of distilled water was added into the above surfactant aqueous solution slowly, resulting in an orange precipitate of the (CTA)2PdBr4 complex that functions as an oxidative template. The mixture was stirred for 5–10 min, and then heated to 90 °C. After another 5 min, 15 μL of pyrrole monomer was added to the above mixture and the mixture gradually turned black in color. The reaction mixture was stirred for a further 12 h at 90 °C and a black precipitate could be formed.
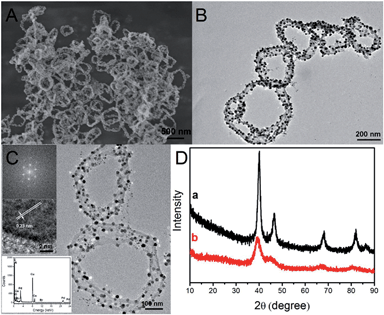
The scanning electron microscopy (SEM) image of the PPy/Pd composites obtained shows a homogeneous nanoring morphology with diameters in the 200–600 nm range (Fig. 1A), which corresponds to the morphology observed in the transmission electron microscopy (TEM) image (Fig. 1B). It is clear that the as-prepared Pd nanoparticles are well distributed on the surface of the conducting polymer. A typical high resolution TEM (HRTEM) image shows that the well distributed nanoparticles with diameters in the range of 4–20 nm (Fig. 1C) and the fringe patterns with a lattice spacing of 0.23 nm correspond well to the {111} planes of pure Pd.25 The energy dispersive X-ray (EDX) spectrum (the inset in Fig. 1C) confirms the presence of elemental C, N and Pd; the signals of Cu and O originated respectively from the carbon-coated copper grid and the adsorption of oxygen. By comparison, the TEM image of PPy/Pd composites synthesized in the absence of CTAB shows the granular morphology as shown in Fig. S1A.† XRD patterns of PPy/Pd composites are shown in Fig. 1D. It is clear that peaks of Pd in PPy/Pd nanorings are in agreement with those in granular PPy/Pd and the diffraction peaks could be attributed to the face-centered cubic structure of crystalline Pd. According to Scherrer's equation, the average sizes of the Pd nanoparticles in PPy/Pd nanorings were calculated to about 7.0 nm. Inductively coupled plasma (ICP) atomic emission spectrometric results showed that the overall weight percentage of Pd in the PPy/Pd composite nanorings was as high as 37.9%. Besides, more detailed information about the molecular structures and surface electronic states of the resulting products has been acquired from Fourier-transform infrared (FT-IR) spectroscopy, Raman spectroscopy and X-ray photoelectron spectroscopy (XPS) (Fig. S1B, S2 and S3†).
During the synthetic process for the fabrication of PPy/Pd composite nanorings, the transparent CTAB solution formed an orange precipitate immediately after Na2PdCl4 was added to it as shown in the inset photograph in Fig. 2. This phenomenon was attributed to the formation of the (CTA)2PdBr4 complex by electrostatic interactions. The stability constant of PdBr42− is about 104 times higher than that of PdCl42−, upon mixing CTAB with Na2PdCl4, the Br− dissociated from CTAB can replace Cl− and bind to Pd2+.The precursor present in solution was characterized by UV-visible spectroscopy to be PdBr42− resulting from the ligand exchange of PdCl42− by bromide from the CTAB (Fig. S4†).26
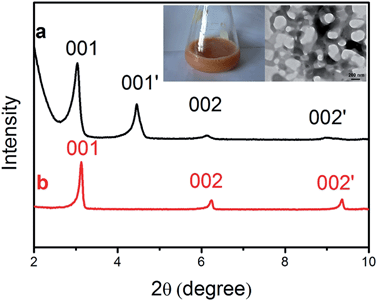 | ||
Fig. 2 Low-angle XRD patterns of (CTA)2PdBr4 precipitated from (a) aqueous solution and (b) a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of water 3 mixture of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol. Insets: a photograph and TEM image of the (CTA)2PdBr4 complex. ethanol. Insets: a photograph and TEM image of the (CTA)2PdBr4 complex. | ||
To elucidate the mechanism for the ring-like morphology of the nanocomposites, further experiments were designed in order to study the structure and morphology of the (CTA)2PdBr4 complex. The TEM image (the inset in Fig. 2) of the complex showed micelles with pore structures and the pores have a similar size to the as-prepared composite nanorings. Low-angle X-ray powder diffraction (XRD) patterns of (CTA)2PdBr4 indicate the typical lamellar mesostructures.27 The precipitate structures from an aqueous solution and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of water
3 mixture of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol have shown two types of lamellar mesostructures, which results in the expansion of the layer spacing of (CTA)2PdBr4 by water molecules.20
ethanol have shown two types of lamellar mesostructures, which results in the expansion of the layer spacing of (CTA)2PdBr4 by water molecules.20
The orange precipitate became black immediately when the pyrrole monomer was dropped into the mixed solution, thereby showing the reaction between (CTA)2PdBr4 and the pyrrole monomer. The probable mechanism of composite nanorings is proposed as shown in Scheme 1. (CTA)2PdBr4 with ordered lamellar structures was formed after the addition of Na2PdCl4 into CTAB. The synthesized (CTA)2PdBr4 with ordered lamellar structures tends to reassemble into ring-like structures to reduce the contact between water and the hydrophobic alkyl chains. After the pyrrole monomer was added into the system, it could enter inside the lamellar (CTA)2PdBr4 and be in situ polymerized by Pd(II) to form PPy. Simultaneously, PdBr42− ions were in situ reduced to Pd(0) nanoparticles and distributed in the PPy matrix uniformly. As the reaction progressed, the (CTA)2PdBr4 would degrade gradually leaving behind the PPy/Pd composites. After the completion of polymerization, PPy/Pd composite nanorings were fabricated.
The current(I)–voltage(V) characteristics of PPy/Pd composite nanorings are non-linear but are symmetric with respect to the polarity of the applied voltage range of +10 to −10 V due to tunnelling and hopping contributions to transport across the barriers (Fig. S5†).28,29 In addition, we evaluated the catalytic activity of the PPy/Pd composite nanorings by catalyzing the reduction of p-nitrophenol into p-aminophenol by NaBH4 in aqueous solution and the kinetic apparent rate constant was calculated to be about 8.5 × 10−3 s−1 (Fig. S6†). For comparison, the catalytic activities of commercial Pd/C, PPy/Pd composites synthesized in the absence of CTAB and commercial Pd black were also tested, and the kinetic apparent rate constants were 2.8 × 10−3 s−1, 2.4 × 10−3 s−1 and 8.4 × 10−4 s−1, respectively (Fig. S7†). It indicated that the catalytic activity of PPy/Pd nanorings for the reduction of p-nitrophenol into p-aminophenol by NaBH4 in aqueous solution was obviously high. Their better catalytic properties might be attributed to the ring-like composite structure formed by the conducting PPy and well dispersed Pd nanoparticles. The PPy not only helps to immobilize Pd nanoparticles into the ring-like nanostructures but also enhances the electron transfer between Pd nanoparticles and conducting PPy due to the special structure. At the same time, the stable ring-like structure could also help to improve the stability of Pd nanoparticles during the catalytic process. Furthermore, this method we have described can be used to synthesize other kinds of composite nanorings. For example, PEDOT/Pd composite nanorings were synthesized using a very similar procedure and characterizations of PEDOT/Pd composite nanorings were also discussed in detail (Fig. S8 and S9†).
Conclusions
In summary, we have described a one-pot fabrication of conducting polymer/noble metal composite nanorings through a facile soft-template method. The lamellar-structured templates play a key role in the formation of ring-like morphology. This is the first time that (CTA)2PdBr4 is used as a template to synthesize conducting polymer composites and conducting polymer/noble metal composite nanorings are successfully fabricated.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51273075 and 21274052) and the National Key Technology Research and Development Program (2013BAC01B02).Notes and references
- C. R. Martin, Acc. Chem. Res., 1995, 28, 61 CrossRef CAS.
- M. X. Wan, Adv. Mater., 2008, 20, 2926 CrossRef CAS.
- D. Li, J. X. Huang and R. B. Kaner, Acc. Chem. Res., 2009, 42, 135 CrossRef CAS PubMed.
- Y. Z. Long, M. M. Li, C. Z. Gu, M. X. Wan, J. L. Duvail, Z. W. Liu and Z. Y. Fan, Prog. Polym. Sci., 2011, 36, 1415 CrossRef CAS PubMed.
- C. Li, H. Bai and G. Q. Shi, Chem. Soc. Rev., 2009, 38, 2397 RSC.
- X. F. Lu, W. J. Zhang, C. Wang, T. C. Wen and Y. Wei, Prog. Polym. Sci., 2011, 36, 671 CrossRef CAS PubMed.
- R. J. Tseng, J. Huang, J. Ouyang, R. B. Kaner and Y. Yang, Nano Lett., 2005, 5, 1077 CrossRef CAS PubMed.
- B. J. Gallon, R. W. Kojima, R. B. Kaner and P. L. Diaconescu, Angew. Chem., Int. Ed., 2007, 46, 7251 CrossRef CAS PubMed.
- X. F. Lu, X. J. Bian, G. D. Nie, C. C. Zhang, C. Wang and Y. Wei, J. Mater. Chem., 2012, 22, 12723 RSC.
- X. J. Bian, X. F. Lu, E. Jin, L. R. Kong, W. J. Zhang and C. Wang, Talanta, 2010, 81, 813 CrossRef CAS PubMed.
- Z. W. Chen, L. B. Xu, W. Z. Li, M. Waje and Y. S. Yan, Nanotechnology, 2009, 17, 5254 CrossRef.
- C. W. Kuo, L. M. Huang, T. C. Wen and A. Gopalan, J. Power Sources, 2006, 160, 65 CrossRef CAS PubMed.
- J. X. Huang, S. Virji, B. H. Weiller and R. B. Kaner, J. Am. Chem. Soc., 2003, 125, 314 CrossRef CAS PubMed.
- J. X. Huang and R. B. Kaner, J. Am. Chem. Soc., 2004, 126, 851 CrossRef CAS PubMed.
- X. Zhang, W. J. Goux and S. K. Manohar, J. Am. Chem. Soc., 2004, 126, 4502 CrossRef CAS PubMed.
- X. Zhang and S. K. Manohar, J. Am. Chem. Soc., 2004, 126, 12714 CrossRef CAS PubMed.
- J. Jang and J. H. Oh, Chem. Commun., 2002, 2200 RSC.
- Z. M. Zhang, Z. X. Wei and M. X. Wan, Macromolecules, 2002, 35, 5937 CrossRef CAS.
- Z. Liu, X. Y. Zhang, S. Poyraz, S. P. Surwade and S. K. Manohar, J. Am. Chem. Soc., 2010, 132, 13158 CrossRef CAS PubMed.
- X. T. Zhang, J. Zhang, Z. F. Liu and C. Robinson, Chem. Commun., 2004, 1852 RSC.
- W. B. Zhong, J. Y. Deng, Y. S. Yang and W. T. Yang, Macromol. Rapid Commun., 2005, 26, 395 CrossRef CAS.
- Y. P. Xue, X. F. Lu, Y. Xu, X. J. Bian, L. R. Kong and C. Wang, Polym. Chem., 2010, 1, 1602 RSC.
- A. Chen, H. Wang and X. Li, Chem. Commun., 2005, 1863 RSC.
- Y. P. Xue, X. F. Lu, X. J. Bian, J. Y. Lei and C. Wang, J. Colloid Interface Sci., 2012, 379, 89 CrossRef CAS PubMed.
- L. Yang, Z. C. Li, X. F. Lu, Y. Tong, G. D. Nie and C. Wang, ChemPlusChem, 2013, 78, 522 CrossRef CAS.
- G. Berhault, M. Bausach, L. Bisson, L. Becerra, C. Thomazeau and D. Uzio, J. Phys. Chem. C, 2007, 111, 5915 CAS.
- Y. D. Li, X. L. Li, Z. X. Deng, B. C. Zhou, S. S. Fan, J. W. Wang and X. M. Sun, Angew. Chem., Int. Ed., 2002, 41, 333 CrossRef CAS.
- J. Hazarika and A. Kumar, Synth. Met., 2013, 175, 155 CrossRef CAS PubMed.
- R. Bhatia, C. S. S. Sangeeth, V. Prasad and R. Menon, Phys. B, 2011, 406, 1727 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, and catalytic reactions. See DOI: 10.1039/c4ta05220j |
| This journal is © The Royal Society of Chemistry 2015 |