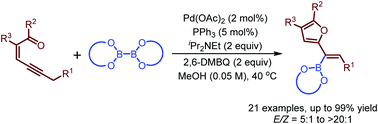Yifan Ping, Taiwei Chang, Kang Wang, Jingfeng Huo and Jianbo Wang
Chem. Commun., 2019,55, 59-62
DOI:
10.1039/C8CC09024F,
Communication
A palladium-catalyzed oxidative borylation reaction of conjugated enynones is developed. This reaction represents a new method for the synthesis of furyl-substituted alkenylboronates. The reaction works well with a series of conjugated enynones. Boryl migratory insertion of the palladium carbene intermediate is proposed as the key step in these transformations.