Unveiling the stimulatory effect of silicon nanoparticles on the direct regeneration and micro-structural developments in Cadaba trifoliata (Roxb.) Wight & Arn.
M.
Manokari
ab,
Mohammad
Faisal
c,
Abdulrahman A.
Alatar
c and
Mahipal S.
Shekhawat
 *a
*a
aBiotechnology Unit, Kanchi Mamunivar Government Institute for Postgraduate Studies and Research, Puducherry – 605 008, India
bDepartment of Botany, Siddha Clinical Research Unit, Central Council for Research in Siddha, Palayamkottai, Tamil Nadu – 627 002, India
cDepartment of Botany & Microbiology, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. E-mail: smahipal3@gmail.com
First published on 24th September 2024
Abstract
Nanosilicon positively affects the growth and development of plants. Investigations were carried out to analyse the effect of applied silicon nanoparticles (SiNPs) on the multiplication and rooting of the shoots of Cadaba trifoliata (Capparaceae). In vitro-raised shoots were cultivated on Murashige and Skoog (MS) medium with 0.5 mg L−1meta-Topolin (mT) and 0.25 mg L−1 indole-3 acetic acid (IAA) along with various concentrations of SiNPs. 2.0 mg L−1 nano-silicon along with optimal plant hormones improved multiplication of shoot numbers and morphology (25 shoots and 13.0 leaves per shoot), leaf area (1.3 × 0.9 cm), biomass (647 mg fresh weight; 114 mg dry weight) production, and photopigments (Chl a: 378.0, Chl b: 513.0, and Cx+c: 60.0 μg g−1 fresh weight). Cent percent of the shoots were rooted in half-strength MS medium with 1.25 mg L−1 indole-3-butyric acid (IBA). Incorporation of 2.0 mg L−1 SiNPs in the medium promoted rooting (8.3 roots per shoot, each 4.93 cm long). Analyses of qualitative microscopic data revealed that the leaves developed from the cloned shoots exhibited well-organized stomatal guard cells and epidermal cells, thick cuticle and epidermis, and increased tissue density in mesophyll and vascular tissues. The stem and root anatomy revealed improvements in vascular tissue density and lignification in xylem vessels and phloem elements. The plantlets were acclimatized under ex vitro conditions for 8 weeks in soilrite and vermicompost mixtures. These findings highlight the potential of SiNPs in improving the regeneration potential for clonal propagation of C. trifoliata.
Environmental significanceSilicon, an abundant element, has environmental implications and finds applications in biological systems. Nano-silicon affects growth and development in plants. We carried out analyses of the effects of concentrations of silicon nanoparticles (SiNPs) on multiplication and rooting of the shoots of Cadaba trifoliata (Capparaceae). A profound impact of optimal SiNPs on in vitro morphometric growth was recorded, promoting biomass, leaf area, photopigments, rhizogenesis, stomata, overall tissue system density, and lignification in cell walls of vascular tissues in the leaves, stems, and roots of C. trifoliata. |
1 Introduction
Nanotechnology is gaining significant interest for its versatile applications in agriculture, including enhancing plant growth, seed germination, nutritional content, yield, and the production of secondary metabolites.1 Research in plant tissue culture has demonstrated that silicon nanoparticles (SiNPs) have a positive impact on in vitro flowering, overall plant growth including leaf area, micro-structural differentiation, chlorophyll development and photosynthesis, alleviating oxidative stress and micro-morphological and physiological disorders, and improving rooting and acclimatization efficiency.2–4 The impact of SiNPs varies based on the specific species and concentration.5Cadaba trifoliata (Roxb.) Wight &Arn., (three-leaf Cadaba) belongs to the family Capparaceae and is found in dry deciduous forests, plains, and coastal areas. The plant is an unarmed tree that can reach a height of 3 to 4 meters. The leaves are arranged in a palmate trifoliate pattern, with oblong or lanceolate leaflets.6 Due to its low population size and narrow geographical distribution, it has been considered a rare and endemic species at the regional level and designated as an intermittent species generally found in southern Peninsular India and Sri Lanka.7–10Cadaba trifoliata roots and other parts are over-exploited for medicinal purposes due to their pharmacological properties and larvicidal activities.11,12
The scarcity of C. trifoliata trees due to insufficient cultivation practices underscores the need to prioritize conservation efforts. Traditional methods for propagating C. trifoliata include both seed and stem-cutting techniques.13,14 However, preserving seeds poses challenges due to the decline in germination rates over time. Additionally, relying on stem cuttings for propagation presents drawbacks such as the availability of planting materials and stringent environmental requirements to make nurseries.15 Therefore, alternative rapid technologies have to be developed to produce saplings of C. trifoliata to restore the natural population.
In vitro propagation is an effective method for quickly multiplying and producing important plant species in large quantities.15 This technology offers the optimal alternative for the consistent and uninterrupted delivery of plants and plant-based active components while efficiently addressing environmental limitations.16 As per the literature survey, in vitro research especially targeting C. trifoliata has not been conducted previously.17–20 Further, in vitro propagation of woody plant species is constrained by challenges such as in vitro recalcitrance, phenolics exudation, slow growth response, and in vitro induced morpho-anatomical abnormalities.21–23
The microscopic analysis of the developments of morpho-anatomy of the vegetative organs of in vitro raised plantlets plays a considerable role in the establishment and successful survival of plantlets.24 It helps in understanding the developmental levels of cultures growing under different chemical and environmental conditions that ultimately improve the survival success of the plants during hardening and in vivo trials.23
Recently, the use of silicon nanoparticles in micropropagation revealed enhancing qualitative and quantitative developments of tissue systems of in vitro grown tree species, which accelerated the production and field survival of plantlets.3,23,25 Therefore, this study aimed to investigate the effects of different concentrations of silicon nanoparticles on the possible changes in the in vitro growth parameters, biomass accumulation, photopigments, and micro-structural developments in the leaves, shoots, and roots of the C. trifoliata tree.
2 Materials and methods
2.1 In vitro application of SiNPs
The cultures were established following the author's previous report on in vitro regeneration of C. trifoliata.20 Briefly, the node explants from a 12 year-old tree (Fig. 1A) were collected and sterilized using 0.1% (w/v) solution of bavistin (BASF India Ltd., Mumbai, India) and 0.1% (w/v) aqueous HgCl2 (HiMedia, Mumbai, India) for 5 min each. The explants were inoculated on Murashige and Skoog medium26 containing 6-(3-hydroxybenzylamino) purine (meta-Topolin, mT) at 1.0 mg L−1 for bud breaking and the shoots were proliferated on 0.5 mg L−1mT and 0.25 mg L−1 IAA (indole-3 acetic acid). These shoots were rooted on a half-strength MS medium containing 1.25 mg L−1 indole-3-butyric acid (IBA).2.2 Impact of SiNPs on the morphological traits
A silicon nanoparticle (SiNP) suspension was prepared using Aerosil300, (hydrophilic silica with a diameter of 10 nm, 270–330 m2 g−1 surface area, and a pH of 3.7–4.5) (manufactured by Evonik Industries, Germany) following Attia and Elhawat27 with necessary modifications. To visualize the effect of SiNP treatment, the proliferated shoots were subcultured on various concentrations (1.0 to 4.0 mg L−1) of SiNPs along with the optimized plant growth regulators (PGRs) (0.5 mg L−1mT and 0.25 mg L−1 IAA), to evaluate the in vitro growth parameters, biomass production, photopigments, and micro-developmental features of the leaves, stems, and roots. Cultures were kept in a growth chamber under controlled conditions: 25 °C temperature, fluorescent lighting providing a light intensity of 45 μmol m−2 s−1, a photoperiod of 12 h, and a relative humidity of 60%. After 4 weeks of cultivation, morphological traits including number of shoots, shoot length (cm), number of leaves, leaf area, fresh weight (FW), and dry weight (DW) of shoots were measured.2.3 Micro-structural developmental characterization of C. trifoliata
To evaluate the SiNP-induced structural characteristics, leaves and stems at the proliferation stage and roots at the rhizogenic stage were selected randomly from both the control (PGRs only) and SiNP-containing media (PGRs + SiNPs). The fresh foliage, stem, and root samples obtained were excised and stored in FAA solution (comprising formaldehyde, acetic acid, and ethyl alcohol) for 24 hours. The samples were dehydrated using a sequence of ethanol solutions. Thin transverse sections, ranging between 10 to 20 μm, were then prepared using a rotary microtome (Almicro, MS-80) to examine the structural changes induced by SiNPs. Following staining with a 1% (w/v) safranin solution, the sections were mounted on slides in glycerine. Photomicrographs were then captured at various magnifications using a light microscope (Leica, DM 750, Leica Microsystems, Heidelberg, Germany) equipped with a digital camera, as indicated by scale bars. These photomicrographs facilitated a comparative analysis of anatomical changes in the samples between the control and SiNP treatment groups. Moreover, the anatomical characterization of dermal, ground, and vascular systems was examined to understand the impact of SiNPs on structural developments.2.4 Determination of leaf chlorophylls
Chlorophyll a, chlorophyll b, and total carotenoids were obtained from fresh leaves (both control and optimal SiNP-derived) in the absence of light. To achieve this, 200 mg of intermediate leaves were pulverized using liquid nitrogen and mixed with 1.5 ml of cooled acetone (80%) in Eppendorf tubes. Afterward, the mixtures were subjected to centrifugation at 15000 revolutions per minute for 10 min at a temperature of 4 °C. The supernatant was collected and the absorbance of chlorophyll a was measured at 663 nm, chlorophyll b at 646 nm, and carotenoids at 470 nm using a UV-visible double-beam spectrophotometer (Systronics India Ltd, Chennai, India, Model 2202) following Saini et al.282.5 Statistical analysis
In this study, the effects of different concentrations of SiNPs on morpho-anatomic growth parameters were conducted based on a completely randomized design with three replications and 24 duplications in each step of plantlet establishment. Data were statistically analyzed by ANOVA and the means were compared using the DMRT test at p-value <0.05. The results were represented as a mean value ± standard deviation.3 Results and discussion
3.1 Impact of SiNPs on shoot proliferation
Bud breaking was induced from the nodal shoot explants following the author's previous report,20 and shoot regeneration was facilitated using 1.0 mg L−1mT (Fig. 1B). The shootlets demonstrated diverse growth responses when exposed to the optimized proliferation medium consisting of 0.5 mg L−1mT and 0.25 mg L−1 IAA with different concentrations of SiNPs (Table 1). Among the various concentrations of SiNPs examined, 2.0 mg L−1 SiNPs resulted in promoting shoot morphological growth (25 shoots measuring 4.8 cm in length) (Table 1; Fig. 1D). In the treatments where SiNPs were implemented optimally, leaf area and shootlet biomass increased while the rate of shoot proliferation was negligible. The combination of 0.5 mg L−1mT and 0.25 mg L−1 IAA (the control medium) without SiNPs produced 23 shoots measuring 6.0 cm in length (Fig. 1C). Conversely, the morphometric characteristics were adversely affected by the elevated concentrations of SiNPs as observed in Table 1. The growth medium containing 2.0 mg L−1 SiNPs in addition to the optimal PGRs exhibited the highest leaf count (13 per shoot) and enhanced leaf area (1.3 cm length × 0.9 cm width), and demonstrated superior performance in terms of biomass acquisition and yielding the increased biomass of 647 mg FW and 114 mg DW (Table 1). In contrast, the control exhibited a decrease in leaf count (7 per shoot), leaf area (0.73 cm × 0.5 cm), and biomass (490 mg FW and 85 mg DW). Among the SiNP concentrations evaluated, 2.0 mg L−1 SiNPs in the medium was deemed optimal based on all proliferation characteristics in this study.| Conc. of SiNPs (mg L−1) | No. of shoots (mean ± SE) | Length of shoots (cm) (mean ± SE) | No. of leaves per shootlet (mean ± SE) | Leaf area (mean ± SE) | Shoot biomass (mg) (mean ± SE) | ||
|---|---|---|---|---|---|---|---|
| Length (cm) | Width (cm) | Fresh weight | Dry weight | ||||
| According to DMRT at p < 0.05, the data in each column followed by a different letter are significantly different. | |||||||
| 0 (control) | 23.0 ± 1.73a | 6.0 ± 0.12a | 7.0 ± 1.15b | 0.73 ± 0.12b | 0.5 ± 0.12b | 490.0 ± 7.1d | 85.0 ± 2.3c |
| 1.0 | 24.0 ± 2.08a | 4.1 ± 0.09c | 9.3 ± 1.33ab | 0.87 ± 0.12b | 0.63 ± 0.07b | 522.0 ± 4.6c | 90.0 ± 1.2c |
| 2.0 | 25.0 ± 1.52a | 4.8 ± 0.12b | 13.0 ± 1.73a | 1.3 ± 0.15a | 0.9 ± 0.06a | 647.0 ± 6.0a | 114.0 ± 5.0a |
| 3.0 | 23.6 ± 1.85a | 4.0 ± 0.33c | 10.7 ± 0.88ab | 0.93± 0.09ab | 0.6 ± 0.06b | 590.0 ± 5.8b | 102.0 ± 3.1b |
| 4.0 | 21.6 ± 1.45a | 3.3 ± 0.15d | 9.0 ± 1.16ab | 0.7 ± 0.10b | 0.43 ± 0.07b | 510.0 ± 7.6c | 89.0 ± 2.3c |
Significant differences were observed in the number of leaves, leaf area, and biomass of shoots between the SiNP concentrations used and the control medium, although the rate of shoot proliferation was not significantly different in the present study. Silicon has been reported to improve many growth parameters including organogenesis, embryogenesis, leaf morphology, physiology, and anatomy of cultures.29 The use of SiNPs in the nutrient mixture was reported to be more effective than foliar application in improving morphometric growth features and secondary metabolites,30,31 as the exogenous administration of SiNPs in the growth media may enhance absorption of nutrients and influence the growth traits of plantlets.
Research indicates that the incorporation of nano-silicon (SiO2-NPs) in the cultivation process can enhance the rate of rooting of shoots, photosynthetic pigments, and overall growth of banana plants.32,33 Furthermore, the inclusion of Si in culture media has proven advantageous, offering benefits such as increased tolerance to water stress, improved photosynthetic capacity, and decreased transpiration.34 Liang et al.35 have also recorded the stimulatory effect of SiO2-NPs on the leaf area, photopigments, and biomass production in cotton seedlings. Similarly, the use of optimal SiNPs has been reported effective in improving organogenesis and alleviating in vitro-induced micro-structural abnormalities in several tree species including Santalum album,25Vitex trifolia,3 and V. negundo.23
3.2 Impact of SiNPs on photosynthetic pigments in vitro
Different concentrations of SiNPs led to variations in the synthesis of photosynthetic pigments in the leaves of in vitro developed shoots. Leaves grown on a medium containing SiNPs showed significantly higher total photosynthetic pigment concentrations compared to those from the control experiments. Chlorophyll pigment development in the foliage of control shoots was found to be less comparatively (Chl a: 180.0, Chl b: 205.0, and Cx+c: 39.0 μg g−1 FW) (Table 2). The leaves derived from 0.5 mg L−1mT + 0.25 mg L−1 IAA and 2.0 mg L−1 SiNPs exhibited higher quantities of total pigments and chlorophylls (Chl a: 378.0, Chl b: 513.0, and Cx+c: 60.0 μg g−1 FW) along with dark-green foliages (Fig. 1D). However, further increase in SiNP concentrations resulted in a reduction in chlorophyll pigment synthesis and an elevation in carotenoid levels in the leaves of C. trifoliata (Table 2).| Conc. of SiNPs (mg L−1) | Photopigments (μg g−1 FW) | ||
|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Total carotenoid (Cx+c) | |
| According to DMRT at p < 0.05, the data in each column followed by a different letter are significantly different. | |||
| 0 (control) | 180.0 ± 3.46e | 205.0 ± 3.61e | 39.0 ± 2.65c |
| 1.0 | 213.0 ± 7.23d | 310.0 ± 5.13d | 44.0 ± 2.31c |
| 2.0 | 378.0 ± 6.93a | 513.0 ± 11.8a | 60.0 ± 1.73b |
| 3.0 | 350.0 ± 5.77b | 470.0 ± 2.89b | 65.0 ± 2.65ab |
| 4.0 | 311.0 ± 7.37c | 418.0 ± 4.36c | 71.0 ± 2.08a |
Studies have demonstrated that silicon positively impacts plant growth and crop yield. The enhanced photopigments in this study is attributed to silicon's ability to improve stem stability and enable leaves to absorb light more effectively, thereby enhancing photosynthesis.36 The beneficial impact of SiO2-NPs on leaf chlorophyll concentration appears to be connected to its function as a cofactor in pigment biosynthesis37 and its ability to block the activity of chlorophyllase, which becomes more pronounced under harsh environmental conditions.38 Previous research emphasized that SiO2-NPs can improve photosynthetic pigments and accelerate the rate of photosynthesis.39 Silicon accumulates in the leaf's width, enhancing leaf strength and increasing chlorophyll contents in the leaf area. It can enhance photosynthesis by maintaining the chloroplast structure and improving the efficiency of photosystem II. Recent studies have demonstrated the beneficial impact of SiNPs on enhancing the photosynthetic parameters in certain plants experiencing drought.2 Silicon deposited in the leaf area can enhance the leaf strength and chlorophylls and improve the light absorption capacity40 and overall health of the plants.
3.3 Effect of exogenous SiNPs on the vegetative anatomy of C. trifoliata
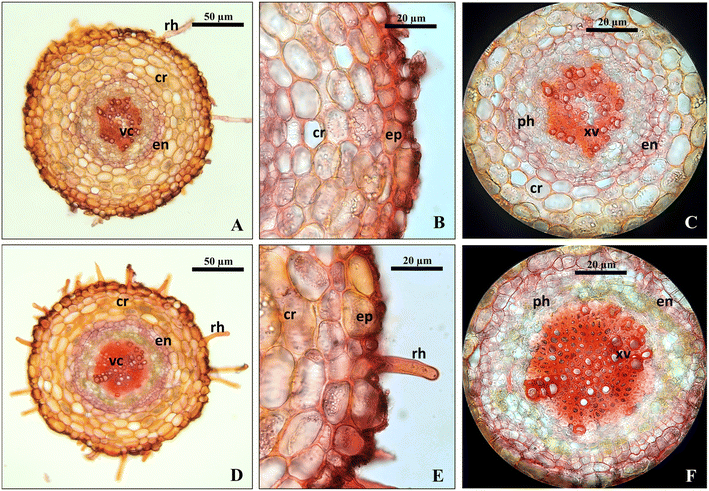
In this study, enhancements in the morpho-anatomical characteristics of the plantlets by incorporating optimal silicon nanoparticles were noticed alongside the pre-optimized plant growth regulators for shoot proliferation. The effect of SiNPs in all the studied parameters was significant in structural developments in the stomata, cuticle, epidermis, hypodermis, endodermis, cortical, and vascular tissues of the leaves and stems. The addition of SiNPs stimulated maximum differentiation in higher tissue density as compared to the control. The thickness of the cuticle, epidermis, and density of mesophyll and vascular tissues were increased by the SiNPs under in vitro conditions over the control leaf and stem samples. The vegetative tissue samples derived from SiNP augmented medium had developed a vascular tissue system with higher lignified vessels and phloem tissues. The formation of more xylem vessels and lignification suggest increased transportation of water by the shoot system under the influence of SiNPs.
Silicon contributes to the formation of xylem cell walls, which play an important role in transporting water into the plant. In addition, the build-up of silicon in the leaf creates a second layer of silicon,41 and decreases transpiration. Similarity, silicon becomes a polymerized gel inside the cells, which strengthens and stabilizes cells and minimizes plant electrolyte leakage.42 Moreover, nano-silicon prevented the formation of increased electrolytes in Rosa damascene.43 According to Sattar et al.,44 silicon supplement enhances the water use efficiency of plants. Similar to the present findings, SiNPs promoted lignification in plant tissues and improved growth in Avena sativa,5Vitex trifolia,3 and Hemidesmus indicus.4
3.4 Influence of SiNPs on morpho-anatomical developments in the root system
In this experiment, the maximum number of roots (100% response, 8.3 roots with 4.93 cm length) were recorded on half-strength MS medium supplemented with 1.25 mg L−1 IBA with 2.0 mg L−1 SiNPs (Table 3; Fig. 1E). On the contrary, the least number of roots per shootlet (95.8% rhizogenesis, 4.8 roots with 4.3 cm length) were formed on media supplemented with 1.25 mg L−1 IBA alone (control). In addition, the roots developed on the control media combination had comparatively underdeveloped morpho-anatomical traits (Fig. 4). Such roots possessed a disturbed outer epidermis, few root hairs, and limited vascular tissues (Fig. 4A–C). Whereas, a continuous dermal layer with numerous root hairs was found in the roots of C. trifoliata when cultivated on the IBA-containing rooting medium along with 2.0 mg L−1 SiNPs (Fig. 4D and E). The cortical layers were arranged more tightly than the control root. Furthermore, the ratio of the central cylinder to the cortical layer was enhanced in these samples (Fig. 4D and F). Silicon NP-mediated roots had an extensive vascular tissue system due to the higher amount of xylem elements.| Conc. of SiNPs (mg L−1) | Rhizogenesis (%) | No. of roots | Length of roots (cm) |
|---|---|---|---|
| According to DMRT at p < 0.05, the data in each column followed by a different letter are significantly different. | |||
| 0 (control) | 95.8 ± 2.40a | 4.8 ± 0.20b | 4.30 ± 0.18ab |
| 1.0 | 97.2 ± 1.40a | 5.0 ± 0.58b | 4.30 ± 0.12ab |
| 2.0 | 100.0 ± 0.0a | 8.3± 0.88a | 4.93 ± 0.09a |
| 3.0 | 87.5 ± 2.43b | 6.0 ± 1.53ab | 3.80 ± 0.15bc |
| 4.0 | 83.3 ± 2.40b | 4.3 ± 0.33b | 3.17 ± 0.44c |
The induction of roots is a crucial stage for successful in vitro propagation. The supplementation of optimal SiNPs was found to enhance the morphometric and structural differentiation at the rhizogenesis stage. Investigations have affirmed that utilization of SiO2 nanoparticles significantly enhanced seedling growth, root collar diameter, main root length, and lateral roots, which improved the quality of seedlings.45 Similarly, in line with the current research, the application of nano-silicon has been shown to augment the rate of shoot proliferation and rooting in pistachio.40 Nano-silicon has promoted adventitious root formation and its structural developments in Hedyotis biflora.46 The improved root structural traits could support the development of an extensive root system to express adaptation to stressful conditions.46 These results support the findings of root microscopic features of Capparaceae species,47,48 which showed that the improved root structural traits promote extensive root systems in the field to express xerophytic adaptation.
3.5 Acclimatization of plantlets
All the rooted shoots were successfully acclimatized to ex vitro conditions. The plantlets were placed for hardening in paper containers containing sterile soilrite and vermicompost, and maintained under the greenhouse environments for up to 4–8 weeks (Fig. 1F). The maximum survivability was noticed in plantlets developed with the help of SiNP augmentation as compared to the control (data not shown). The addition of silicon to the culture media can enhance plant growth by boosting the levels of hemicellulose and lignin, hence augmenting the rigidity of the cell wall. These modifications enhance survival during acclimatization. It has an indirect effect of increasing photosynthetic capacity, decreasing transpiratory rates, and enhancing the mechanical resistance of plant cells.49,50 The utilization of SiNPs has been instrumental in fostering stress tolerance and enhancing plant adaptation across diverse crops. Recent studies have demonstrated that treating plants with SiNPs leads to an increase in morphological traits, biochemical parameters, and antioxidant activities under stress conditions.51 Furthermore, the application of SiO2-NPs has been proven effective in promoting root development, improving gas exchange, and bolstering drought resistance in plants.52 Overall, the incorporation of SiNPs in the medium has exhibited promising outcomes in the context of rooting, hardening, and facilitating plant's adaptation to various stresses.4 Conclusion
The study established that the exogenous application of SiNPs coupled with optimized PGRs considerably enhanced the in vitro morpho-structural developments in Cadaba trifoliata plantlets. The use of SiNPs positively impacted plantlet development, with a significant effect observed at a concentration of 2.0 mg L−1. The potential impact of optimal SiNPs on in vitro morphometric growth was attributed to the enhancement in biomass, leaf area, photopigments, rhizogenesis, stomata, overall tissue system density, and lignification in cell walls of vascular tissues in leaves, stems, and roots. It is suggested that nano-silicon has beneficial impacts on the growth and development of the in vitro propagation process and could be utilized for the propagation and conservation of Cadaba trifoliata and other members of the family Capparaceae. Additional research is needed to explore the potential synergistic impact of SiNPs and PGRs on enhancing the morpho-anatomy of tree species.Human and animal rights
This research did not involve experiments with human or animal participants.Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.Data availability
The authors claim that the data and information in this article are all derived from this research and have been indicated in the manuscript.Author contributions
MM, MSS, MF, and AAA: conceptualization, investigation, and methodology. MM: data compilation and hardening of the plants. MSS, MM, MF, and AAA: writing of original draft, statistics, data interpretation, and revision of the manuscript. All authors have read and approved the final manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The work was financially supported by the Researchers Supporting Project (RSP2024R86), King Saud University, Riyadh, Saudi Arabia.References
- S. A. Elsayh, R. N. Arafa, G. A. Ali, W. Abdelaal, R. A. Sidky and T. I. Ragab, Impact of silver nanoparticles on multiplication, rooting of shoots and biochemical analyses of date palm Hayani cv. By in vitro, Biocatal. Agric. Biotechnol., 2022, 43, 102400, DOI:10.1016/j.bcab.2022.102400
.
- L. M. Mahmoud, M. Dutt, A. M. Shalan, M. E. El-Kady, M. S. El-Boray, Y. M. Shabana and J. W. Grosser, Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress, S. Afr. J. Bot., 2020, 132, 155–163, DOI:10.1016/j.sajb.2020.04.027
.
- M. Cokul Raj, M. Manokari, N. Arumugam, A. Dey, M. Faisal, A. A. Alatar, A. Alok and M. S. Shekhawat, Silicon nanoparticles mediated in vitro flowering and study of pollen viability in Vitex negundo L, Silicon, 2023, 15, 4861–4871, DOI:10.1007/s12633-023-02397-4
.
- M. Manokari, M. Faisal, A. A. Alatar, D. Barboni and M. S. Shekhawat, Silicon nanoparticles on the enhanced micropropagation of Hemidesmus indicus (L.) R. Br. ex Schult, Silicon, 2024, 16, 775–782, DOI:10.1007/s12633-023-02714-x
.
- G. Asgari-Targhi, A. Iranbakhsh and Z. O. Ardebili, Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum, Plant Physiol. Biochem., 2018, 127, 393–402, DOI:10.1016/j.plaphy.2018.04.013
.
-
K. M. Matthew, Flora of Tamilnadu Carnatic, The Rapinat Herbarium, St. Joseph's College, Tiruchirapalli, TN, India, 1983 Search PubMed
.
- S. E. S. Kumar, S. S. Yeragi, K. N. Babu and S. G. Nair, Sacred grove flora of Kerala – I new plant records for Kerala, J. Econ. Taxon. Bot., 2002, 26, 141–143 Search PubMed
.
- N. Balachandran, K. Rajendiran and W. Gastmans, Endemic plants of tropical dry evergreen forest, Southern India, Biodiv. Res. Conserv., 2018, 52, 11–23, DOI:10.2478/biorc-2018-0015
.
-
A. A. Mao and S. S. Dash, Flowering Plants of India an Annotated Checklist (Dicotyledons), Botanical Survey of India, 2020, vol. 1, pp. 1–970 Search PubMed
.
-
D. Narayanasamy and B. Natesan, Endemic vascular plants from the coromandel coast of Tamil Nadu, Southern India, Intech Open, 2021, DOI:10.5772/intechopen.94333
.
- R. Mythreyi, E. Sasikala, A. Geetha and V. Madhavan, Antibacterial activity of leaves of Cadaba trifoliata (Roxb.)Wt. & Arn, Indian J. Pharm. Sci., 2009, 71, 115–116, DOI:10.4103/0250-474X.54271
.
- S. Rajkumar, A. Jebanesan and R. Nagarajan, Synergistic effect of Andrographis echioides and Cadaba trifoliata leaf extracts against larvae of dengue mosquito Aedes aegypti L, Asian Pac. J. Trop. Biomed., 2011, 2, S1588–S1591, DOI:10.1016/S2221-1691(12)60458-4
.
- A. Rajuri, P. Sanjay, K. Rajender, C. Ragini, A. Vijay Kumar and G. Raju, Pharmacognostic studies of Cadaba trifoliata Roxb(Capparaceae) leaves, Int. J. Pharmacogn. Phytochem. Res., 2012, 4, 151–156 Search PubMed
.
-
S. K. Rao, K. S. Raja, D. Kumar, S. R. Arun and G. K. Bhat, Flora of Peninsular India, https://peninsula.ces.iisc.ac.in/plants.php?name=Cadaba trifoliata, 2019, Accessed on 6 March 2024 Search PubMed
.
- A. Hasnain, S. A. Hasan Naqvi, S. I. Ayesha, F. Khalid, M. Ellahi, S. Iqbal, M. Z. Hassan, A. Abbas, R. Adamski, D. Markowska, A. Baazeem, G. Mustafa, M. Moustafa, M. E. Hasan and A. M. M. Abdelhamid, Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches, Front. Plant Sci., 2022, 13, 1009395, DOI:10.3389/fpls.2022.1009395
.
- A. Wijerathna-Yapa and J. Hiti-Bandaralage, Tissue culture-a sustainable approach to explore plant stresses, Life, 2023, 13, 780, DOI:10.3390/life13030780
.
- H. Abbas and M. Qaiser,
In vitro conservation of Cadaba heterotricha stocks, an endangered species in Pakistan, Pak. J. Bot., 2010, 42, 1553–1559 CAS
.
- D. Lodha, A. K. Patel and N. S. Shekhawat, A high-frequency in vitro multiplication, micromorphological studies and ex vitro rooting of Cadaba fruticosa (L.) Druce (Bahuguni): A multipurpose endangered medicinal shrub, Physiol. Mol. Biol. Plants, 2015, 21, 407–415, DOI:10.1007/s12298-015-0310-6
.
- S. Y. Juliet, K. Kalimuthu, V. Chinnadurai and R. Krishnasamy, Effect of growth regulators on direct shoot formation from leaf explant and antioxidant activity of in situ and in vitro plants of Cadaba fruticosa - an endemic medicinal plant, Eur. J. Med. Plants, 2017, 18, 1–10, DOI:10.9734/EJMP/2017/32507
.
- M. Manokari, M. Faisal, M. M. Alatar and M. S. Shekhawat,
In vitro regeneration and structural and physiological modifications in the foliages of Cadaba trifoliata (Roxb.) Wight &Arn.: an endemic tree of the family Capparaceae, Russ. J. Plant Physiol., 2024, 71, 122 CrossRef CAS
.
- M. K. Rai, P. Asthana, V. S. Jaiswal and U. Jaiswal, Biotechnological advances in guava (Psidium guajava L.): recent developments and prospects for further research, Trees, 2010, 24, 1–12, DOI:10.1007/s00468-009-0384-2
.
- U. Sharma, V. Kataria and N. S. Shekhawat, In vitro propagation, ex vitro rooting and leaf micromorpholo,gy of Bauhinia racemosa Lam.: A leguminous tree with medicinal values, Physiol. Mol. Biol. Plants, 2017, 23, 969–977, DOI:10.1007/s12298-017-0459-2
.
- M. Cokul Raj, M. Manokari, A. Dey, M. Faisal, A. A. Alatar, R. K. Singh and M. S. Shekhawat, Improvements in morphometric and structural traits of Vitex trifolia L. to exogenous application of nano-silicon in vitro, S. Afr. J. Bot., 2023, 160, 613–621, DOI:10.1016/j.sajb.2023.07.049
.
- J. P. R. Martins, V. Verdoodt, M. Pasqual and M. De Proft, Impacts of photoautotrophic and photomixotrophic conditions on in vitro propagated Billbergia zebrine (Bromeliaceae), Plant Cell, Tissue Organ Cult., 2015, 123, 121–132 CrossRef CAS
.
- M. Manokari, A. Dey, M. Faisal, A. A. Alatar, R. K. Singh and M. S. Shekhawat, Silicon nanoparticles (SiNPs) positively affect morpho-structural differentiation in micropropagated plantlets of Santalum album L, Silicon, 2023, 15, 6843–6850, DOI:10.1007/s12633-023-02558-5
.
- T. Murashige and F. Skoog, A revised medium for rapid growth and bio assays with tobacco tissue cultures, Physiol. Plant., 1962, 15, 473–497 CrossRef CAS
.
- E. A. Attia and N. Elhawat, Combined foliar and soil application of silica nanoparticles enhances the growth, flowering period and flower characteristics of marigold (Tagetes erecta L.), Sci. Hortic, 2021, 282, 110015, DOI:10.1016/j.scienta.2021.110015
.
-
R. S. Saini, K. D. Sharma and O. P. Dhankhar, Laboratory manual of analytical in horticulture, Agrobios, India, 2001 Search PubMed
.
- M. Sahebi, M. M. Hanafi and P. Azizi, Application of silicon in plant tissue culture, In Vitro Cell. Dev. Biol. Plant, 2016, 52, 226–232 CrossRef CAS
.
- R. Suriyaprabha, G. Karunakaran, K. Kavitha, R. Yuvakkumar, V. Rajendran and N. Kannan, Application of silica nanoparticles in maize to enhance fungal resistance, IET Nanobiotechnol., 2014, 8, 133–137, DOI:10.1049/iet-nbt.2013.0004
.
- M. Mukarram, M. M. A. Khan and F. J. Corpas, Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield, J. Hazard. Mater., 2021, 412, 125254, DOI:10.1016/j.jhazmat.2021.125254
.
- B. N. S. Costa, A. Rúbio Neto, E. A. Chagas, P. C. Chagas, M. Pasqual and W. A. Vendrame, Influence of silicon and in vitro culture systems on the micropropagation and acclimatization of “Dwarf Cavendish” banana, Acta Sci., Agron., 2020, 43, e47490, DOI:10.4025/actasciagron.v43i1.47490
.
- D. F. C. da Silva, P. D. de Oliveira Paiva and R. C. Herrera,
et al., Effectiveness of silicon sources for in vitro development of gerbera, Plant Cell, Tissue Organ Cult., 2020, 141, 77–85, DOI:10.1007/s11240-020-01768-8
.
- M. Riaz, S. Zhao, M. Kamran, N. U. Rehman, F. Mora-Poblete, C. Maldonado, M. H. Saleem, A. Parveen, A. A. Al-Ghamdi, F. M. Al-Hemaid, S. Ali and M. S. Elshikh, Effect of nano-silicon on the regulation of ascorbate glutathione contents, antioxidant defense system and growth of copper stressed wheat (Triticum aestivum L.) seedlings, Front. Plant Sci., 2022, 13, 986991, DOI:10.3389/fpls.2022.986991
.
- Y. Liang, H. Liu, Y. Fu, P. Li, S. Li and Y. Gao, Regulatory effects of silicon nanoparticles on the growth and photosynthesis of cotton seedlings under salt and low-temperature dual stress, BMC Plant Biol., 2023, 23, 504, DOI:10.1186/s12870-023-04509-z
.
- A. Samuels, A. Glass, D. Ehret and J. Menzies, The effects of silicon supplementation on cucumber fruit: changes in surface characteristics, Ann. Bot., 1993, 72, 433–440 CrossRef CAS
.
- A. Misra, A. K. Srivastava, N. K. Srivastava and A. Khan, Zn-acquisition and its role in growth, photosynthesis, photosynthetic pigments, and biochemical changes in essential monoterpene oil (s) of Pelargonium graveolens, Photosynthetica, 2005, 43, 153–155 CrossRef CAS
.
- J. Feng, Q. Shi, X. Wang, M. Wei, F. Yang and H. Xu, Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L, Sci. Hortic., 2010, 123, 521–530 CrossRef CAS
.
- J. H. Mejias, F. Salazar, L. Pérez Amaro, S. Hube, M. Rodriguez and M. Alfaro, Nanofertilizers: a cutting-edge approach to increase nitrogen use efficiency in grasslands, Front. Environ. Sci., 2021, 9, 635114, DOI:10.3389/fenvs.2021.635114
.
- M. Moradipour, R. Saberi-Riseh, R. Mohammadinejad and A. Hosseini, Nano-encapsulation of plant growth-promoting rhizobacteria and their metabolites using alginate-silica nanoparticles and carbon nanotube improves ucb1 Pistachio micropropagation, J. Microbiol. Biotechnol., 2019, 29, 1096–1103, DOI:10.4014/jmb.1903.03022
.
-
A. K. da Silva Lobato, E. M. Silva Guedes, D. J. Marqeus and C. F. de Oliveira Neto, Silicon: a beneficial element to improve tolerance in plants exposed to water deficiency, Response of organisms to water stress, ed., Akinci S., 2013, DOI:10.5772/53765
.
- Y. Liang, W. Sun, Y.-G. Zhu and P. Christie, Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review, Environ. Pollut., 2007, 147, 422–428 CrossRef CAS
.
- S. H. Hajizadeh, S. Azizi, F. Rasouli and O. Kaya, Evaluation of nano-silicon efficiency on compatible solutes and nutrient status of Damask rose affected by in vitro simulated drought stress, Chem. Biol. Technol. Agric., 2023, 10, 1–19, DOI:10.1186/s40538-023-00397-5
.
- A. Sattar, M. A. Cheema, A. Sher, M. Ijaz, A. Wasaya and T. A. Yasir,
et al., Foliar applied silicon improves water relations, stay green and enzymatic antioxidants activity in late sown wheat, Silicon, 2020, 12, 223–230 CrossRef CAS
.
- L. Bao-shan, D. shao-qi, L. Chun-Hui, F. Li-Jun, Q. Shu-Chun and Y. Min, Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings, J. For. Res., 2004, 15, 138–140, DOI:10.1007/BF02856749
.
- M. Manokari, M. Cokul Raj, A. Dey, M. Faisal, A. A. Alatar, N. Joshee and M. S. Shekhawat, Improvements in Morpho-Anatomical Traits of Adventitious Roots of Hedyotis biflora (L.) Lam. using Silicon Nanoparticles, Silicon, 2023, 15, 5747–5755, DOI:10.1007/s12633-023-02484-6
.
- L. Gan, C. Zhang, Y. Yin, Z. Lin, Y. Huang, J. Xiang, C. Fu and M. Li, Anatomical adaptations of the xerophilous medicinal
plant, Capparis spinosa, to drought conditions, Hortic., Environ. Biotechnol., 2013, 54, 156–161, DOI:10.1007/s13580-013-0162-3
.
- S. A. Zokian, Morphological, anatomical study and geographical distribution in Iraq of Capparis spinosa L, Iraqi J. Sci., 2015, 56, 100–104 Search PubMed
.
- A. Valente, R. Morais, C. Couto and J. H. Correia, Modeling, simulation and testing of a silicon soil moisture sensor based on the dual-probe heat-pulse method, Sens. Actuators, A, 2004, 115, 434–439 CrossRef CAS
.
- S. D. R. Soares, M. Pasqual, A. G. de Araujo, E. M. de Castro, F. Pereira and F. T. Braga, Leaf anatomy of orchids micropropagated with different silicon concentrations, Acta Sci., Agron., 2012, 34, 413–421, DOI:10.4025/actasciagron.v34i4.15062
.
- M. González-Moscoso, A. Juárez-Maldonado and G. Cadenas-Pliego,
et al., Silicon nanoparticles decrease arsenic translocation and mitigate phytotoxicity in tomato plants, Environ. Sci. Pollut. Res., 2022, 29, 34147–34163, DOI:10.1007/s11356-021-17665-2
.
- K. K. Verma, X.-P. Song, M. Singh, H.-R. Huang, R. Bhatt, L. Xu, V. Kumar and Y.-R. Li, Influence of nanosilicon on drought tolerance in plants: An overview, Front. Plant Sci., 2022, 13, 1014816, DOI:10.3389/fpls.2022.1014816
.
| This journal is © The Royal Society of Chemistry 2025 |