 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterocycles in reactions with boradigermaallyl†
Ralf H.
Kern
,
Paul L.
Schmiedel
,
Hartmut
Schubert
and
Lars
Wesemann
 *
*
Institut für Anorganische Chemie, Eberhard Karls Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany. E-mail: lars.wesemann@uni-tuebingen.de
First published on 27th February 2025
Abstract
Reactions of boradigermaallyl, which can also be regarded as a chloroborylene stabilzed by two germylene donors, with thiophene, furan, pyridazine and 2,2′-bipyridine are presented. Insertion of the boron atom into the heterocycles is observed and the resulting heterocycles continue to react with the bis(germylene) molecule.
The chemistry of low valent boron compounds is in the case of free borylenes dominated by matrix experiments and high temperature gas-phase synthesis.1–8 Preparative studies of borylene chemistry are concerned with trapping experiments with particular interest currently focused on Lewis base adducts of borylenes (examples of Lewis bases: CAAC,9 CO,10 isonitriles,10 oxazol-2-ylidene,11 silylenes12,13).9–11,14–20 Furthermore, borylene transition metal complexes have also been studied extensively and display a unique reactivity.21–24 We are interested in low valent tetrylene chemistry and have prepared intramolecular tetrylene phosphine Lewis pairs and bis(tetrylenes) of germanium and tin.25–28 Stabilization of low valent boron in these chelating vicinities leads to phosphine stabilized germa- and stannaborenes and boradigermaallyl 1 (Scheme 1).28–30 Chloro substituted boradigermaallyl, which can also be regarded as chloroborylene stabilized by two germylene donors, is a highly reactive molecule and shows at room temperature an insertion reaction with benzene. In this publication we present the reactions of boradigermaallyl with the organic heterocycles thiophene, furan, pyridazine and 2,2′-bipyridine.
 | ||
| Scheme 1 Boradigermaallyl 1 in reactions with heterocycles thiophene, furan, pyridazine, and 2,2′-bipyridine. | ||
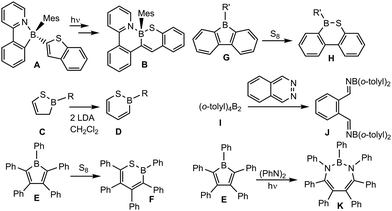
Boron insertion into thiophenes was observed by photoinitiated transformation of a N,C-chelated boron compound bearing at boron a benzothienyl substituent in 2-position A to yield a 1,2-benzothiaborinine B derivative (Chart 1).31 2H-1,2-Thiaborinine D has been synthesized by Ashe III and co-workers using a carbenoid ring expansion of 2,5-dihydro-1,2-thiaborole C.32 Sulphur insertion into boroles was employed by Martin et al. for pentaphenylborole E in reaction with sulphur at 65 °C.33,34 In the case of 9-borafluorene G derivatives Finze, Braunschweig and co-workers incorporated sulphur under UV light irradiation.35 Low valent boron chemistry has not yet been employed for insertion reactions of thiophene. However, Crimmin et al. studied the reaction of a low valent aluminium(I) complex [{HC(CMeNDipp)2}Al] (Dipp = 2,6-di-isopropylphenyl) with thiophenes.36,37 At 60 °C formation of a mixture of ring-expansion products, insertion of one and two aluminium fragments, and desulphurisation was observed.36
 | ||
| Chart 1 Syntheses of 1,2-thiaborinine derivatives and splitting of NN-double bonds by electrophilic boron compounds. | ||
Boradigermaallyl 1 was treated with 1.2 equiv. of thiophene in pentane and shows an immediate reaction, which is documented by a spontaneous colour change from turquoise to almost colorless (Scheme 1). The solution was stirred for another four days at 40 °C to ensure complete conversion. The reaction product 2 was isolated at −38 °C as a colorless solid in a yield of 70%. The molecular structure (Fig. 1, left top) features the insertion of the boron atom into a C–S bond of the thiophene ring. The Cl–Ge–B unit could be the product of an oxidative addition of the B–Cl moiety at a germylene. Finally, the other germylene unit exhibits a connectivity to the six membered SBC4 ring, which could be the result of a (1+4) cycloaddition between germylene and diene moiety of the thiaborinine cycle. The 11B NMR signal of 2 was observed as a very broad signal at 83 ppm and can be compared with the signal for the 1,2-thiaborinine F at 50.8 ppm.33 Because of the missing electronic delocalization of a boron neighboring double bond like in F the 11B NMR signal of 2 was observed at much higher frequency. The B–S bond length of 1.778(3) Å can be compared with B–S distances observed in H [1.775(7)–1.796(7) Å], F [1.7934(17) Å], and Mes2BSMe [1.787(6) Å] which exhibit partial B–S double bond character.33,35,38 The found B–Ge1 distance of 2.060(3) Å is similar to a single bond between these elements.29 The homologous furan was also treated with boradigermaallyl (Scheme 1). In the product compound 3 a BCl unit is inserted into a C–O bond of the furan ring and furthermore a germylene exhibits an oxidative addition with the C–O moiety of the oxaborinine to give a seven membered oxagermaborepin ring compound. Finally, a (1+4) cycloaddition of the diene unit with another germylene was observed (Fig. 1). 1,2-Oxaborinines have been synthesized by ring closure treating 2-methoxy-3,3′-bithiophene with BCl3, the carbenoid ring-expansion route starting with 1,2-oxaborol and oxygen insertion into boroles.39–41 To date oxaborepins have been synthesized via insertion of a CO unit of aldehydes, ketones or ketenes into boroles and boriranes.34,42–45 In 3 a B–O bond length of 1.332(2) Å was found which is slightly shorter than B–O distances observed in oxaborinines [B–O 1.384(6), 1.380(2) Å].40,41 The Ge–O bond in 3 [1.8218(12) Å] is comparable with a Ge–O single bond.46
Reactions of 1,2-diazenes such as pyridazine and benzo[c]cinnoline with Lewis acidic diborane(4) compounds, o-phenylbisborate and 9,10-dihydro-9,10-diboraanthracene lead to the formation of cyclic BN adducts.47–51 Yamashita, Lin and co-workers explored the reactivity of tetra(o-tolyl)diborane(4) (Chart 1, I) and reported adduct formation with pyridazine and a N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-splitting reaction towards phthalazine to give 1,2-bis(borylimidoyl)benzene (J).51 Splitting of a N
N-splitting reaction towards phthalazine to give 1,2-bis(borylimidoyl)benzene (J).51 Splitting of a N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-bond and insertion of a boron atom was presented by Martin and co-worker treating pentaphenylborole E with azobenzene derivatives under photolytic conditions to give a BN2C4 seven membered boracycle K.52 In the case of the benzo[c]cinnoline reaction with pentaphenylborole insertion of a PhB unit into the N–N bond was observed to give a bicyclic molecule with a 1,3,2-diazaborepin moiety.52 Boradigermaallyl was treated with three equivalents of pyridazine in cyclohexane at room temperature. The color of the solution changed after addition of pyridazine from turquoise to orange-red in a period of 15 minutes. To complete the conversion the solution has been stirred for another three days to yield a microcrystalline yellow-orange solid of 4 (Scheme 1). Crystals suitable for X-ray diffraction were obtained from toluene (Fig. 1).
N-bond and insertion of a boron atom was presented by Martin and co-worker treating pentaphenylborole E with azobenzene derivatives under photolytic conditions to give a BN2C4 seven membered boracycle K.52 In the case of the benzo[c]cinnoline reaction with pentaphenylborole insertion of a PhB unit into the N–N bond was observed to give a bicyclic molecule with a 1,3,2-diazaborepin moiety.52 Boradigermaallyl was treated with three equivalents of pyridazine in cyclohexane at room temperature. The color of the solution changed after addition of pyridazine from turquoise to orange-red in a period of 15 minutes. To complete the conversion the solution has been stirred for another three days to yield a microcrystalline yellow-orange solid of 4 (Scheme 1). Crystals suitable for X-ray diffraction were obtained from toluene (Fig. 1).
Due to strong broadening, an 11B NMR signal for 4 could not be observed in the 11B NMR spectrum. The NMR signals for the CH unit of C5 (Fig. 1) were observed in the 1H and 13C{1H} NMR spectra at 4.23–4.27 and 47.3 ppm, indicating an increase of the coordination number. The boron atom in 4 is trigonal planar coordinated by three nitrogen atoms (N1–N3), showing a slight pyramidalization (∑angles N1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 359.8, N2
359.8, N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 346.2, N3
346.2, N3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356.6°), and the BN-distances [1.424(3), 1.434(3), 1.443(3) Å] are comparable with BN3 motifs, which exhibit delocalization of the electron pairs at nitrogen into the empty p-orbital at the boron atom.53–56 The found Ge–N bond lengths [1.8527(17), 1.8715(18) Å] are similar to single bonds between these elements.57 The structure of the 1,3,2-diazaborepin moiety in 4 can be compared with the diazaborepin structures published by Martin et al.52 Interestingly, Martin did not observe an NN bond cleavage in the reaction of unsubstituted pyridazine with pentaphenylborol, even at elevated temperatures. In a presumed mechanistic sequence for the formation of 4 (see ESI†) an adduct between the pyridazine nitrogen atoms and the germanium atoms could be the first step. Due to Ge–N adduct formation the borylene electron pair should be localized to a higher degree at the boron atom resulting in an increase of reactivity at boron and formation of a diazaboriridine.58 The chloride atom at the boron atom is substituted by a second equivalent of pyridazine followed by chloride germanium bond formation. The N–N bond opens, and the other germanium atom reacts as a nucleophile with the carbon atom of the coordinated, neighbouring pyridazine to form a Ge–C bond.
356.6°), and the BN-distances [1.424(3), 1.434(3), 1.443(3) Å] are comparable with BN3 motifs, which exhibit delocalization of the electron pairs at nitrogen into the empty p-orbital at the boron atom.53–56 The found Ge–N bond lengths [1.8527(17), 1.8715(18) Å] are similar to single bonds between these elements.57 The structure of the 1,3,2-diazaborepin moiety in 4 can be compared with the diazaborepin structures published by Martin et al.52 Interestingly, Martin did not observe an NN bond cleavage in the reaction of unsubstituted pyridazine with pentaphenylborol, even at elevated temperatures. In a presumed mechanistic sequence for the formation of 4 (see ESI†) an adduct between the pyridazine nitrogen atoms and the germanium atoms could be the first step. Due to Ge–N adduct formation the borylene electron pair should be localized to a higher degree at the boron atom resulting in an increase of reactivity at boron and formation of a diazaboriridine.58 The chloride atom at the boron atom is substituted by a second equivalent of pyridazine followed by chloride germanium bond formation. The N–N bond opens, and the other germanium atom reacts as a nucleophile with the carbon atom of the coordinated, neighbouring pyridazine to form a Ge–C bond.
2,2′-Bipyridine shows a redox reaction with low valent boron compounds like borylene transition metal complexes and abstracts an RB unit under formation of BN bonds.59 To abstract the BCl unit from boradigermaallyl 1 the reaction with 2,2′-bipyridine was investigated. After 2 days stirring at room temperature and filtration red to purple-coloured crystals were obtained from hexane (Scheme 1). In contrast to the borylene abstraction from metal borylene complexes we observed in reaction of the allyl-compound 1 with 2,2′-bipyridine an insertion of the BCl unit into a C–N bond and a hitherto unknown redox reaction of the bipyridine with a diarylgermylene moiety. Interestingly, the bis(germylene), boradigermaallyl 1 without BCl unit, does not react with 2,2′-bipy. The bipyridine is reduced to the dianion and exhibits two Ge–N bonds [1.8455(16), 1.8721(18) Å] with distances lying in the range of anionic amide coordination at germanium.57,60 Longer Ge–N bond lengths were observed for bipyridine coordination at GeCl2 [2.0738(16), 2.0637(15) Å].61 The bond length between the two carbon atoms C5–C6, connecting the two pyridine units, of 1.361(3) Å in 5 is shorter than the corresponding C–C bond in [bipyGeCl2] [1.474(3) Å] and comparable with the reduced bipy in DurB(bipy) [1.387(3) Å] (Dur = 2,3,5,6-tetramethylphenyl).59,61 The B–N interatomic distance of 1.402(3) Å in 5 is comparable with compounds exhibiting BN moieties conjugated with unsaturated organic units.62 The 1H NMR signals of the six membered C5N cycle were found in the range of 4.29–5.59 ppm, and are thus at considerably lower frequencies than those observed for the 1H NMR signals of [bipyGeCl2] (7.51–8.84 ppm).61 The signal in the 11B NMR for compound 5 was found at 40.9 ppm and is similar to the resonance observed for PhBCl[N(H)Dipp] at 40 ppm.63
In conclusion, as a highly reactive compound boradigermaallyl shows insertion reactions with thiophene, furan, pyridazine and 2,2′-bipyridine. The low valent germylene units participate in the reactions, leading to the formation of products of an oxidative addition of a BCl and B–O unit, as well as a (1+4) cycloaddition with an organic diene. Pyridazine adds a boron atom between the two nitrogen atoms to yield a seven membered ring compound. 2,2′-Bipyridine does not abstract a BCl unit into the NN-pocket. Instead bipy inserts a BCl unit into a CN-bond and furthermore shows a previously unknown redox reaction with a diarylgermylene to give a Ge(IV)diamide and a reduced bipy derivative. With this exciting reactivity study of boradigermaallyl towards heterocycles we are looking forward to experiments with further groups of compounds.
R. H. K. experiments, writing of manuscript and ESI,† P. L. S. synthesis of 2, H. S. crystallographic investigations, L. W. supervision, funding acquisition, manuscript writing and review.
L. W. thanks the German research foundation (DFG) for a grant (WE 1876/16-1).
Data availability
Full experimental details are provided as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- B. Xu, H. Beckers, H. Ye, Y. Lu, J. Cheng, X. Wang and S. Riedel, Angew. Chem., Int. Ed., 2021, 60, 17205–17210 Search PubMed.
- H. F. Bettinger, J. Am. Chem. Soc., 2006, 128, 2534–2535 CrossRef CAS PubMed.
- P. L. Timms, Acc. Chem. Res., 1973, 6, 118–123 Search PubMed.
- R. W. Kirk and P. L. Timms, J. Am. Chem. Soc., 1969, 91, 6315–6318 CrossRef CAS.
- P. L. Timms, J. Am. Chem. Soc., 1968, 90, 4585–4589 CrossRef CAS.
- P. L. Timms, J. Am. Chem. Soc., 1967, 89, 1629–1632 CrossRef CAS.
- J. A. J. Pardoe, N. C. Norman and P. L. Timms, Polyhedron, 2002, 21, 543–548 CrossRef CAS.
- C. R. Brazier, J. Mol. Spectrosc., 1996, 177, 90–105 CrossRef CAS.
- R. Kinjo, B. Donnadieu, M. A. Celik, G. Frenking and G. Bertrand, Science, 2011, 333, 610–613 CrossRef CAS PubMed.
- H. Braunschweig, R. D. Dewhurst, F. Hupp, M. Nutz, K. Radacki, C. W. Tate, A. Vargas and Q. Ye, Nature, 2015, 522, 327–330 CrossRef CAS PubMed.
- L. Kong, Y. Li, R. Ganguly, D. Vidovic and R. Kinjo, Angew. Chem., Int. Ed., 2014, 53, 9280–9283 CrossRef CAS PubMed.
- H. Wang, L. Wu, Z. Lin and Z. Xie, Angew. Chem., Int. Ed., 2018, 57, 8708–8713 CrossRef CAS PubMed.
- H. Wang, L. Wu, Z. Lin and Z. Xie, J. Am. Chem. Soc., 2017, 139, 13680–13683 CrossRef CAS PubMed.
- B. Pachaly and R. West, Angew. Chem., Int. Ed. Engl., 1984, 23, 454–455 CrossRef.
- M. Ito, N. Tokitoh, T. Kawashima and R. Okazaki, Tetrahedron Lett., 1999, 40, 5557–5560 CrossRef CAS.
- A. Meller, U. Seebold, W. Maringgele, M. Noltemeyer and G. M. Sheldrick, J. Am. Chem. Soc., 1989, 111, 8299–8300 CrossRef CAS.
- P. Bissinger, H. Braunschweig, K. Kraft and T. Kupfer, Angew. Chem., Int. Ed., 2011, 50, 4704–4707 CrossRef CAS PubMed.
- W. J. Grigsby and P. P. Power, J. Am. Chem. Soc., 1996, 118, 7981–7988 CrossRef CAS.
- F. Dahcheh, D. Martin, D. W. Stephan and G. Bertrand, Angew. Chem., Int. Ed., 2014, 53, 13159–13163 CrossRef CAS PubMed.
- A. D. Ledet and T. W. Hudnall, Dalton Trans., 2016, 45, 9820–9826 RSC.
- H. Braunschweig, C. Kollann and D. Rais, Angew. Chem., Int. Ed., 2006, 45, 5254–5274 CrossRef CAS PubMed.
- H. Braunschweig, R. D. Dewhurst and A. Schneider, Chem. Rev., 2010, 110, 3924–3957 CrossRef CAS PubMed.
- H. Braunschweig, R. D. Dewhurst and V. H. Gessner, Chem. Soc. Rev., 2013, 42, 3197–3208 RSC.
- J. T. Goettel and H. Braunschweig, Coord. Chem. Rev., 2019, 380, 184–200 CrossRef CAS.
- J. Schneider, K. M. Krebs, S. Freitag, K. Eichele, H. Schubert and L. Wesemann, Chem. – Eur. J., 2016, 22, 9812–9826 CrossRef CAS PubMed.
- J. Henning and L. Wesemann, Angew. Chem., Int. Ed., 2012, 51, 12869–12873 CrossRef CAS PubMed.
- J. Henoch, A. Auch, F. Diab, K. Eichele, H. Schubert, P. Sirsch, T. Block, R. Pöttgen and L. Wesemann, Inorg. Chem., 2018, 57, 4135–4145 CrossRef CAS PubMed.
- R. H. Kern, M. Schneider, K. Eichele, H. Schubert, H. F. Bettinger and L. Wesemann, Angew. Chem., Int. Ed., 2023, 62, e202301593 CrossRef CAS PubMed.
- D. Raiser, C. P. Sindlinger, H. Schubert and L. Wesemann, Angew. Chem., Int. Ed., 2020, 59, 3151–3155 CrossRef CAS PubMed.
- M. Zweigart, K. Eichele, H. Schubert, C. P. Sindlinger and L. Wesemann, J. Am. Chem. Soc., 2023, 145, 12452–12458 CrossRef CAS PubMed.
- S. K. Mellerup, C. Li, J. Radtke, X. Wang, Q.-S. Li and S. Wang, Angew. Chem., Int. Ed., 2018, 57, 9634–9639 CrossRef CAS PubMed.
- A. D. Rohr, M. M. Banaszak Holl, J. W. Kampf and A. J. AsheIII, Organometallics, 2011, 30, 3698–3700 CrossRef CAS.
- S. Yruegas and C. D. Martin, Chem. – Eur. J., 2016, 22, 18358–18361 CrossRef CAS PubMed.
- X. Su, J. J. Baker and C. D. Martin, Chem. Sci., 2020, 11, 126–131 RSC.
- T. Bischof, N. Wieprecht, S. Fuchs, L. Endres, I. Krummenacher, M. Michel, C. Mihm, H. Braunschweig and M. Finze, Inorg. Chem., 2023, 62, 21329–21335 CrossRef CAS PubMed.
- J. S. McMullen, A. J. P. White and M. R. Crimmin, Chem. Commun., 2023, 59, 14681–14684 RSC.
- L. Zhang, S. Kaukver, J. McMullen, A. J. P. White and M. R. Crimmin, Organometallics, 2023, 42, 1711–1716 CrossRef CAS.
- M. T. Ashby and N. A. Sheshtawy, Organometallics, 1994, 13, 236–243 CrossRef CAS.
- L. Menduti, C. Baldoli, S. Manetto, M. Bolte, H.-W. Lerner, G. Longhi, C. Villani, E. Licandro and M. Wagner, Angew. Chem., Int. Ed., 2023, 62, e202215468 CrossRef CAS PubMed.
- J. Chen, Z. Bajko, J. W. Kampf and A. J. Ashe, Organometallics, 2007, 26, 1563–1564 CrossRef CAS.
- S. Yruegas, D. C. Patterson and C. D. Martin, Chem. Commun., 2016, 52, 6658–6661 RSC.
- K. Huang and C. D. Martin, Inorg. Chem., 2015, 54, 1869–1875 CrossRef CAS PubMed.
- K. R. Bluer, L. E. Laperriere, A. Pujol, S. Yruegas, V. A. K. Adiraju and C. D. Martin, Organometallics, 2018, 37, 2917–2927 CrossRef CAS.
- T. Bischof, L. Beßler, I. Krummenacher, L. Erhard, H. Braunschweig and M. Finze, Chem. – Eur. J., 2023, 29, e202300210 CrossRef CAS PubMed.
- Y. Wei, J. Wang, W. Yang, Z. Lin and Q. Ye, Chem. – Eur. J., 2023, 29, e202203265 CrossRef CAS PubMed.
- P. Kitschke, A. A. Auer, A. Seifert, T. Rüffer, H. Lang and M. Mehring, Inorg. Chim. Acta, 2014, 409, 472–478 CrossRef CAS.
- A. Lorbach, M. Bolte, H.-W. Lerner and M. Wagner, Chem. Commun., 2010, 46, 3592–3594 RSC.
- Z. Lu, H. Quanz, O. Burghaus, J. Hofmann, C. Logemann, S. Beeck, P. R. Schreiner and H. A. Wegner, J. Am. Chem. Soc., 2017, 139, 18488–18491 CrossRef CAS PubMed.
- J. Ruhl, N. Oberhof, A. Dreuw and H. A. Wegner, Angew. Chem., Int. Ed., 2023, 62, e202300785 CrossRef CAS PubMed.
- Y. Katsuma, H. Asakawa and M. Yamashita, Chem. Sci., 2018, 9, 1301–1310 RSC.
- Y. Katsuma, L. Wu, Z. Lin, S. Akiyama and M. Yamashita, Angew. Chem., Int. Ed., 2019, 58, 317–321 CrossRef CAS PubMed.
- V. A. K. Adiraju and C. D. Martin, Chem. – Eur. J., 2017, 23, 11437–11444 CrossRef CAS PubMed.
- G. J. Bullen, Dalton Trans., 1981, 511–514 RSC.
- K. Huang, J. L. Dutton and C. D. Martin, Chem. – Eur. J., 2017, 23, 10532–10535 CrossRef CAS PubMed.
- M. A. Rodriguez and T. T. Borek, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, o3341–o3343 CrossRef CAS.
- K. Lee, C. M. Donahue and S. R. Daly, Dalton Trans., 2017, 46, 9394–9406 RSC.
- M. J. Evans, F. M. Burke, P. M. Chapple and J. R. Fulton, Inorg. Chem., 2021, 60, 8293–8303 CrossRef CAS PubMed.
- R. Boese and U. Klingebiel, J. Organomet. Chem., 1986, 306, 295–302 CrossRef CAS.
- S. Liu, M.-A. Légaré, J. Seufert, D. Prieschl, A. Rempel, L. Englert, T. Dellermann, V. Paprocki, A. Stoy and H. Braunschweig, Inorg. Chem., 2020, 59, 10866–10873 CrossRef CAS PubMed.
- M. F. Lappert, M. J. Slade, J. L. Atwood and M. J. Zaworotko, Chem. Commun., 1980, 621–622 Search PubMed.
- F. Cheng, J. M. Dyke, F. Ferrante, A. L. Hector, W. Levason, G. Reid, M. Webster and W. Zhang, Dalton Trans., 2010, 39, 847–856 RSC.
- M. Franceschini, M. Crosta, R. R. Ferreira, D. Poletto, N. Demitri, J. P. Zobel, L. González and D. Bonifazi, J. Am. Chem. Soc., 2022, 144, 21470–21484 Search PubMed.
- T. Chivers, C. Fedorchuk and M. Parvez, Inorg. Chem., 2004, 43, 2643–2653 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2420519–2420522. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc00639b |
| This journal is © The Royal Society of Chemistry 2025 |

