Open Access Article
Open Access ArticleEfficient circularly polarized multiple resonance thermally activated delayed fluorescence from B,N-embedded hetero[8]helicene enantiomers†
Tingting Huang‡
a,
Li Yuan‡c,
Xueying Lua,
Yupei Qua,
Cheng Qu a,
Yincai Xu
a,
Yincai Xu *a,
You-Xuan Zheng
*a,
You-Xuan Zheng *c and
Yue Wang
*c and
Yue Wang *ab
*ab
aState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: ycxu17@mails.jlu.edu.cn; yuewang@jlu.edu.cn
bJihua Laboratory, 28 Huandao Nan Road, Foshan, 528200, Guangdong Province, P. R. China
cState Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, P. R. China. E-mail: yxzheng@nju.edu.cn
First published on 28th August 2024
Abstract
Helicene-based circularly polarized multiple resonance thermally activated delayed fluorescence (CP-MR-TADF) materials are promising for ultra-high-definition and 3D displays, but most of them encounter potential problems such as easy racemization during the thermal deposition process, low luminous efficiency, and low luminescence dissymmetry factor (glum), making the development of efficient circularly polarized organic light-emitting diodes (CP-OLEDs) a significant challenge. Here, we report a pair of CP-MR-TADF enantiomers with high-order B,N-embedded hetero[8]helicene, (P/M)-BN-TP-ICz, by fusing two MR chromophores, DtCzB and indolo[3,2,1-jk]carbazole (ICz). BN-TP-ICz exhibits green emission in toluene with a peak of 531 nm and a full-width at half-maximum (FWHM) of 36 nm. The optimized CP-OLEDs with enantiomers (P/M)-BN-TP-ICz exhibit green emission with peaks of 540 nm, FWHMs of 38 nm and Commission Internationale de L'Eclairage coordinates of (0.33, 0.65). Moreover, they showcase maximum external quantum efficiencies (EQEs) of 32.0%, with gELs of +6.49 × 10−4 and −7.74 × 10−4 for devices based on (P)-BN-TP-ICz- and (M)-BN-TP-ICz, respectively.
Introduction
Chirality is a ubiquitous phenomenon in nature, a fundamental property of chemical substances.1 In the fields of chemistry and materials research, chiral small molecules–a long-standing sustainable development interest and subject–are deeply fascinating to scientists.2 Chiral molecules can serve as important mediators for chirality, and its constituent factors mainly include point chirality, axial chirality, planar chirality, and helical chirality.3 Helicenes, as the most representative molecules of helical chirality, are essentially polycyclic aromatic hydrocarbons (PAHs) formed with ortho-fused aromatic/hetero rings.4 The large steric hindrance between their “head” and “tail” compels them to adopt a helical three-dimensional topology, thereby inducing the generation of helical chirality.5 The intrinsic helical chirality and unique three-dimensional π-conjugated structure of molecules endow them with excellent circular dichroism (CD) and circularly polarized luminescence (CPL) characteristics.6 Recently, helical chiral materials have demonstrated extraordinary research value and broad application prospects in the realm of optoelectronics, especially in circularly polarized organic light-emitting diodes (CP-OLEDs).7With the increasing diversification of lifestyle and the continuous evolution of display technology, the development of ultra-high-definition (UHD) displays with a wide color gamut has become the focus of cutting-edge OLED displays.8 Therefore, integrating CPL with high-color-purity multiple resonance thermally activated delayed fluorescence (MR-TADF) materials has become a prevalent trend to create CP-MR-TADF emitters with multiple figures-of-merit.9 CP-MR-TADF materials can directly emit CPL, avoiding energy loss caused by the need for circular polarizers and quarter-wave plates, and they can reduce glare and enhance contrast, clarity, and expressiveness of images in practical applications. Furthermore, they address the issue of low color purity encountered with traditional CPL materials.10 Currently, the actively researched helicene-type CP-MR-TADF materials are mainly focused on [6]helicene or even lower-order helicenes.11 Higher-order helicenes can easily overcome the problem of the low energy barrier of racemization, thereby avoiding the risk of configuration instability during vacuum thermal deposition and significantly improving repeatability.12 Additionally, the helical chiral π-conjugated system can participate in the distribution of frontier molecular orbitals (FMOs) of molecules, favoring π-electron flow, which facilitates enhancement of optical rotation and luminescence dissymmetry factor (glum).13
The general approach for constructing high-order helicenes is to increase the number of benzene rings in the helicene,14 which not only poses challenges to molecular synthetic methodology but also usually accelerates the intersystem crossing (ISC) process from the lowest singlet (S1) to the lowest triplet (T1) excited state, leading to the accumulation of non-radiative triplet exciton transition and a significant reduction in photoluminescence quantum yields (ΦPLs). Additionally, the extended persistent π-conjugated structure may destroy the alternating distribution pattern of FMOs and lead to an unpredictable broadening of the emission spectrum or even an uncontrollable loss of TADF characteristics.15 Therefore, it is an ongoing pursuit to develop CP-MR-TADF materials with narrow full-width at half-maximum (FWHM), high ΦPL, decent glum, and high energy barrier of racemization from large-sized higher-order helicenes.16
Our group has implemented the edge-topology molecular-engineering strategy to expand the sub-resonance skeleton of MR molecules and successfully developed an efficient helicene-type CP-MR-TADF emitter BN-Py.17 This work provides a molecular construction template and synthetic methodology for the subsequent development of helical chiral CP-MR-TADF materials. In this contribution, following the well-defined paradigm, we initially made a tentative work to introduce a pure carbon PAH into the star MR-TADF molecule DtCzB,18 and constructed a hetero[7]helicene-type molecule, BN-DBC, with a moderate energy barrier of racemization (Scheme 1). It exhibits yellow emission with a peak of 548 nm, a broad FWHM of 44 nm, and a low ΦPL of 55% in toluene solution. Unfortunately, it lacks TADF characteristics, limiting its electroluminescence (EL) performance in devices. Subsequently, we further stitched a unique MR chromophore with a rigid planar structure independent of the B,N-containing MR framework, indolo[3,2,1-jk]carbazole (ICz), with the DtCzB fragment to develop a CP-MR-TADF molecule with high-order B,N-embedded hetero[8]helicene, BN-TP-ICz, with a high energy barrier of racemization. It displays vivid green emission with a peak of 531 nm, a narrow FWHM of 36 nm, and a high ΦPL of 93% in toluene solution. The optimized CP-OLEDs with enantiomers (P/M)-BN-TP-ICz exhibit green emission with peaks of 540 nm, FWHMs of 38 nm and Commission Internationale de L'Eclairage (CIE) coordinates of (0.33, 0.65). Moreover, they showcase maximum external quantum efficiencies (EQEs) of 32.0%, with electroluminescence dissymmetry factors (gELs) of +6.49 × 10−4 and −7.74 × 10−4 for devices based on (P)-BN-TP-ICz and (M)-BN-TP-ICz, respectively. The successful construction of BN-TP-ICz effectively provides a new perspective for the future research on high-order helicene-type CP-MR-TADF materials.
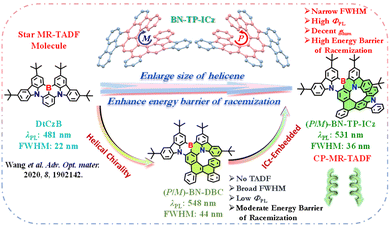 | ||
| Scheme 1 Schematic diagram of molecular structure evolution of chiral hetero[n]helicene based on the parent molecule DtCzB and the corresponding photophysical properties. | ||
Results and discussion
Synthesis and characterization
Both BN-DBC and BN-TP-ICz were prepared from the substrate DtCzB via the classic synthetic trilogy (Scheme S1†). DtCzB moiety was readily converted to DtCzB-Bpin through Miyaura borylation,19 which then underwent Suzuki coupling with commercially available 9-(2-bromophenyl)fluorene and lab-synthesized 2-(2-iodophenyl)indolo[3,2,1-jk]carbazole (IP-ICz) (derived from 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)indolo[3,2,1-jk]carbazole and 1-bromo-2-iodobenzene) to give the key precursors BN-PPhen and BN-P-ICz. Finally, hetero[7]helicene-embedded BN-DBC and hetero[8]helicene-embedded BN-TP-ICz were developed through intramolecular Scholl oxidative coupling. The main disparity in the synthetic procedures of these two target molecules lies in the oxidants adopted in the cyclization step, with the former employing iron(III) chloride, while the latter being induced by [bis(trifluoroacetoxy)iodo]benzene and boron trifluoride etherate. The molecular structures of all intermediates and target compounds were comprehensively characterized using NMR, mass spectrometry, and elemental analysis (Fig. S1–S7†). In addition, BN-TP-ICz exhibits outstanding thermal stability with a high decomposition temperature (Td, corresponding to 5% weight loss) of 502 °C (Fig. S8†), which is advantageous for its application in vacuum thermal deposition OLEDs.For comparison, the FMO distribution of BN-DBC was calculated (Fig. S9†), and its fundamental photophysical properties were characterized (Fig. S10, S11 and Table S1†). Compound BN-DBC in toluene solution (1 × 10−5 M) and PMMA (polymethyl methacrylate) film (1 wt% doping concentration) exhibits yellow emission with peaks of 548 and 553 nm, broad FWHMs of 44 and 50 nm, and low ΦPLs of 55% and 60%, respectively. Furthermore, the lowest-excited singlet–triplet energy splittings (ΔESTs) were estimated to be 0.32 and 0.31 eV, respectively, which implies that BN-DBC likely does not exhibit TADF properties. The triplet spin density distribution (TSDD) is primarily on the dibenzo[g,p]chrysene group, which may reveal a substantial decrease in T1 energy (Fig. S12†). Further analysis of the fluorescence lifetimes in toluene solution and PMMA doped film show mono-exponential transient photoluminescence (PL) decay with only nanosecond-scale prompt fluorescence detected (Fig. S13†). The combined theoretical calculations and experimental results indicate the absence of TADF properties in BN-DBC, which is therefore only used as a reference molecule and the properties are not further explored in this work. The associated experimental results of BN-DBC demonstrate that it would be challenging to achieve the desired goals only by embedding a large-sized pure carbon PAH helicene into the MR-TADF molecule DtCzB.
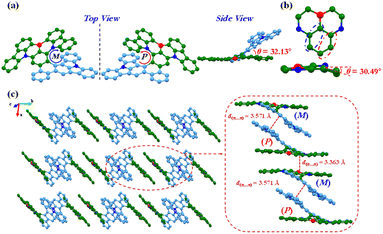
A single crystal of racemic BN-TP-ICz was acquired via vacuum vapor-phase deposition (Fig. S14, S15 and Table S2†). Single-crystal X-ray crystallographic analysis reveals that the crystal BN-TP-ICz belongs to the triclinic crystal system and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, and the π-conjugated framework consists of 54 sp2 hybridized carbon atoms, 1 coordinated boron atom and 3 embedded nitrogen atoms (C54BN3), exhibiting an unequivocal non-planar hetero[8]helicene structure (Fig. 1a). Viewed from the side, the dihedral angle between the planes containing the DtCzB moiety and ICz moiety is 32.13°, giving BN-TP-ICz a highly folded and distorted conformation and exhibiting an angular two-dimensional topology. The bulky steric hindrance between ICz and DtCzB pulls and deforms the N–B–N-substituted central phenyl ring in DtCzB, forming a torsion angle of 30.49° (Fig. 1b). In the three-dimensional stacking structure of the crystal, BN-TP-ICz adopts a regular and dense linear strand arrangement along the b-axis, with two adjacent molecules of (P)- and (M)-enantiomers appearing in pairs, placed in an inverted manner (Fig. 1c). As a result, there are abundant π⋯π interactions in the crystal, including the π⋯π interactions between ICz planes of the paired molecules (P)-BN-TP-ICz and (M)-BN-TP-ICz, and the π⋯π interactions between DtCzB planes of molecule (P)-BN-TP-ICz and molecule (M)-BN-TP-ICz of the neighboring pair, with the distances of 3.571 and 3.363 Å, respectively.
space group, and the π-conjugated framework consists of 54 sp2 hybridized carbon atoms, 1 coordinated boron atom and 3 embedded nitrogen atoms (C54BN3), exhibiting an unequivocal non-planar hetero[8]helicene structure (Fig. 1a). Viewed from the side, the dihedral angle between the planes containing the DtCzB moiety and ICz moiety is 32.13°, giving BN-TP-ICz a highly folded and distorted conformation and exhibiting an angular two-dimensional topology. The bulky steric hindrance between ICz and DtCzB pulls and deforms the N–B–N-substituted central phenyl ring in DtCzB, forming a torsion angle of 30.49° (Fig. 1b). In the three-dimensional stacking structure of the crystal, BN-TP-ICz adopts a regular and dense linear strand arrangement along the b-axis, with two adjacent molecules of (P)- and (M)-enantiomers appearing in pairs, placed in an inverted manner (Fig. 1c). As a result, there are abundant π⋯π interactions in the crystal, including the π⋯π interactions between ICz planes of the paired molecules (P)-BN-TP-ICz and (M)-BN-TP-ICz, and the π⋯π interactions between DtCzB planes of molecule (P)-BN-TP-ICz and molecule (M)-BN-TP-ICz of the neighboring pair, with the distances of 3.571 and 3.363 Å, respectively.
Computational simulations
To gain an in-depth understanding of the geometrical structure and electronic characteristics of BN-TP-ICz, and considering that enantiomers have almost identical attributes except for chiroptical properties, (P)-BN-TP-ICz was selected for density functional theory (DFT) and time-dependent DFT (TDDFT) calculations. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) distribution, HOMO–LUMO energy level gap (Egap), and oscillator strength (f) of (P)-BN-TP-ICz are illustrated in Fig. 2a. In the optimized ground (S0) state, (P)-BN-TP-ICz shows a highly distorted molecular conformation derived from the congested steric hindrance between DtCzB and ICz moieties. Due to the inherent MR characteristics of DtCzB and ICz, (P)-BN-TP-ICz generally retains the typical characteristics of the MR framework, with an alternating HOMO/LUMO distribution. The HOMO is predominantly localized on the nitrogen atoms and ortho/para-positions of the carbon atoms connected to nitrogen atoms in DtCzB moiety, while the LUMO is concentrated on the boron atom and ortho/para-positions of the carbon atoms connected to the boron atom in the DtCzB moiety, inducing a short-range charge transfer (SRCT) effect. In addition, the HOMO is partially extended to the ICz moiety, and the LUMO is partially diffused into the benzene ring connected to the para-carbon position of the B-substituted phenyl ring, inducing a long-range charge transfer (LRCT) effect.20 This amalgamation of SRCT and LRCT states in (P)-BN-TP-ICz is not only anticipated to facilitate a bathochromic shift in the spectrum but also preserve the narrowband emission. The π-electron properties of (P)-BN-TP-ICz were analyzed with the localized orbital locator (LOL) function (Fig. S16†).21 The results show that π-electrons in all-carbon benzene rings exhibit more pronounced six-center and six-electron delocalization than other rings, indicating a decent π-conjugation degree; in heterocycles containing boron or nitrogen atoms, π-electron flow is suppressed to some extent. Therefore, it can be concluded that the incorporation of heteroatoms significantly influences the molecular orbital distribution of the entire molecule and induces FMO separation. Furthermore, the hole–electron analysis map delineates well-separated holes and electrons in (P)-BN-TP-ICz (Fig. S17†).22 The alternating distribution pattern of holes and electrons within the DtCzB fragment aligns with the alternating FMO distribution, indicating SRCT excitation from S0 to S1 state. In addition, the hole is also propagated into the ICz fragment, and the electron is also spread into the benzene ring connected to the para-carbon position of the B-substituted phenyl ring, facilitating LRCT excitation from S0 to S1 state.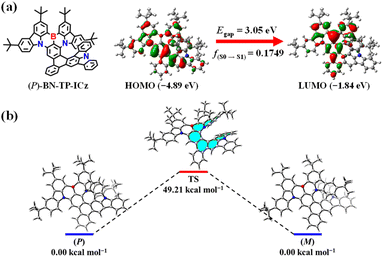 | ||
| Fig. 2 (a) HOMO and LUMO distribution, HOMO–LUMO energy level gap, and oscillator strength of (P)-BN-TP-ICz. (b) Energy barrier of the racemization of enantiomers (P/M)-BN-TP-ICz. | ||
Configurational stability is the paramount stereochemical feature of helicenes and their derivatives, which will directly impact their applications in chiroptics and optoelectronic devices. Therefore, we calculated the energy barrier of racemization of BN-TP-ICz (Fig. 2b).23 Analysis of the transition states of (P/M)-BN-TP-ICz reveals an energy barrier of racemization up to 49.21 kcal mol−1, which is higher than that of (P/M)-BN-DBC (37.25 kcal mol−1, Fig. S18†), and is adequate to support chiral resolution and further research on chiroptical performances under thermal evaporation. Chiral resolution of BN-TP-ICz was favorably achieved, and a pair of enantiomers (P/M)-BN-TP-ICz with high optical purity were obtained (enantiomeric excess (ee) value >99.0%, Fig. S19†). The formation of the high energy barrier of racemization can be further elucidated by analyzing the intramolecular non-covalent interactions (NCIs) within (P)-BN-TP-ICz as a function of reduced density gradient (RDG) (Fig. S20†).24 It is visually visible from the RDG isosurface that these NCIs are mainly concentrated in the spatial overlap region of head-to-tail rings of the hetero[8]helicene, demonstrating a robust steric hindrance effect that stems from the highly distorted and folded conformation of (P)-BN-TP-ICz. Consequently, during the configurational transition of (P/M)-BN-TP-ICz, overcoming these crowded steric hindrances becomes crucial, contributing to the high energy barrier of racemization of BN-TP-ICz.
Photophysical properties
Preliminary photophysical properties of BN-TP-ICz were characterized in toluene solution (1 × 10−5 M). Racemic BN-TP-ICz exhibits intense ultraviolet-visible (UV-vis) absorption peaking at 510 nm, attributed to a strong intramolecular charge transfer (ICT) transition excited state. It displays vibrant green fluorescence emission with a peak of 531 nm, a narrow FWHM of 36 nm, and CIE coordinates of (0.29, 0.66) (Fig. 3a and Table 1). The well-distinguishable mirror-symmetrical relationship between the absorption and emission spectra of BN-TP-ICz, along with a small Stokes shift of only 21 nm, indicates minimal geometric changes between S0 and S1 states. The fusion of DtCzB and ICz provides the molecular composite with a rigid framework, as evidenced by its root-mean-square deviation (RMSD) of 0.1507 Å (Fig. S21†) and low recombination energy of 0.22 eV (Fig. S22†). Furthermore, the rigid structure and MR effect endow the BN-TP-ICz molecule with a high ΦPL of 93%. Utilizing fluorescence and phosphorescence spectra at 77 K (Fig. S23†), the ΔEST of BN-TP-ICz was estimated to be 0.19 eV, which is sufficient to provide a channel for the reverse intersystem crossing (RISC) process of triplet excitons and promote the formation of TADF. The HOMO and LUMO energy levels of BN-TP-ICz were estimated to be −5.06 and −2.69 eV, respectively (Fig. S24†).| Compound | λabsa [nm] | λemb [nm] | FWHMc [nm] | ES1d [eV] | ET1e [eV] | ΔESTf [eV] | Egg [eV] | HOMOh [eV] | LUMOh [eV] | ϕPLi [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| a Peak wavelength of the lowest energy absorption band.b Peak wavelength of the PL spectrum in toluene solution (1 × 10−5 M, 298 K).c Full-width at half-maximum.d Singlet energy estimated from the onset of the fluorescence spectrum in toluene solution (1 × 10−5 M, 77 K).e Triplet energy estimated from the onset of the phosphorescence spectrum in a frozen toluene matrix (1 × 10−5 M, 77 K).f ΔEST = ES1 − ET1.g Optical band gap estimated from the absorption edge of the UV-vis spectrum.h Determined from cyclic voltammetry using the formula: EHOMO = −(Eox + 4.8) eV and ELUMO = −(Ered + 4.8) eV.i Absolute photoluminescence quantum yield measured with an integrating sphere in N2-bubbling toluene solution (1 × 10−5 M, 298 K). | ||||||||||
| BN-TP-ICz | 510 | 531 | 36 | 2.44 | 2.25 | 0.19 | 2.32 | −5.06 | −2.69 | 93 |
Subsequently, the solid-state thin film of BN-TP-ICz doped in 9-(2-(9-phenyl-9H-carbazol-3-yl)phenyl)-9H-3,9′-bicarbazole (PhCzBCz) with 5 wt% doping concentration was prepared via vacuum thermal evaporation for further investigation of photophysical properties. The doped film of BN-TP-ICz emits green light with a peak of 540 nm and a FWHM of 43 nm (Fig. S25†). The bi-exponential transient PL decay curve, composed of nanosecond and microsecond components, observed at room temperature and under a vacuum atmosphere (Fig. S26a†), exhibits typical TADF features of BN-TP-ICz. It demonstrates prompt fluorescence decay with a lifetime (τp) of 5.8 ns and delayed fluorescence decay with a lifetime (τd) of 138.5 μs. Moreover, it is observed from the temperature-dependent transient PL decay curves that both the intensity and proportion of the delayed fluorescence component significantly increase as the temperature increases from 80 to 320 K (Fig. S26b†), intuitively substantiating TADF properties. Derived from the photophysical data of the doped film, the calculated rate constants show that the rate constant of prompt fluorescence (kF) of BN-TP-ICz is 13.2 × 107 s−1, significantly higher than its rate constant of internal conversion (kIC) of 1.15 × 107 s−1, indicating that the non-radiative energy loss is small; the corresponding rate constant of RISC (kRISC) is 0.61 × 104 s−1 (Table S3†). These characteristics are consistent with the general photophysical behavior of MR-TADF materials.
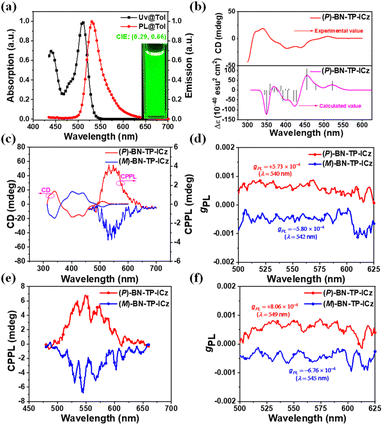
To assess the chiroptical properties of enantiomers (P/M)-BN-TP-ICz in S0 and S1 states, their CD and CPL spectra were studied. The absolute configurations of the two components separated by chiral high performance liquid chromatography (HPLC) were confirmed by comparing the experimentally tested and theoretically simulated CD spectra of (P)-BN-TP-ICz (Fig. 3b),25 identifying the first and second fractions as (M)-BN-TP-ICz and (P)-BN-TP-ICz, respectively. The enantiomers (P/M)-BN-TP-ICz exhibit distinct mirror symmetry in both CD and circularly polarized photoluminescence (CPPL) spectra in toluene solution (Fig. 3c). In the higher energy absorption bands at 313–362 and 362–489 nm, the CD spectra display intense Cotton effects, which correspond to the intrinsic localized state absorption of B,N-embedded hetero[8]helicene. In the lower energy region between 489 and 541 nm, the weak Cotton effect originates from the chirality transfer from the B,N-embedded hetero[8]helicene to the chromophore DtCzB. The photoluminescence dissymmetry factors (gPLs) of (P)-BN-TP-ICz and (M)-BN-TP-ICz in toluene solution are +5.73 × 10−4 and −5.80 × 10−4, respectively (Fig. 3d). To date, helicene-based CPL emitters have attracted widespread attention. CPL brightness (BCPL), as a comprehensive indicator parameter of CPL performance, can easily compare the CPL activity of different compounds. Here, we calculated the BCPL of (P)-BN-TP-ICz and compared it with the representative single-helicene-based CPL emitters reported previously to fully grasp the overall research progress in this field (Table S6†).26 The results show that (P)-BN-TP-ICz exhibits decent CPL performance. The CPPL spectra of enantiomers (P/M)-BN-TP-ICz in the thermally deposited doped film (temperature: <160 °C, pressure: <5 × 10−4 Pa, thickness: 100 nm) also showcase good mirror symmetry, with gPLs of +8.06 × 10−4 and −6.76 × 10−4, respectively (Fig. 3e and f). Next, the optical purity of the residual enantiomers (P/M)-BN-TP-ICz samples after thermal deposition was further examined. Chiral HPLC analysis reveals that the ee values (>99.0%) are essentially consistent with those of the samples without thermal deposition treatment (Fig. S27†), indicating no racemization during the vacuum thermal deposition process and excellent configurational stability. The above results experimentally validate the rationality of incorporating ICz into DtCzB to form the BN-TP-ICz molecule with B,N-embedded hetero[8]helicene to construct CP-MR-TADF materials, laying a solid foundation for further research on CP-OLEDs.
Device performances
The CP-OLEDs were fabricated via vacuum thermal deposition with the configuration of [ITO/TAPC (50 nm)/TCTA (5 nm)/PhCzBCz: x wt% (P/M)-BN-TP-ICz (30 nm)/TmPyPB (30 nm)/LiF (1 nm)/Al (100 nm) (x = 3, 5, 8)] (Fig. S28†). In this setup, Al and ITO (indium tin oxide) were used as the cathode and anode, respectively. To balance hole and electron mobility, TAPC (1,1-bis[(di-4-tolylamino)phenyl]cyclohexane) and TmPyPB (3,3′-[5′'-[3-(3-pridinyl)phenyl][1,1′:3′,1′′-terphenyl]-3,3′′-diyl]bispyridine) with excellent charge carrier transport properties were employed as the hole transporting layer (HTL) and electron transporting layer (ETL), respectively, while TCTA (tris(4-carbazolyl-9-ylphenyl)amine) acted as the electron blocking layer (EBL) to confine the exciton recombination zone. Additionally, PhCzBCz with a highly twisted configuration and high triplet energy (ET1 = 2.96 eV) was identified as the host of the emitting layer (EML) to suppress intermolecular stacking quenching and ensure efficient energy transfer.27 Fig. 4, S29† and Table 2 summarize a range of curves and parameters of devices, including EL spectra, EQE–luminance (L) curves, as well as curves depicting the correlation between current density (J), voltage (V), current efficiency (CE), power efficiency (PE), and luminance, providing a comprehensive evaluation of the holistic EL performances.| x wt% | λema [nm] | FWHMb [nm] | CIE(x, y)c | Vond [V] | Lmaxe [cd m−2] | CEmaxf [cd A−1] | PEmaxg [lm W−1] | EQEh [%] |
|---|---|---|---|---|---|---|---|---|
| a EL peak wavelength.b Full-width at half-maximum.c Commission Internationale de L'Eclairage coordinates (value taken at 100 cd m−2).d Turn-on voltage at 1 cd m−2.e Maximum luminance.f Maximum current efficiency.g Maximum power efficiency.h Maximum external quantum efficiency, and values at 100 and 1000 cd m−2, respectively. (Note: (A) denotes the device with (P)-BN-TP-ICz, (B) denotes the device with (M)-BN-TP-ICz. | ||||||||
| 3 (A) | 536 | 38 | (0.32, 0.66) | 3.1 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
130.0 | 131.7 | 31.1/19.5/6.6 |
| 5 (A) | 540 | 38 | (0.33, 0.65) | 3.0 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 940 |
135.0 | 140.7 | 32.0/20.3/6.1 |
| 8 (A) | 540 | 40 | (0.34, 0.64) | 3.0 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 240 |
123.8 | 129.7 | 30.7/18.6/4.5 |
| 3 (B) | 540 | 38 | (0.32, 0.66) | 3.0 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
121.9 | 119.7 | 30.0/18.5/5.8 |
| 5 (B) | 540 | 38 | (0.33, 0.65) | 3.0 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
128.7 | 134.8 | 32.0/20.1/5.3 |
| 8 (B) | 540 | 39 | (0.34, 0.65) | 3.0 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 710 |
113.2 | 115.9 | 31.2/19.9/5.2 |
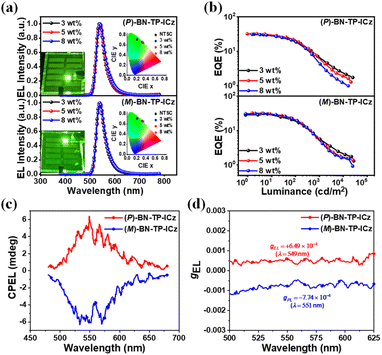
Owing to the similar photophysical properties of enantiomers (P/M)-BN-TP-ICz, they also exhibit almost the same EL performances in devices. All the single host-based devices demonstrate effective carrier injection and transport, with turn-on voltages (Von at 1 cd m−2) of ≤3.1 V. They achieve complete energy transfer at 3 wt% doping concentration, but the optimal EL performances are attained when the doping concentration increases to 5 wt%. At the optimal doping concentration, (P/M)-BN-TP-ICz-based devices show green emission with peaks of 540 nm and narrow FWHMs of 38 nm, and CIE coordinates of (0.33, 0.65). (P)-BN-TP-ICz- and (M)-BN-TP-ICz-based devices achieve maximum EQEs of 32.0% and 32.0%, maximum CEs of 135.0 and 128.7 cd A−1, and maximum PEs of 140.7 and 134.8 lm W−1, respectively, which are much better than those of the BN-DBC-based single-host device with an identical device structure at 5 wt% doping concentration (maximum EQE = 4.7%, CE = 17.2 cd A−1 and PE = 16.4 lm W−1, Fig. S30† and Table S4†). The devices based on emitter (P/M)-BN-TP-ICz have remarkably maximum EQEs, principally because the EML PhCzBCz: 5 wt% BN-TP-ICz simultaneously has a high ϕPL of up to 92% (Table S3†) and dominant horizontal emitting dipole orientation ratio (Θ//) of up to 80% (Fig. S31†). The calculated S0 → S1 transition dipole moment (TDM) of molecule (P)-BN-TP-ICz reveals that its TDM direction is mainly along the x-axis, and the TDM vector has a minor Z component (Fig. S32†), indicating that when anchored on the surface of a thin film, the emitter (P/M)-BN-TP-ICz may have a natural tendency to preferentially align the molecular axis horizontally. When the doping concentration increases to 8 wt%, the EL performances of the devices show an observable decrease, which is potentially attributed to molecular aggregation-induced quenching and confirmed by the appreciable crystal π⋯π stacking. Furthermore, the devices with 5 wt% doping concentration (P/M)-BN-TP-ICz exhibit good spectral stability with the increase in driving voltage (Fig. S33†), suggesting that there is only one stationary exciton recombination zone located in the EML within the device architecture, which is beneficial for its practical applications. More importantly, (P/M)-BN-TP-ICz-based devices with 5 wt% doping concentration shows distinct mirror circularly polarized electroluminescence (CPEL) spectra signal with decent gELs of +6.49 × 10−4 and −7.74 × 10−4 for (P)-BN-TP-ICz and (M)-BN-TP-ICz, respectively. In summary, (P/M)-BN-TP-ICz-based devices not only exhibit excellent EL performance but also show good CPEL characteristics, which verifies the scientificity of fusing ICz into DtCzB to construct CP-MR-TADF materials with B,N-embedded hetero[8]helicene, making their application in UHD CP-OLED displays attractive. In addition, since the efficiency roll-off of BN-TP-ICz-based devices is relatively large, the use of the sensitization strategy can alleviate the device efficiency roll-off to a certain extent.28 Related research is provided in the ESI (Fig. S34–S37 and Table S5).†
Conclusions
In conclusion, we have successfully developed a pair of CP-MR-TADF enantiomers with a high-order B,N-embedded hetero[8]helicene, BN-TP-ICz. The molecule fuses two high-performance MR chromophores, DtCzB and ICz, which are endowed with the advantages of narrowband emission and high efficiency. The enantiomers (P/M)-BN-TP-ICz exhibit significant configurational stability and high energy barrier of racemization. The optimized CP-OLEDs based on single host PhCzBCz and enantiomers (P/M)-BN-TP-ICz demonstrate green emission with peaks of 540 nm, FWHMs of 38 nm, CIE coordinates of (0.33, 0.65), and maximum EQEs of 32.0%. Moreover, they showcase distinct mirror CPEL spectra signal with decent gELs of +6.49 × 10−4 and −7.74 × 10−4 for (P)-BN-TP-ICz and (M)-BN-TP-ICz, respectively. The successful construction of BN-TP-ICz not only enriches the structural diversity of MR-TADF materials, but also presents a straightforward and viable synthetic strategy for constructing large-sized helicene-type CP-MR-TADF materials with excellent configuration stability, which provides conceptual guidance for the construction of narrowband chiral emitters in future UHD CP-OLED displays.Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. The data include experimental results, characterization data, and any computational models used in the analysis. The following specific data types are available:Raw and processed data: experimental measurements, including raw data from spectroscopic, NMR, and other characterization techniques.
ESI material†: additional information such as detailed experimental procedures, intermediate data, and supplementary figures.
Requests for data should be directed to [yuewang@jlu.edu.cn]. The data will be made available in a timely manner to ensure transparency and reproducibility of the results presented in this study.
Author contributions
Conceptualization, Yincai Xu; methodology, Tingting Huang and Li Yuan; validation, Yincai Xu, You-Xuan Zheng and Yue Wang; formal analysis, Yincai Xu and Tingting Huang and Li Yuan; investigation, Tingting Huang and Li Yuan; resources, Xueying Lu; data curation, Yupei Qu and Cheng Qu; writing – original draft, Tingting Huang and Yincai Xu; writing – review & editing, Yincai Xu; supervision, You-Xuan Zheng and Yue Wang.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21935005 and 92256304), the National Key R&D Program of China (2020YFA0714601), Jihua Laboratory (X190321TF190) and Foshan Science and Technology Innovation Team Special Project (1920001000128).Notes and references
- M. Liu, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304–7397 CrossRef CAS PubMed.
- (a) L. Frédéric, A. Desmarchelier, L. Favereau and G. Pieters, Adv. Funct. Mater., 2021, 31, 2010281 CrossRef; (b) P. Peluso and B. Chankvetadze, Chem. Rev., 2022, 122, 13235–13400 CrossRef CAS PubMed.
- J. R. Brandt, F. Salerno and M. J. Fuchter, Nat. Rev. Chem, 2017, 1, 0045 CrossRef CAS.
- (a) E. VanderDonckt, J. Nasielski, J. R. Greenleaf and J. B. Birks, Chem. Phys. Lett., 1968, 2, 409–410 CrossRef CAS; (b) Y. Shen and C. F. Chen, Chem. Rev., 2012, 112, 1463–1535 CrossRef CAS PubMed.
- K. Dhbaibi, L. Favereau and J. Crassous, Chem. Rev., 2019, 119, 8846–8953 CrossRef CAS PubMed.
- (a) J. A. Schellman, Chem. Rev., 1975, 75, 323–331 CrossRef CAS; (b) D.-W. Zhang, M. Li and C.-F. Chen, Chem. Soc. Rev., 2020, 49, 1331–1343 RSC.
- (a) M. Li, S.-H. Li, D. Zhang, M. Cai, L. Duan, M.-K. Fung and C.-F. Chen, Angew. Chem., Int. Ed., 2018, 57, 2889–2893 CrossRef CAS PubMed; (b) L. Xu, H. Liu, X. Peng, P. Shen, B. Z. Tang and Z. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202300492 CrossRef CAS PubMed; (c) Z.-G. Wu, H.-B. Han, Z.-P. Yan, X.-F. Luo, Y. Wang, Y.-X. Zheng, J.-L. Zuo and Y. Pan, Adv. Mater., 2019, 31, 1900524 CrossRef PubMed; (d) S.-Y. Yang, Y.-K. Wang, C.-C. Peng, Z.-G. Wu, S. Yuan, Y.-J. Yu, H. Li, T.-T. Wang, H.-C. Li, Y.-X. Zheng, Z.-Q. Jiang and L.-S. Liao, J. Am. Chem. Soc., 2020, 142, 17756–17765 CrossRef CAS PubMed; (e) J.-K. Li, X.-Y. Chen, Y.-L. Guo, X.-C. Wang, A.-C. Sue, X.-Y. Cao and X.-Y. Wang, J. Am. Chem. Soc., 2021, 143, 17958–17963 CrossRef CAS PubMed.
- (a) R. M. Soneira, Inf. Disp., 2016, 32, 26–31 Search PubMed; (b) International Telecommunication Union, Recommendation ITU-R BT.2020, Parameter values for ultrahigh definition television systems for production and international program exchange, https://www.itu.int/rec/R-REC-BT.2020-2-201510-I/en.
- (a) H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed; (b) T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed; (c) Y. Xu, Q. Wang, X. Cai, C. Li and Y. Wang, Adv. Mater., 2021, 33, 2100652 CrossRef CAS PubMed; (d) Z.-P. Yan, L. Yuan, Y. Zhang, M.-X. Mao, X.-J. Liao, H.-X. Ni, Z.-H. Wang, Z. An, Y.-X. Zheng and J.-L. Zuo, Adv. Mater., 2022, 34, 2204253 CrossRef CAS PubMed; (e) X. Wu, J.-W. Huang, B.-K. Su, S. Wang, L. Yuan, W.-Q. Zheng, H. Zhang, Y.-X. Zheng, W. Zhu and P.-T. Chou, Adv. Mater., 2022, 34, 2105080 CrossRef CAS PubMed; (f) Z. Chen, C. Zhong, J. Han, J. Miao, Y. Qi, Y. Zou, G. Xie, S. Gong and C. Yang, Adv. Mater., 2022, 34, 2109147 CrossRef CAS PubMed; (g) Y. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai and T. Hatakeyama, Nat. Photonics, 2019, 13, 678–682 CrossRef CAS; (h) S.-Y. Yang, S.-N. Zou, F.-C. Kong, X.-J. Liao, Y.-K. Qu, Z.-Q. Feng, Y.-X. Zheng, Z.-Q. Jiang and L.-S. Liao, Chem. Commun., 2021, 57, 11041–11044 RSC; (i) W. Yang, N. Li, J. Miao, L. Zhan, S. Gong, Z. Huang and C. Yang, CCS Chem., 2022, 4, 3463–3471 CrossRef CAS; (j) Y. Yang, N. Li, J. Miao, X. Cao, A. Ying, K. Pan, X. Lv, F. Ni, Z. Huang, S. Gong and C. Yang, Angew. Chem., Int. Ed., 2022, 61, e202202227 CrossRef CAS PubMed.
- (a) M. Grell, M. Oda, K. S. Whitehead, A. Asimakis, D. Neher and D. D. C. Bradley, Adv. Mater., 2001, 13, 577–580 CrossRef CAS; (b) L. Wan, J. Wade, X. Shi, S. Xu, M. J. Fuchter and A. J. Campbell, ACS Appl. Mater. Interfaces, 2020, 12, 39471–39478 CrossRef CAS PubMed; (c) D. Tan, J. Dong, T. Ma, Q. Feng, S. Wang and D. T. Yang, Angew. Chem., Int. Ed., 2023, 62, e202304711 CrossRef CAS PubMed; (d) P. Li, W. Li, Q. Lv, R. Chen and C. Zheng, J. Mater. Chem. C, 2023, 11, 4033–4041 RSC.
- (a) P. Ravat, R. Hinkelmann, D. Steinebrunner, A. Prescimone, I. Bodoky and M. Juricek, Org. Lett., 2017, 19, 3707–3710 CrossRef CAS PubMed; (b) Y. Zhang, D. Zhang, T. Huang, A. J. Gillett, Y. Liu, D. Hu, L. Cui, Z. Bin, G. Li, J. Wei and L. Duan, Angew. Chem., Int. Ed., 2021, 60, 20498–20503 CrossRef CAS PubMed; (c) J. M. dos Santos, D. Sun, J. M. Moreno-Naranjo, D. Hall, F. Zinna, S. T. J. Ryan, W. Shi, T. Matulaitis, D. B. Cordes, A. M. Z. Slawin, D. Beljonne, S. L. Warriner, Y. Olivier, M. J. Fuchter and E. Zysman-Colman, J. Mater. Chem. C, 2022, 10, 4861–4870 RSC; (d) Y. Xu, Q. Wang, X. Song, Y. Wang and C. Li, Chem.–Eur. J., 2023, 29, e202203414 CrossRef CAS PubMed.
- (a) J. Barroso, J. L. Cabellos, S. Pan, F. Murillo, X. Zarate, M. A. Fernandez-Herrera and G. Merino, Chem. Commun., 2018, 54, 188–191 RSC; (b) M. Krzeszewski, H. Ito and K. Itami, J. Am. Chem. Soc., 2022, 144, 862–871 CrossRef CAS PubMed; (c) J. Tan, X. Xu, J. Liu, S. Vasylevskyi, Z. Lin, R. Kabe, Y. Zou, K. Mullen, A. Narita and Y. Hu, Angew. Chem., Int. Ed., 2023, 62, e202218494 CrossRef CAS PubMed.
- (a) T. Katayama, S. Nakatsuka, H. Hirai, N. Yasuda, J. Kumar, T. Kawai and T. Hatakeyama, J. Am. Chem. Soc., 2016, 138, 5210–5213 CrossRef CAS PubMed; (b) Y. Nakakuki, T. Hirose, H. Sotome, H. Miyasaka and K. Matsuda, J. Am. Chem. Soc., 2018, 140, 4317–4326 CrossRef CAS PubMed; (c) J. L. Greenfield, J. Wade, J. R. Brandt, X. Shi, T. J. Penfold and M. J. Fuchter, Chem. Sci., 2021, 12, 8589–8602 RSC.
- (a) Y.-F. Wu, L. Zhang, Q. Zhang, S.-Y. Xie and L.-S. Zheng, Org. Chem. Front., 2022, 9, 4726–4743 RSC; (b) G.-F. Huo, W.-T. Xu, Y. Han, J. Zhu, X. Hou, W. Fan, Y. Ni, S. Wu, H.-B. Yang and J. Wu, Angew. Chem., Int. Ed., 2024, 63, e202403149 CrossRef CAS PubMed.
- F. Zhang, F. Rauch, A. Swain, T. B. Marder and P. Ravat, Angew. Chem., Int. Ed., 2023, 62, e202218965 CrossRef CAS PubMed.
- (a) L. Yuan, Z.-L. Tu, J.-W. Xu, H.-X. Ni, Z.-P. Mao, W.-Y. Xu and Y.-X. Zheng, Sci. China: Chem., 2023, 66, 2612–2620 CrossRef CAS; (b) G. Meng, J. Zhou, X. S. Han, W. Zhao, Y. Zhang, M. Li, C. F. Chen, D. Zhang and L. Duan, Adv. Mater., 2024, 36, 2307420 CrossRef CAS PubMed; (c) W.-C. Guo, W.-L. Zhao, K.-K. Tan, M. Li and C.-F. Chen, Angew. Chem., Int. Ed., 2024, 63, e202401835 CrossRef CAS PubMed.
- Q. Wang, L. Yuan, C. Qu, T. Huang, X. Song, Y. Xu, Y.-X. Zheng and Y. Wang, Adv. Mater., 2023, 35, 2305125 CrossRef CAS PubMed.
- (a) Y. Zhang, D. Zhang, J. Wei, Z. Liu, Y. Lu and L. Duan, Angew. Chem., Int. Ed., 2019, 58, 16912–16917 CrossRef CAS PubMed; (b) Y. Xu, Z. Cheng, Z. Li, B. Liang, J. Wang, J. Wei, Z. Zhang and Y. Wang, Adv. Opt. Mater., 2020, 8, 1902142 CrossRef CAS; (c) M. Yang, I. S. Park and T. Yasuda, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed.
- Y. Xu, C. Li, Z. Li, J. Wang, J. Xue, Q. Wang, X. Cai and Y. Wang, CCS Chem., 2022, 4, 2065–2079 CrossRef CAS.
- (a) A. Pershin, D. Hall, V. Lemaur, J.-C. Sancho-Garcia, L. Muccioli, E. Zysman-Colman, D. Beljonne and Y. Olivier, Nat. Commun., 2019, 10, 597 CrossRef CAS PubMed; (b) S. Wu, W. Li, K. Yoshida, D. Hall, S. M. Suresh, T. Sayner, J. Gong, D. Beljonne, Y. Olivier, I. D. W. Samuel and E. Z.- Colman, ACS Appl. Mater. Interfaces, 2022, 14, 22341–22352 CrossRef CAS PubMed.
- T. Lu and Q. Chen, Theor. Chem. Acc., 2020, 139, 25 Search PubMed.
- Z. Liu, T. Lu and Q. Chen, Carbon, 2020, 165, 468–475 CrossRef CAS.
- (a) S. Grimme and S. D. Peyerimhoff, Chem. Phys., 1996, 204, 411–417 CrossRef CAS; (b) R. H. Janke, G. Haufe, E.-U. Würthwein and J. H. Borkent, J. Am. Chem. Soc., 1996, 118, 6031–6035 CrossRef CAS.
- T. Lu and Q. Chen, Chem.: Methods, 2021, 1, 231–239 CAS.
- (a) T. Otani, A. Tsuyuki, T. Iwachi, S. Someya, K. Tateno, H. Kawai, T. Saito, K. S. Kanyiva and T. Shibata, Angew. Chem., Int. Ed., 2017, 56, 3906–3910 CrossRef CAS PubMed; (b) Y.-Y. Ju, L. Chai, K. Li, J.-F. Xing, X.-H. Ma, Z.-L. Qiu, X.-J. Zhao, J. Zhu and Y.-Z. Tan, J. Am. Chem. Soc., 2023, 145, 2815–2821 CrossRef CAS PubMed.
- L. Arrico, L. Di Bar and F. Zinna, Chem.–Eur. J., 2021, 27, 2920–2934 CrossRef CAS PubMed.
- Y. Xu, C. Li, Z. Li, Q. Wang, X. Cai, J. Wei and Y. Wang, Angew. Chem., Int. Ed., 2020, 59, 17442–17446 CrossRef CAS PubMed.
- (a) C. Murawski, K. Leo and M. C. Gather, Adv. Mater., 2013, 25, 6801–6827 CrossRef CAS PubMed; (b) H. Nakanotani, T. Higuchi, T. Furukawa, K. Masui, K. Morimoto, M. Numata, H. Tanaka, Y. Sagara, T. Yasuda and C. Adachi, Nat. Commun., 2014, 5, 4016 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2361156. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc03854a |
| ‡ T. Huang and L. Yuan contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |