Open Access Article
Open Access ArticleIridium/chiral phosphoramidite–olefin complex-catalysed enantioselective [3+2] annulation of ortho-ketoarylboron compounds with conjugated dienes†
Ryota
Yabe
and
Takahiro
Nishimura
 *
*
Department of Chemistry, Graduate School of Science, Osaka Metropolitan University, Sumiyoshi, Osaka 558-8585, Japan. E-mail: tnishi@omu.ac.jp
First published on 18th October 2024
Abstract
The iridium-catalysed enantioselective [3+2] annulation of ortho-ketoarylboron compounds with conjugated dienes proceeded to give chiral indanol derivatives bearing an all-carbon quaternary stereogenic center in high yields with high enantioselectivity. Chiral phosphoramidite–olefin ligands were effective for the high reactivity and enantioselectivity.
Indane derivatives, which have a fused structure of benzene ring and five-membered ring, are important structural motifs in several chiral drugs and natural products.1 Transition-metal-catalysed stereoselective [3+2] annulation is one of the useful methods for the synthesis of indane derivatives, and ortho-functionalized arylboron reagents have often been used with unsaturated hydrocarbons such as allenes, alkynes and conjugated dienes for the [3+2] annulation.2 In this respect, we reported iridium-catalyzed [3+2] annulation of ortho-carbonylated arylboronic acids with 1,3-dienes giving indanol derivatives with high regio- and diastereoselectivity (Scheme 1a).3 The reaction proceeded via an ortho-carbonylaryliridium(I) species, which is generated from a hydroxoiridium complex and the corresponding arylboronic acids. The enantioselective annulation was also achieved by use of a chiral diene ligand (Scheme 1b).4,5 However, the successful reaction was limited to ortho-formylarylboron compounds, and ortho-ketoarylboron reagents could not be applied to the annulation because of the low reactivity. In addition, high reaction temperature to promote the reaction can probably cause ligand exchange between the chiral diene ligand and the 1,3-diene, which exists in excess to the Ir catalyst. Unfortunately, the use of chiral diphosphine ligands, which strongly coordinate to the iridium, inhibited the reaction. It follows that new ligands bearing appropriate coordination ability are required for the realization of the iridium-catalyzed asymmetric annulation. In this context, we focused on chiral phosphine–olefin hybrid ligands,6,7 which have stronger coordination ability than diene ligands, and are unique ligands that combine the coordination properties of both phosphines and olefins.7,8 Here we report that iridium/chiral phosphine–olefin complexes are effective in catalysing asymmetric [3+2] annulation of ortho-keto arylboron compounds with 1,3-dienes.
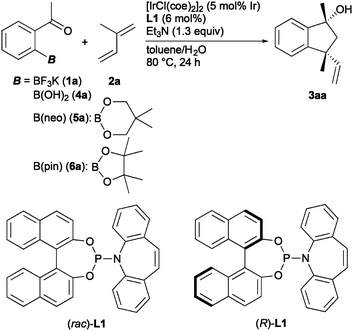
In the first set of experiments, annulation of ortho-acetylphenylboron compound 1a with isoprene (2a) was examined under several reaction conditions (Table 1). Treatment of 1a with 2a (3.0 equiv.) in toluene/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 80 °C for 24 h in the presence of [IrCl(coe)2]2 (5 mol% of Ir, coe = cyclooctene), (rac)-L1 (6 mol%), and Et3N (1.3 equiv.) gave the corresponding product cis-3aa exclusively as a single isomer in 58% yield (entry 1).9 The reaction in 1,4-dioxane or 1,2-dichloroethane instead of toluene decreased the yield of 3aa (entries 2 and 3). The lower diastereoselectivity (cis
1) at 80 °C for 24 h in the presence of [IrCl(coe)2]2 (5 mol% of Ir, coe = cyclooctene), (rac)-L1 (6 mol%), and Et3N (1.3 equiv.) gave the corresponding product cis-3aa exclusively as a single isomer in 58% yield (entry 1).9 The reaction in 1,4-dioxane or 1,2-dichloroethane instead of toluene decreased the yield of 3aa (entries 2 and 3). The lower diastereoselectivity (cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans = 73
trans = 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27) was observed in the reaction without triethylamine, which was added to inhibit the hydrolysis reaction of 1a by the iridium catalysis (entry 4).10 Water was necessary for the reaction (entry 5). The reaction without ligand (rac)-L1, where Ir(I)/isoprene complex is formed, did not procced (entry 6). Fortunately, the use of (R)-L17a gave 3aa with good enantioselectivity (85% ee, entry 7). It was found that a rhodium complex with (rac)-L1 also promoted the present reaction albeit with low yield (20%, entry 8). Boron functional groups influenced the yield and enantioselectivity (entries 9–11). Commercially available ortho-acetylboronic acid (4a) showed slightly lower reactivity compared with potassium aryltrifluoroborate 1a (entry 9). Boron esters can also be applied to the present reaction (entries 10 and 11), and, in particular, pinacol ester 6a displayed excellent reactivity, thus giving 3aa in 79% yield with 86% ee (entry 11). The absolute configuration of 3aa obtained using (R)-L1 was determined to be (1R,3R) by HPLC analysis of a separately synthesized (1R,3R)-3aa (see the ESI† for details).
27) was observed in the reaction without triethylamine, which was added to inhibit the hydrolysis reaction of 1a by the iridium catalysis (entry 4).10 Water was necessary for the reaction (entry 5). The reaction without ligand (rac)-L1, where Ir(I)/isoprene complex is formed, did not procced (entry 6). Fortunately, the use of (R)-L17a gave 3aa with good enantioselectivity (85% ee, entry 7). It was found that a rhodium complex with (rac)-L1 also promoted the present reaction albeit with low yield (20%, entry 8). Boron functional groups influenced the yield and enantioselectivity (entries 9–11). Commercially available ortho-acetylboronic acid (4a) showed slightly lower reactivity compared with potassium aryltrifluoroborate 1a (entry 9). Boron esters can also be applied to the present reaction (entries 10 and 11), and, in particular, pinacol ester 6a displayed excellent reactivity, thus giving 3aa in 79% yield with 86% ee (entry 11). The absolute configuration of 3aa obtained using (R)-L1 was determined to be (1R,3R) by HPLC analysis of a separately synthesized (1R,3R)-3aa (see the ESI† for details).
| Entry | Changes from standard conditions | Yieldb (%) | eec (%) |
|---|---|---|---|
a Reaction conditions: 1a or 4a–6a (0.20 mmol), 2a (0.60 mmol, 3.0 equiv.), [IrCl(coe)2]2 (0.0050 mmol, 5 mol% of Ir), L1 (0.012 mmol, 6 mol%) and Et3N (0.26 mmol, 1.3 equiv.) in toluene (0.8 mL) and H2O (0.8 mL) at 80 °C for 24 h under N2 atmosphere. Unless otherwise stated, the ratio of diastereomers is >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
b Determined by 1H NMR.
c Determined by chiral HPLC analysis.
d The product was obtained as a mixture of diastereomers (cis/trans = 73 1.
b Determined by 1H NMR.
c Determined by chiral HPLC analysis.
d The product was obtained as a mixture of diastereomers (cis/trans = 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27). 27).
|
|||
| 1 | No changes | 58 | — |
| 2 | 1,4-Dioxane instead of toluene | 43 | — |
| 3 | ClCH2CH2Cl instead of toluene | 31 | — |
| 4 | Without Et3N | 43d | — |
| 5 | Without H2O | 15 | — |
| 6 | Without (rac)-L1 | <1 | — |
| 7 | (R)-L1 instead of (rac)-L1 | 66 | 85 |
| 8 | [RhCl(coe)2]2 instead of [IrCl(coe)2]2 | 20 | — |
| 9 | 4a instead of 1a with (R)-L1 | 55 | 87 |
| 10 | 5a instead of 1a with (R)-L1 | 31 | 86 |
| 11 | 6a instead of 1a with (R)-L1 | 79 | 86 |
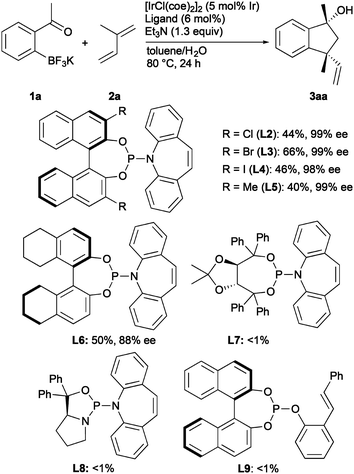
Screening results of chiral phosphoramidite–olefin ligands using 1a as the substrate are shown in Scheme 2. We took an approach to revise the binaphthyl moiety due to easy synthesis and availability. The use of phosphoramidite–olefin ligands with a 3,3′-disubstituted binaphthyl moiety (L2–L5) gave 3aa with excellent enantioselectivity (98–99% ee). In particular, L3 substituted with a 3,3′-dibromo-substituted binaphthyl showed the best reactivity and enantioselectivity (66%, 99% ee). As the steric hindrance of the 3,3′-substituents further increased, the product yield decreased (L4 and L5). H8-Binol backbone in L6 did not improve the enantioselectivity. The phosphoramidite–olefin ligand derived from (R,R)-TADDOL (L7)7b or L-prolinol (L8)7b did not induce the catalytic activity. Phosphite–olefin ligand L9,8 which is used for Rh-catalyzed asymmetric 1,4-additions, could not be applied to the present annulation.
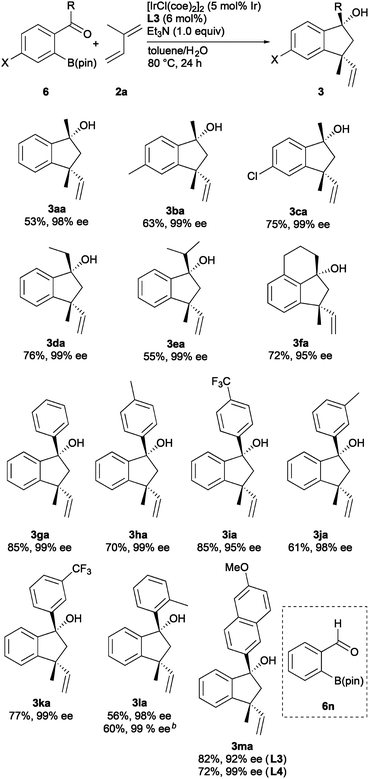
The scope of ortho-ketoarylboron esters 6, some of which are readily available by C–H borylation,11 is shown in Scheme 3.12 The reaction of 6a with isoprene (2a) gave 3aa in 53% yield with 98% ee. Para-substituted acetophenone derivatives 6b and 6c underwent the annulation to give the corresponding products 3ba and 3ca, respectively, with high enantioselectivity (99% ee). Arylboron esters 6d and 6e substituted with ethyl and isopropyl groups reacted efficiently to give the corresponding products 3da and 3ea in 76 and 55% yields, respectively, with complete enantioselectivity. The reaction of α-tetralone derivative 6f gave tricyclic product 3fa in 72% yield with 95% ee. Boron ester 6g bearing a benzoyl group is also a good substrate to give the corresponding indanol 3ga in 85% yield with 99% ee. Para-, meta-, and ortho-substituted benzoylphenylboron esters 6h–6l efficiently participated in the reaction to give the corresponding products 3ha–3la in good yields (56–85%) with excellent selectivity (95–99% ee). The reaction using 1.0 mmol of 6l gave 3la in 60% yield with 99% ee. In the reaction of arylboron ester 6m, which has a 6-methoxynaphthyl group, the use of L4 was effective in displaying the high enantioselectivity (99% ee) of the product 3ma. Unfortunately, the use of o-formylphenylboron ester 6n resulted in the formation of unidentified complex mixtures.
The results obtained for the reaction of several 1,3-dienes with 6g are shown in Scheme 4. 2-Substituted 1,3-dienes such as myrcene (2b), myrcenol (2c), and acetylated myrcenol 2d could be applied to the reaction of 6g to give the corresponding products 3gb–3gd with high enantioselectivity. The reactions of 3-methyl-1,3-pentadiene (2e) and 2,3-dimethyl-1,3-butadiene (2f) also displayed high enantioselectivity, albeit with the somewhat low yields. Unfortunately, however, dienes 2g–2j were not applicable to the present reaction.
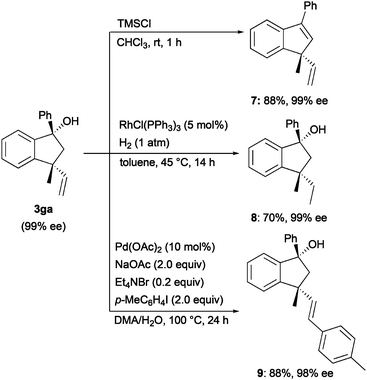
Indanol 3ga was converted into several compounds while maintaining the stereochemistry (Scheme 5). Dehydration of 3ga with trimethylsilyl chloride (TMSCl) in CHCl3 gave indene 7 in 88% yield.13 Rh(I)-catalysed hydrogenation of 3ga gave indanol 8 in 70% yield.14 Mizoroki–Heck reaction of indanol 3ga with p-iodotoluene gave 9 in 80% yield.15
In summary, we have developed the asymmetric [3+2] annulation of ortho-ketoarylboron compounds with 1,3-dienes catalysed by the iridium/chiral phosphoramidite–olefin complex. The use of a new chiral phosphoramidite–olefin ligand with a 3,3′-bromo-substituted binaphthyl structure showed almost complete enantioselectivity of the annulation products bearing an all-carbon quaternary center.
This work was supported by JSPS KAKENHI Grant Number JP19H02721 and JP24K08416.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- For bioactive and natural compounds with an indane structure, see: (a) L. Lasko, C. Jakob and R. Edalji, et al. , Nature, 2017, 550, 128 CrossRef CAS PubMed; (b) R. M. McMillan, J.-M. Girodeau and S. J. Foster, Br. J. Pharmacol., 1990, 101, 501 CrossRef CAS PubMed; (c) S.-H. Kim, S. H. Kwon, S.-H. Park, J. K. Lee, H.-S. Bang, S.-J. Nam, H. K. Kwon, J. Shin and D.-C. Oh, Org. Lett., 2013, 15, 1834 CrossRef CAS PubMed; (d) K. Vougogiannopoulou, N. Fokialakis, N. Aligiannis, C. Cantrell and A.-L. Skaltsounis, Org. Lett., 2010, 12, 1908 CrossRef CAS PubMed.
- For the annulation of ortho-functionalized arylboron reagents with unsaturated hydrocarbons, see: (a) X. Yu and X. Lu, Org. Lett., 2009, 11, 4366 CrossRef CAS PubMed; (b) H. Panchal, C. Clarke, C. Bell, S. N. Karad, W. Lewis and H. W. Lam, Chem. Commun., 2018, 54, 12389 RSC; (c) T. Kumon, M. Shimada, J. Wu, S. Yamada and T. Konno, Beilstein J. Org. Chem., 2020, 16, 2193 CrossRef CAS PubMed; (d) J. L. L. F. Regueira, L. F. Silva Jr. and R. A. Pilli, Org. Lett., 2020, 22, 6262 CrossRef CAS PubMed; (e) A. Zhu, Y. Sun, J. Lai, Z. Chen, X. Bu, Y.-N. Yue, M. Ma and F. Xue, J. Org. Chem., 2022, 87, 788 Search PubMed; (f) S.-Y. Liang, T.-Y. Zhang, Z.-C. Chen, W. Du and Y.-C. Chen, Org. Lett., 2024, 26, 1483 CrossRef CAS PubMed.
- T. Nishimura, Y. Yasuhara and T. Hayashi, J. Am. Chem. Soc., 2007, 129, 7506 CrossRef CAS PubMed.
- T. Nishimura, Y. Yasuhara, M. Nagaosa and T. Hayashi, Tetrahedron: Asymmetry, 2008, 19, 1778 CrossRef CAS.
- Similar annulation of 1,3-dienes catalysed by Ir(I) complexes, see: (a) R. Yabe, Y. Ebe and T. Nishimura, Chem. Commun., 2021, 57, 5917 RSC; (b) M. Hatano and T. Nishimura, Angew. Chem., Int. Ed., 2015, 54, 10949 CrossRef CAS PubMed; (c) Y. Ebe, M. Hatano and T. Nishimura, Adv. Synth. Catal., 2015, 357, 1425 CrossRef CAS; (d) Y. Ebe and T. Nishimura, J. Am. Chem. Soc., 2014, 136, 9284 CrossRef CAS PubMed; (e) T. Nishimura, M. Nagamoto, Y. Ebe and T. Hayashi, Chem. Sci., 2013, 4, 4499 RSC; (f) T. Nishimura, Y. Ebe and T. Hayashi, J. Am. Chem. Soc., 2013, 135, 2092 CrossRef CAS PubMed.
- (a) K. Sakamoto and T. Nishimura, Chem. Commun., 2022, 58, 11783 RSC; (b) K. Murakami, K. Sakamoto and T. Nishimura, Synthesis, 2022, 4753 CAS.
- For selected examples, see; (a) C. Defieber, M. A. Ariger, P. Moriel and E. M. Carreira, Angew. Chem., Int. Ed., 2007, 46, 3139 CrossRef CAS PubMed; (b) R. Mariz, A. Briceño, R. Dorta and R. Dorta, Organometallics, 2008, 27, 6605 CrossRef CAS; (c) T. J. Hoffman and E. M. Carreira, Angew. Chem., Int. Ed., 2011, 50, 10670 CrossRef CAS PubMed; (d) Z. Li, Z. Cao and H. Du, Org. Biomol. Chem., 2011, 9, 5369 RSC.
- Y.-N. Yu and M.-H. Xu, Org. Chem. Front., 2014, 1, 738 RSC.
- The mechanism of the present reaction has been proposed in previous work (ref. 3) from the observed regio- and stereoselectivity.
- It is not clear why the bases affect the diastereoselectivity. See also ESI† for the results using other bases.
- H. Itoh, T. Kikuchi, T. Ishiyama and N. Miyaura, Chem. Lett., 2011, 40, 1007 CrossRef CAS.
- The corresponding ketones formed by hydrolysis of 6 were also observed.
- H. Firouzababi, N. Iranpoor, H. Hazarkhani and B. Karimi, Synth. Commun., 2003, 33, 3653 CrossRef.
- M. Shigeno, T. Yamamoto and M. Murakami, Chem. – Eur. J., 2009, 15, 12929 CrossRef CAS PubMed.
- (a) T. Mizoroki, K. Mori and A. Ozaki, Bull. Chem. Soc. Jpn., 1971, 44, 581 CrossRef CAS; (b) R. F. Heck and J. P. Nolley, J. Org. Chem., 1972, 37, 2320 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, and compound characterization data. See DOI: https://doi.org/10.1039/d4cc04238g |
| This journal is © The Royal Society of Chemistry 2024 |