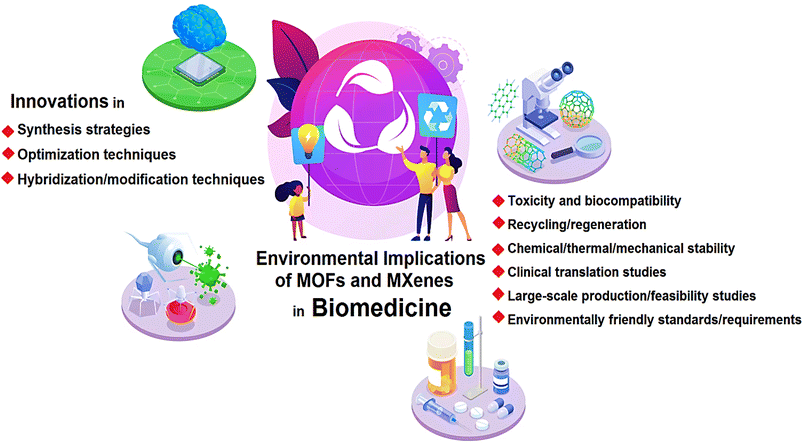
Open Access Article
Open Access ArticleEnvironmental implications of metal–organic frameworks and MXenes in biomedical applications: a perspective
Bahareh Farasati Far a,
Navid Rabiee
a,
Navid Rabiee *bc and
Siavash Iravani
*bc and
Siavash Iravani *d
*d
aDepartment of Chemistry, Iran University of Science and Technology, Tehran, 1684611367, Iran
bSchool of Engineering, Macquarie University, Sydney, New South Wales 2109, Australia. E-mail: nrabiee94@gmail.com
cCentre for Molecular Medicine and Innovative Therapeutics, Murdoch University, Perth, WA 6150, Australia
dIndependent Researcher, W Nazar ST, Boostan Ave, Isfahan, Iran. E-mail: siavashira@gmail.com
First published on 24th November 2023
Abstract
Metal–organic frameworks (MOFs) and MXenes have demonstrated immense potential for biomedical applications, offering a plethora of advantages. MXenes, in particular, exhibit robust mechanical strength, hydrophilicity, large surface areas, significant light absorption potential, and tunable surface terminations, among other remarkable characteristics. Meanwhile, MOFs possess high porosity and large surface area, making them ideal for protecting active biomolecules and serving as carriers for drug delivery, hence their extensive study in the field of biomedicine. However, akin to other (nano)materials, concerns regarding their environmental implications persist. The number of studies investigating the toxicity and biocompatibility of MXenes and MOFs is growing, albeit further systematic research is needed to thoroughly understand their biosafety issues and biological effects prior to clinical trials. The synthesis of MXenes often involves the use of strong acids and high temperatures, which, if not properly managed, can have adverse effects on the environment. Efforts should be made to minimize the release of harmful byproducts and ensure proper waste management during the production process. In addition, it is crucial to assess the potential release of MXenes into the environment during their use in biomedical applications. For the biomedical applications of MOFs, several challenges exist. These include high fabrication costs, poor selectivity, low capacity, the quest for stable and water-resistant MOFs, as well as difficulties in recycling/regeneration and maintaining chemical/thermal/mechanical stability. Thus, careful consideration of the biosafety issues associated with their fabrication and utilization is vital. In addition to the synthesis and manufacturing processes, the ultimate utilization and fate of MOFs and MXenes in biomedical applications must be taken into account. While numerous reviews have been published regarding the biomedical applications of MOFs and MXenes, this perspective aims to shed light on the key environmental implications and biosafety issues, urging researchers to conduct further research in this field. Thus, the crucial aspects of the environmental implications and biosafety of MOFs and MXenes in biomedicine are thoroughly discussed, focusing on the main challenges and outlining future directions.
1. Introduction
Metal–organic frameworks (MOFs) and MXenes are two classes of materials that have gained considerable attention in recent years due to their exceptional properties and potential applications in various fields of biomedicine.1 MOFs are porous materials composed of metal ions or clusters coordinated to organic ligands,2 while MXenes are two-dimensional (2D) transition metal carbides, nitrides, and carbonitrides.3 MXenes offer a range of advantages in the field of biomedicine, including high surface area, biodegradability, electrical conductivity, antibacterial properties, tunable surface chemistry, enhanced imaging capabilities, and stimuli-responsive behavior.4–6 These characteristics make them promising candidates for various biomedical applications, paving the way for innovative and efficient therapeutic and diagnostic approaches.7–9 These materials are highly attractive for a wide range of biomedical applications, including drug delivery,10,11 tissue engineering,12,13 (bio)sensing,8,14–16 and imaging.17,18 MOFs can be designed to encapsulate and deliver therapeutic agents, providing controlled release and targeted delivery to specific sites in the body.19 Meanwhile, MXenes possess excellent electrical conductivity and have been employed in biosensors for sensitive and rapid detection of biomarkers.16,20 Notably, both MOFs and MXenes have shown great promises in bioimaging applications, enabling enhanced contrast and precise imaging of biological tissues.21Numerous promising nanomaterials with potential biomedical uses have emerged in recent years, representing a rapid development in the field of nanomaterials.22,23 The special characteristics and wide range of possible uses of MOFs and MXenes have made them the focus of much interest. Because of their high porosity and wide surface area, MOFs are well-suited to serve as carriers for drug delivery and preserving active biomolecules. However, MXenes are expected to rise due to their impressive mechanical strength, hydrophilicity, wide surface areas, strong light absorption potential, and variable surface terminations.24 The structural composition and properties of MOFs and MXenes are still very different, although they are both finding applications in biomedical and environmental application.
The expanding application of MOFs and MXenes in biomedicine requires a comprehensive approach to addressing their environmental effects, which is why they have been chosen as the subject of this perspective.25 Despite their differences, these materials are being investigated for uses such as drug delivery, imaging, biosensing, and tissue engineering, all of which highlight the need of understanding their similarities. This strategy allows us to emphasize the vital significance of identifying the environmental effects of the production, utilization, and disposal of nanomaterials in the biomedical setting, and developing strategies to minimize those impacts.26,27
This viewpoint serves to highlight the unifying issue of environmental responsibility and awareness across these different materials, despite the fact that MOFs and MXenes may differ greatly in their synthesis processes, physicochemical features, and environmental effects. Therefore, our goal in covering both MOFs and MXenes is to give the reader a more complete picture of the rapidly developing field of nanomaterials in biomedicine and to highlight the importance of environmentally sustainable methods in this field.
While the biomedical applications of MOFs and MXenes hold great promise, it is essential to consider the potential environmental implications associated with their synthesis, utilization, and eventual disposal (Fig. 1). As with any new technology or material, it is crucial to evaluate the environmental impact to ensure sustainable development and minimize any potential risks to human health and the ecosystem. The synthesis and manufacturing processes of MOFs and MXenes involve the use of various chemicals, solvents, and energy-intensive procedures.28 For instance, the synthesis of MOFs often requires the use of organic solvents, which can have adverse effects on human health and the environment.29 These solvents may pose risks of toxicity, flammability, and the release of volatile organic compounds (VOCs) into the atmosphere, contributing to air pollution and potential harm to ecosystems.30 Similarly, the production of MXenes may involve harsh chemical treatments and energy-intensive processes to remove the metallic layers from their parent compounds.31 The use of strong acids or etchants in the synthesis of MXenes raises concerns regarding the release of hazardous chemicals and the potential contamination of water sources if proper waste management practices are not implemented.32,33 Furthermore, the large-scale production of MOFs and MXenes necessitates the use of significant amounts of resources, including metals and organic ligands. The extraction and processing of these raw materials may have negative environmental impacts, including habitat destruction, water pollution, and energy consumption.34–36 It is crucial to assess the sustainability of these processes and explore alternative methods that minimize the environmental footprint. So far, many reviews have been published regarding the biomedical applications of MOFs and MXenes.37–40 Herein, important aspects regarding the environmental implications and biosafety of MOFs and MXenes in biomedicine are deliberated, focusing on main challenges and future directions. In this perspective, it has been tried to express the key issues in environmental implications of them to encourage researchers for more research in this field.
2. Environmental implications in manufacturing of MXenes and MOFs
Various biological applications, from biosensing and drug transport to cancer theranostics and antibacterial treatments, have incorporated MOFs and MXenes because of their exceptional features. However, it is equally important to consider the environmental implications associated with their synthesis, use, and eventual disposal as is their potential for improving healthcare outcomes.2.1. Synthesis methods and environmental impact
The synthesis process for MOFs and MXenes is a serious environmental problem. Strong acids and high temperature treatments are commonplace in MXene production. These processes can have negative environmental effects and emit toxic byproducts if not well controlled. Researchers must prioritize cleaning up their synthesis processes to protect the environment. There needs to be a system in place to manage waste and reduce the amount of toxic byproducts released during production.41Nature serves as a direct source of inspiration for rational design strategies. The structural similarity between natural active components has motivated researchers to explore bioinspired molecules for applications in biomedical sciences. By leveraging the knowledge of the structural characteristics of these natural components, specific nanomaterial preparation techniques can be employed to create similar active sites within nanostructures, resulting in more effective therapeutic outcomes.42 However, recent advancements in understanding MOF synthesis and growth principles have revealed the possibility of effectively incorporating natural active components into MOF backbones through diversification of metal nodes or organic ligands.43 For instance, porphyrin-based MOFs can be modified with functional groups to optimize their optical and electronic properties, as exemplified in the case of MnO2-coated porphyrin MOFs created to facilitate the oxidation of GSH by MnO2, resulting in improved PDT, activated magnetic resonance imaging (MRI), and controlled release of doxorubicin for guided MRI drug-PDT dual-therapy.44 The incorporation of active components stabilized by MOFs provides an alternative approach to constructing versatile platforms for photodynamic therapy (PDT), sonodynamic therapy (SDT), and catalytic therapy.45 For instance, Chen et al.46 introduced a porous Zr(IV)-based porphyrinic MOF (PZM) nanosystem modified with α-cyano-4-hydroxycinnamate (CHC) to inhibit the expression of lactate-proton symporter, monocarboxylate transporter 1 (MCT1), thereby reducing lactate uptake in tumor cells. This alteration shifted the energy supply from lactate-fueled aerobic respiration to anaerobic glycolysis, consequently facilitating PDT in cancer treatment by decreasing oxygen consumption in tumor cells. Notably, the PZM nanosystems were coated with hyaluronic acid (HA) for CD44-targeting and hyaluronidase-induced intracellular drug release. Both in vitro and in vivo studies have demonstrated the favorable biocompatibility and enhanced PDT efficacy of the HA-coated PZM nanosystems in tumor cells.46 Researchers have developed a cancer-targeted cascade bioreactor called mCGP by embedding glucose oxidase and catalase in the cancer cell membrane-camouflaged porphyrin MOF known as PCN-224.47 The surface functionalization of mCGP mimics biological processes, enhancing its ability to target and remain within cancer cells. Once inside the cancer cells, mCGP promoted oxygenation of the microenvironment by catalyzing the conversion of endogenous H2O2 into O2. This process, combined with light irradiation, could increase the breakdown of intracellular glucose and the production of cytotoxic singlet oxygen (1O2). As a result, mCGP exhibited significant synergistic effects in long-term cancer starvation therapy and PDT, effectively inhibiting cancer growth after a single administration. This cascade bioreactor holds promise for the development of complementary approaches to spatiotemporally controlled cancer treatment.47 Zhang et al.48 introduced Cu(II)-nano-MOF that could be easily absorbed by breast cancer cells, leading to the production of high levels of ROS when exposed to light. Simultaneously, the presence of this MOF resulted in a significant reduction in intracellular glutathione levels. This combined effect could synergistically increase ROS concentration and promote apoptosis, thereby enhancing the effectiveness of PDT. Importantly, the prepared MOF exhibited a similar impact to the commercial antitumor drug camptothecin in a mouse model of breast cancer, primarily through the direct adsorption of glutathione.48 Furthermore, MOF-derived nitrogen-rich carbon nanostructures, prepared through pyrolysis treatment, offer substrate materials capable of realizing bioinspired design. Abundant nitrogen sites within these structures can anchor metal atoms, forming biomimetic M–Nx sites.49 MOF-based nanomaterials inspired by natural components create a suitable microenvironment for the optimal functioning of bioinspired active components, addressing concerns regarding the low stability and deactivation of natural active components as well as the environmental implications. Moreover, the ordered and periodic arrangement of the porous framework structure in MOFs provides attachment sites for introducing numerous active components, while the well-defined active structure forms the foundation for uncovering chemical reaction mechanisms.50–52
The adoption of circular economy principles in the synthesis of these materials offers a promising avenue for mitigating these environmental challenges. The circular economy approach aims to reduce waste, promote resource efficiency, and minimize environmental harm by prioritizing the reuse, recycling, and recovery of materials.53,54 In conventional synthesis routes, significant amounts of precursors, solvents, and by-products are often wasted, resulting in environmental pollution and resource depletion. By embracing circular economy principles, waste generation can be minimized through various strategies.55 One approach is the development of greener and more efficient synthesis processes that optimize the use of raw materials and minimize waste generation. In addition, the recycling and reuse of precursors, solvents, and by-products can further reduce waste and conserve resources.56,57 For instance, the utilization of waste streams from other industrial processes as feedstocks for MOF or MXene synthesis can contribute to waste reduction and promote resource circularity.
The circular economy emphasizes the efficient use of resources to minimize the extraction and consumption of virgin materials. In the context of MOFs and MXenes, circular economy principles can be applied to enhance resource efficiency in several ways. Firstly, the selection of sustainable and abundant raw materials for MOF and MXene synthesis reduces reliance on scarce resources. For instance, the use of bio-based or renewable precursors as alternatives to conventional synthetic precursors can contribute to resource efficiency and reduce the environmental footprint. Secondly, the recycling and recovery of MOFs and MXenes at the end of their life cycle can further optimize resource utilization. By implementing effective separation and recovery techniques, valuable components can be extracted from spent MOFs and MXenes, reducing the need for new material production. Circular economy principles play a crucial role in minimizing the environmental impact of MOF and MXene synthesis by promoting the use of less hazardous materials and reducing the release of pollutants.58 The substitution of toxic solvents or reagents with safer alternatives can significantly improve the environmental profile of the synthesis processes. In addition, the implementation of closed-loop systems, where waste streams are treated and recycled within the manufacturing process, prevents the discharge of harmful substances into the environment. By minimizing environmental pollution and reducing the overall ecological footprint, the circular economy approach contributes to the sustainable development of MOFs and MXenes. In an impressive study, to assess the cumulative energy demand (CED) and environmental impacts of lab-scale synthesis of MXene (Ti3C2Tx), a “cradle to gate” life cycle assessment (LCA) was conducted.59 The application of electromagnetic interface shielding was selected, and the LCA of MXene synthesis was compared to aluminum and copper foils. The investigation of MXene synthesis focused on various aspects including precursor fabrication, selective etching, delamination processes, laboratory location, energy mix, and raw material type. The findings revealed that more than 70% of the environmental impacts originated from the electricity consumption during the synthesis processes in the laboratory. In comparison, the production of 1.0 kg of industrial-scale aluminum and copper foil emitted 23.0 kg and 8.75 kg of CO2, respectively, while the synthesis of 1.0 kg of lab-scale MXene released 428.10 kg of CO2. The study indicates that electricity usage has a greater impact than chemical usage, suggesting that incorporating recycled resources and renewable energy can enhance the sustainability of MXene synthesis. Understanding the LCA of MXenes contributes to the industrialization of this material, enabling informed decisions for its production.59
2.2. Biocompatibility and toxicity
Biocompatibility and toxicity are crucial considerations in the context of MOFs and MXenes, as these materials are intended for use within living organisms. Understanding the impact of these nanomaterials on human health and the environment is essential. While MOFs and MXenes hold great promise, there is still ongoing research to thoroughly assess their biocompatibility and potential toxicity. It is imperative to conduct comprehensive studies to determine any potential risks and to develop strategies for safe and sustainable applications.602.3. Application and disposal considerations
Environmental concerns extend beyond the synthesis process to the application and eventual disposal of MOFs and MXenes in biomedical settings. For instance, the fate of these materials in the human body and their potential release into the environment post-use requires careful examination. Therefore, researchers should consider strategies for ensuring the safe utilization and disposal of these materials to minimize any adverse effects on the environment and public health.613. Environmental implications in biomedical applications of MXenes and MOFs
While the employment of MOFs and MXenes in biomedicine holds great promise, several challenges need to be addressed to ensure their safe and sustainable implementation. In addition, exploring future perspectives in this field can guide researchers toward overcoming these challenges and maximizing the potential benefits of these materials. In this section, we will discuss some of the key challenges and provide insights into the future perspectives of MOFs and MXenes in biomedical applications. From our perspective, some of the important challenges that need to be addressed in this field are (i) biocompatibility and toxicity, (ii) stability, (iii) degradation, (iv) scalability, (v) controlled release and drug loading efficiency, and (vi) manufacturing processes.The cytotoxicity of MOFs and MXene depends on several factors, including their composition, size, surface properties, and degradation behavior.20,62–64 Numerous studies have investigated the cytotoxicity of various MOFs and some of the MXenes using different cell lines and experimental conditions.65–68 MOFs have shown relatively low cytotoxicity in many cases.19,30,63,69 However, it is important to note that some MOFs have exhibited cytotoxic effects, particularly at higher concentrations or prolonged exposure times. The cytotoxicity mechanisms associated with MOFs can be attributed to several factors.70 One factor is the release of metal ions or organic ligands from the MOF structure, which can induce toxicity or interfere with cellular processes.71 In addition, the size and surface charge of MOFs can influence their cellular uptake and interactions with intracellular components, potentially leading to cytotoxic effects.72 Moreover, the potential presence of impurities or residual reactants from the synthesis process can contribute to the observed cytotoxicity.73,74 The cytotoxicity mechanisms of MXenes are still not fully understood and require further investigation. Some studies have suggested that MXenes can induce oxidative stress in cells, leading to cytotoxic effects.75 The surface chemistry and functional groups of MXenes can play a role in modulating their cytotoxicity by affecting their interactions with cells and cellular components. In addition, the lateral size and morphology of MXenes can influence their cellular uptake and subsequent intracellular responses.60,76 To enhance the biocompatibility of MOFs and MXenes and mitigate potential cytotoxicity and eliminate the potential environmental problems, several strategies can be explored, including (i) using green and sustainable precursors, (ii) surface modifications with natural and biocompatible components like polymers and leaf extracts, (iii) reducing the size of the material, (iv) using natural ligands/linkers on the surface, and (v) using green solvents.
After the employment of MOFs and MXenes for biomedical purposes, these materials may be excreted from the body or disposed of through waste streams. If not properly managed, the release of MOFs and MXenes into the environment could pose potential risks. Their persistence and potential for bioaccumulation raise concerns about their long-term impacts on ecosystems and organisms.77–79 To address the environmental implications associated with MOFs and MXenes, researchers and industries must work together to develop sustainable strategies. These strategies include the adoption of green chemistry principles such as using benign solvents, using green and sustainable precursors, reducing energy consumption, and implementing efficient waste management practices. Moreover, the exploration of recycling and reusing MOFs and MXenes can contribute to a circular economy approach, minimizing waste and resource consumption.80,81
3.1. Biosensing
In biosensing, MXene-based materials have emerged as promising candidates because of their unique properties such as high electrical conductivity, large surface area, unique mechanical properties, biocompatibility, and tunable surface properties.82–84 MXenes possess a high surface area, which allows for efficient immobilization of biomolecules such as enzymes, antibodies, or DNA probes. This enhanced surface area facilitates a higher loading capacity of biomolecules, leading to improved sensitivity and detection limits in biosensing.85,86 In addition, MXenes exhibit excellent electrical conductivity, which enables direct electron transfer between the immobilized biomolecules and the electrode surface, eliminating the need for additional redox mediators. This direct electron transfer enhances the efficiency and speed of the biosensing process, resulting in rapid and real-time detection of target analytes.87,88 Notably, MXenes have exceptional mechanical and chemical stability, making them highly resistant to degradation and providing long-term stability for biosensing applications. This stability ensures the reliability and reproducibility of biosensor performance, even under harsh conditions. MXenes can be also easily functionalized or modified with various functional groups, allowing for the specific recognition and binding of target analytes. This functionalization enhances the selectivity and specificity of biosensors, reducing the chances of false-positive or false-negative results. Lastly, MXenes are compatible with different transduction methods, including optical, electrochemical, and mass-based techniques. This versatility enables the development of diverse biosensing platforms, catering to different analytical needs and applications.89–92 MXene-based and MOF-based materials offer desirable properties such as high surface area, tunable pore sizes, and excellent chemical stability, which can enhance the sensitivity and selectivity of biosensors. This, in turn, enables improved detection and analysis of biological molecules, leading to advancements in medical diagnostics and research. While MXenes and MOFs do have unique relevance for their biological applications in biosensing, it is crucial to consider the environmental implications associated with their manufacturing. By addressing these concerns and adopting sustainable practices, researchers can ensure the responsible use of these materials in biomedicine while minimizing their impact on the environment.The integration of MXene nanosheets with other components can be applied for developing highly sensitive and selective biosensors for the detection of various analytes. These biosensors offer rapid response times, excellent stability, and wide detection ranges, making them suitable for applications in clinical diagnostics and environmental monitoring. In one study, dual-signal electrochemical biosensor was developed for the detection of neutrophil gelatinase-associated lipocalin (NGAL) through the fabrication of MXene-loaded polyaniline nanocomposites as the sensing platform to anchor gold (Au) nanoparticles and immobile primary antibodies.93 Thus, a novel approach was introduced for the early diagnosis of acute kidney injury (AKI) by designing a sensitive dual-signal sandwich-type electrochemical immunosensor for detecting NGAL. After the preparation of an Au NP-loaded copper MOF decorated with a single-walled carbon nanohorn nanocomposite, they bound the NGAL affinity peptide (Pep) to form a Pep/Au/Cu-MOF/SWNH nanocomposite probe. The probe demonstrated two distinct signals for NGAL detection: a square wave voltammetry (SWV) signal and a current–time curve signal; the SWV signal represented electron transfer between Cu2+ and Cu+ of the Cu-MOF, while the current–time curve signal resulted from the reduction of H2O2 by the Au/Cu-MOF/SWNH nanocomposite, exhibiting high electrocatalytic activity. The developed immunosensor exhibited excellent analytical performance, with a linear detection range of 0.00001–10 ng mL−1 for NGAL and detection limits of 0.0074 pg mL−1 (SWV) and 0.0405 pg mL−1. The researchers further validated the immunosensor by detecting NGAL in a sample, highlighting its potential applications in the diagnosis of acute kidney injury. This study offering a sensitive and reliable method based on MXene and MOF for early AKI diagnosis, which could ultimately contribute to improved patient outcomes and treatment strategies.93
3.2. Cancer therapy and drug delivery
MXene- and MOF-based nanosystems have been studied for targeted cancer therapy and drug delivery (Fig. 2).94,95 The incorporation of therapeutic agents within MXene nanosheets can enable the controlled release and targeted delivery of drugs/therapeutic agents to specific tissues or cells. This approach enhances drug efficacy while minimizing side effects. The evolution of η values is important which represent the loading and release efficiencies of the drugs, providing crucial insights into the performance of MXene-based drug delivery systems. MXene nanosheets have been also explored for photothermal tumor ablation as a promising strategy for cancer therapy. These materials exhibited remarkable photothermal conversion efficiency; they can generate localized hyperthermia upon exposure to near-infrared (NIR) light, resulting in the selective destruction of tumor cells while sparing healthy tissues. In this context, efficient tumor tissue penetration is vital in effective cancer therapy. MXene-based nanosystems can penetrate deep into tumor tissues, ensuring efficient delivery of therapeutic agents and enhancing treatment outcomes. Several optimization processes have been explored to enhance the penetration of MXene nanosheets such as surface modification and size control, providing enhanced tumor targeting and improved therapeutic efficacy.96–98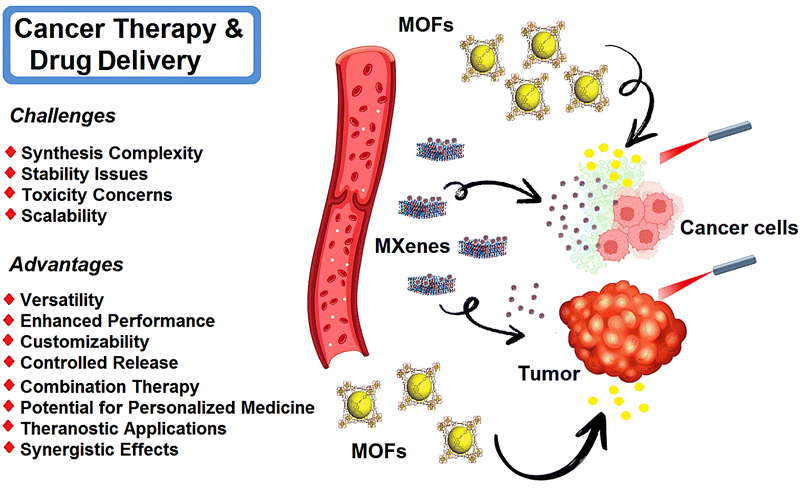 | ||
| Fig. 2 Schematic illustration of MXene- and MOF-based materials for targeted cancer therapy and drug delivery, with advantages and important challenges. | ||
MOFs can serve as effective drug carriers, providing controlled and targeted delivery of therapeutic agents.99,100 They can encapsulate a wide range of drugs, such as chemotherapeutic agents, immunotherapeutic agents, and nucleic acids. They can be employed in PDT, a minimally invasive treatment that utilizes light to activate photosensitizers and generate reactive oxygen species to destroy cancer cells. In this context, MOFs can serve as carriers for photosensitizers, providing protection and controlled release of these agents at the tumor site, enhancing the efficacy of PDT.101 The porous structure of MOFs allows for high drug loading capacity, ensuring efficient delivery to the target site.102 In addition, MOFs enable the co-delivery of multiple drugs or therapeutic agents. This approach allows for combination therapy, where different drugs with complementary mechanisms of action can be delivered simultaneously.103,104 Combination therapy can enhance treatment efficacy, overcome drug resistance, and target multiple pathways involved in cancer progression.105 MOF-based materials can be engineered to exhibit tunable drug release profiles. By modifying the structure or incorporating stimuli-responsive components, the release of drugs can be controlled based on specific triggers such as changes in pH, temperature, or external stimuli. This controlled release mechanism enhances therapeutic efficacy and reduces side effects.106,107 MOFs can be functionalized with targeting ligands or antibodies to selectively bind to cancer cells or specific tissues. This targeted delivery approach improves the specificity of drug delivery, reducing off-target effects and enhancing the accumulation of therapeutic agents at the desired site. Zhao et al.108 developed the lanthanide-doped upconversion nanoparticles (DUCNP)@Mn-MOF nanocarrier, which effectively loaded and delivered a cytotoxic antitumor agent called 3-F-10-OH-evodiamine (FOE). By combining the pH-responsive and peroxidase-like properties of Mn-MOF with the unique optical features of DUCNPs, the prepared system displayed synergistic chemodynamic and chemotherapeutic effects. This nanocarrier successfully addressed the limitations of FOE, including unfavorable physicochemical features and limited in vivo potency. It also exhibited excellent tumor targeting capability by responding to the tumor microenvironment. Consequently, this MOF-based nanosystem exhibited selective and bioavailable drug delivery features, holding promise for cancer therapy. In a mouse breast cancer model, DUCNP@Mn-MOF/FOE could effectively inhibit tumor growth without causing noticeable toxicity.108
While MOF- and MXene-based materials show promising potential in the field of bio- and nanomedicine, it is important to consider their impact on the environment. Conducting a LCA can provide valuable insights into the environmental impacts of MXenes and MOFs throughout their entire life cycle, including synthesis, usage, and disposal.59 This analysis can help identify areas where improvements can be made to reduce their environmental footprint. The toxicity and biocompatibility of MXenes and MOFs need to be thoroughly investigated for their clinical applications in drug delivery and cancer therapy.40,109–111 While these materials may offer advantages in drug delivery and therapy, any potential adverse effects on the environment and human health should be carefully evaluated. Long-term studies are necessary to understand their behavior and potential risks.19 In addition, the synthesis of MOF- and MXene-based systems may involve the use of various chemicals and energy-intensive processes.59 These processes can potentially generate waste and emissions, contributing to environmental pollution. It is crucial to develop sustainable and eco-friendly synthesis methods to minimize the environmental impact.20,32,112–114
3.3. Antibacterial applications
MXene-based composites have demonstrated excellent potential as antibacterial agents, showing great promises in photothermal antibacterial therapy.115,116 MXene-based nanosystems are able to disrupt bacterial cell membranes and inhibit bacterial growth. Mechanism studies revealed several antibacterial strategies, including MXene's inherent antibacterial properties with the photothermal effects as well as the release of reactive oxygen species (ROS), leading to synergistic antibacterial effects. These findings pave the way for the development of effective antibacterial systems to combat drug-resistant bacteria.115,116 Indeed, the mechanism by which MXene destroys bacterial cell membranes and inhibits bacterial growth is not fully understood. However, it is believed that antimicrobial properties of MXenes are attributed to its ability to disrupt the integrity of bacterial cell membranes. This disruption can lead to leakage of cellular contents, loss of membrane potential, and ultimately cell death. In addition, high surface area and unique physicochemical properties of MXenes may contribute to their antimicrobial activity by promoting interactions with bacterial cells and interfering with essential cellular processes.117–120 Further research is needed to fully elucidate the specific mechanisms involved. On the other hand, the antibacterial performance of MOFs is typically associated with physical damage to bacterial cells; MOFs can act as a reservoir of metal ions, which are released to inhibit the growth of bacteria.121 In this context, there are some strategies to enhance the antibacterial effects of MOF-based systems, which include size modulation, pore size modulation, and adjustment of coordination environment of active sites as well as the design of MOF-based composites by combining MOFs with other materials such as nanoparticles, antibiotics, phytochemicals, and polymers.122,123 The surface modification of MOFs can be accomplished by applying photosensitizers with antibacterial purposes; MOF-based systems can also be employed as carriers to transport the agents into the pathogenic bacteria. In addition, the release of antibacterial metal ions or organic ligands from MOF-based systems has shown efficient synergistic antibacterial effects.124 Overall, MOFs have great potential for use in antibacterial materials, and it is hoped that their antibacterial qualities will garner more interest in the fields of water and environmental treatment, biomedical science, and materials science, leading to more research and practical applications of MOFs.124 Fig. 3 indicates the effects of MXene's optical properties, distribution, and persistence on its antimicrobial attributes. The efficacy of MXenes in photothermal conversion is emphasized, suggesting that they may enhance antibacterial effects via NIR-induced mechanisms and impede bacterial cell growth via photothermal therapy. In addition, the figure illustrates how enhanced dispersion in water can elevate biocidal rates, though the oxidation of MXene may exert a detrimental influence on this aspect.125 Fig. 4 provides a diagrammatic representation of the structural components comprising a Gram-negative bacterial cell (Fig. 4a). Insights into probable scenarios deriving from these interactions are provided by the interaction results between the outer membrane of the bacterial cell and a bacteriophage, Ti3C2Tx MXene, and Ti3C2Tx MXene loaded with bacteriophage, as illustrated in Fig. 4b.125,126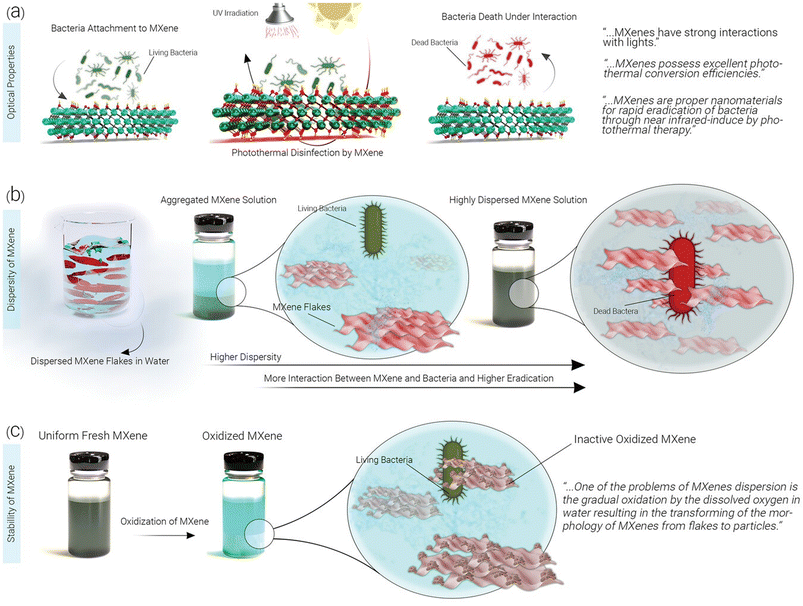 | ||
| Fig. 3 The impact of (a) optical characteristics, (b) distribution, and (c) endurance of MXenes on their antimicrobial features. The substantial photothermal conversion efficacy of MXenes could intensify the antibacterial effect through NIR-induced processes, thereby impeding bacterial cells via photothermal therapy. Enhanced dispersion of nanomaterials in water would amplify the available surface area for engaging with bacteria, leading to elevated biocidal rates. Nonetheless, the oxidation of MXene could exert a detrimental influence on this aspect. Reproduced from ref. 125 with permission from Wiley-VCH GmbH, copyright 2023. | ||
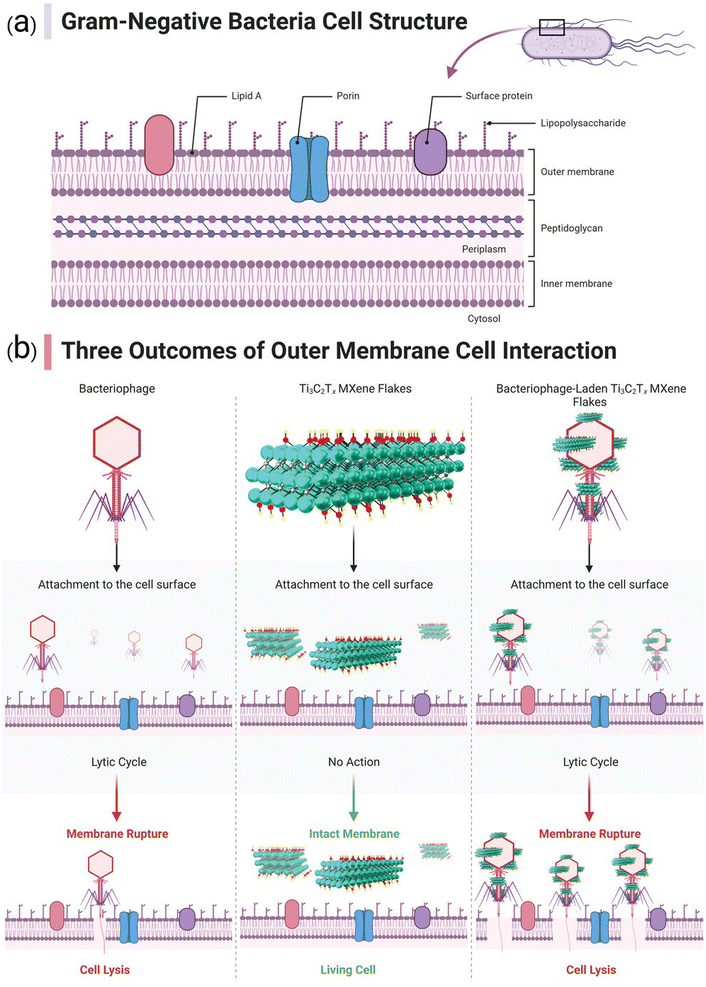 | ||
| Fig. 4 (a) Diagram representing the structural components of a Gram-negative bacterial cell. (b) Depiction of three possible outcomes resulting from the interaction between the outer membrane of the bacterial cell and a bacteriophage, Ti3C2Tx MXene, and Ti3C2Tx MXene loaded with bacteriophage. Reproduced from ref. 125 with permission from Wiley-VCH GmbH, copyright 2023; reproduced from ref. 126 with permission from Elsevier, copyright 2021. | ||
4. Challenges and future perspectives
To facilitate the development of effective biosafety materials and solve biosafety-related problems, the integration of biosafety and materials science ought to be considered. Despite great potential of MXenes and MOFs in biomedicine, their implications in the environment should be systematically evaluated.4.1. MXenes
There is a vital need for further systematic analysis of the biological effects of MXenes, given their great potential for biomedical and environmental applications.113 MXenes with their unique mechanical properties, large surface area, and substantial light absorption potential have been widely explored for biomedical applications. However, there is still a long way to their clinical uses, which is due to the lack of accurate and pre-clinical tests as well as clinical translation studies and systematic toxicological analyses. Designing next-generation systems with biomedical applications requires comprehensive in vitro and in vivo assessments, which for MXenes is still in their early stages and requires more elaborative studies.127Biodegradability is one of the crucial factor in biosafety evaluation of MXenes before their biomedical and clinical applications.113,127 Since the toxicity and biocompatibility of MXenes are dependent on size, dose, and surface coating, the employment of optimization techniques can help to improve their biosafety.128 With employment of surface modification/functionalization strategies such as the adjustment of surface terminations, surface-initiated polymerization, the utilization of small or macromolecules, and single heteroatom techniques, their biosafety as well as their electronic, magnetic, optical, mechanical, and thermal properties can be significantly improved.129
The controlled deterioration of materials such as Ti3C2Tx MXene presents promising prospects in particular domains of biomedicine. Significantly, the regulation of therapeutic agent release is an essential stipulation in drug delivery systems. The degradation of MXene, which is initiated by environmental conditions such changes in pH or the presence of particular enzymes, can be applied to accomplish regulated and exact drug release.132 Therefore, the utilization of oxidative degradation of MXenes in the development of intelligent drug delivery systems that adapt to certain biochemical or physiological circumstances in a biological system may serve as an advantage. It is important to understand the mechanisms and kinetics by which MXene degrades in various biomedical settings. These insights enable the optimization of material degradation rates to correspond with the temporal demands of various applications, therefore increasing their efficacy.41
The environmental ramifications and biosafety of MXenes in biomedical applications are significant concerns that are prompted by their oxidative breakdown. Carbon and titanium dioxide, which are byproducts of disintegration, have the ability to enter the environment and exert an impact on ecosystems.133 Furthermore, a thorough evaluation is necessary to determine the toxicity of these results, whether they constitute byproducts of material degradation or are produced after their discharge. It is imperative that stakeholders and researchers assess the prospective advantages and disadvantages linked to the regulated degradation of MXenes in the field of biomedicine, ensuring that their assessment is consistent with wider environmental and biosafety concerns.134 By adopting this comprehensive strategy, it provides that the beneficial application of MXenes does not jeopardize the environment, human health, or the intended therapeutic objectives.
4.2. MOFs
MOF-based systems have been designed with unique properties to address major contemporary challenges in the environmental and biomedical fields. Various MOFs have been introduced with drug delivery, imaging, photodynamic therapy, antibacterial, and targeted cancer therapy applications, which can improve fundamental issues in drug development and therapeutic processes. However, it is necessary to analyze the biosafety of MOFs before their clinical applications, as it was indicated in the in vitro and in vivo toxicity of micron/nanoscale Mg-MOF74. As a result, nanoscale Mg-MOF74 exhibited good biocompatibility with lower cardiotoxicity, demonstrating that the reduction in particle size can be considered as a valuable tactic to improve and expand medical applications of MOFs.135,136 Besides, studies revealed that MOFs had a dose-dependent response against cell lines; but, the prepared MOFs have no significant toxicity (in vitro).137Beyond the synthesis step, it is important to consider the destiny of MOFs and MXenes when they are applied within biological systems and when they are released into the environment after use. Accordingly, it is crucial to carefully assess methods for safe and environmentally friendly use and disposal of these materials in biomedical systems. The full environmental impact of these materials, from processing to final disposal, will be considered in the analysis.138
Given their proposed use within living organisms, biosafety of MOFs and MXenes in biomedical applications becomes of critical importance. To ensure patient safety and identify any potential downsides, a thorough investigation into their biosafety profile is required. In addition, studies will be conducted in vitro and in vivo to identify if MOFs and MXenes are biocompatible.139 Moreover, a full evaluation of current studies on probable toxicity profiles, comprising known toxicological parameters, will be done. Moreover, case studies or real-world examples can be used to highlight the complexities of environmental issues and suggest possible solutions.
The fascinating properties of MOFs such as high surface area/porosity, adjustable pore sizes, and micro-porous structures can enhance the loading of biomolecules and the encapsulation of different pharmaceuticals/therapeutic agents. But, these features also may raise concerns about the potential toxicity of MOFs.140 The toxicity of MOFs is mostly due to the degradation and the materials released from them. The toxic effects of MOFs are likely due to the presence of metal ions and functional groups in the organic ligands. In this context, some parameters such as the particle size, functionalized groups, and ligands as well as the solvent system applied for fabricating MOFs and the types of cross-linkers/metals should be considered since they may promote toxicity in MOFs.141 In addition, the selection of solvents for manufacturing MOFs is an important criterion, which can also have toxic effects. Solvents could be confined in the porous MOFs, leading to short- and long-term health effects.140 It appears that toxicological studies of MOFs are still in their early stages, and more studies are needed to evaluate their environmental implications, particularly the biosafety issues.
5. Conclusion
This review on the environmental implications of MOFs and MXenes in biomedical applications presents several novel insights compared to previous works. It goes beyond the traditional focus on synthesis and applications by emphasizing the environmental fate of MOFs and MXenes, their impact on ecological systems, and the evaluation of their biodegradability and biocompatibility. In addition, the review incorporates life cycle assessments to analyze their overall environmental sustainability and identifies strategies for environmental mitigation. By providing a comprehensive understanding of these materials' environmental implications, the review contributes to advancing sustainable and environmentally friendly applications of MOFs and MXenes in the biomedical field. The employment of MOFs and MXenes in biomedical applications holds tremendous potential for revolutionizing disease treatment, drug delivery, and diagnostics. However, to fully realize their benefits, it is crucial to consider the environmental implications associated with their synthesis and use. This perspective article discussed the role of different green and sustainable approaches in the synthesis of MOFs and MXenes, highlighting their positive impact on environmental aspects. By embracing the green and sustainable strategies including circular economy principles, waste generation in MOF and MXene synthesis can be significantly reduced. Strategies such as the development of greener synthesis processes, recycling and reuse of precursors and by-products, and the utilization of waste streams contribute to waste reduction and resource circularity. Moreover, these strategies promote resource efficiency by selecting sustainable and abundant raw materials and enabling the recycling and recovery of MOFs and MXenes at the end of their life cycle. Circular economy principles also play a vital role in conserving energy during MOF and MXene synthesis. Optimizing synthesis routes, adopting energy-efficient technologies, and integrating renewable energy sources minimize the environmental impact associated with energy consumption. Furthermore, the use of less hazardous materials and the implementation of closed-loop systems prevent the release of pollutants, thus reducing the overall environmental footprint.Overall, considering the environmental impact is crucial when evaluating the overall suitability of MXenes and MOFs for biomedical applications.
5.1. Sustainability
The manufacturing processes of MXenes and MOFs can involve the use of various chemicals and energy-intensive procedures. It is important to assess and minimize the environmental footprint of these processes to ensure sustainable production. By adopting greener manufacturing methods, researchers can reduce the carbon footprint and potential ecological damage associated with the production of these materials.5.2. Toxicity, biocompatibility, and biodegradability
While MXenes and MOFs hold great potential in biomedicine, it is imperative to understand their potential toxicity, biocompatibility, and biodegradability. The environmental implications arise when these materials are released into the environment, either during production or after use. If not properly managed, these materials could potentially accumulate in ecosystems and impact biodiversity. Thus, it is pivotal to study and mitigate any potential adverse effects on the environment.5.3. Life cycle assessment (LCA)
A comprehensive LCA is essential to evaluate the environmental impact of MXenes and MOFs throughout their entire life cycle, from raw material extraction to manufacturing, use, and disposal. This assessment helps identify areas for improvement and enables the development of more sustainable processes.Conflicts of interest
The author(s) declare no competing interest.References
- H. Ding, Y. Li, M. Li, K. Chen, K. Liang, G. Chen, J. Lu, J. Palisaitis, P. O. Å. Persson, P. Eklund, L. Hultman, S. Du, Z. Chai, Y. Gogotsi and Q. Huang, Science, 2023, 379, 1130–1135 CrossRef CAS PubMed.
- M. Ding, X. Cai and H.-L. Jiang, Chem. Sci., 2019, 10, 10209–10230 RSC.
- X. Zhan, C. Si, J. Zhou and Z. Sun, Nanoscale Horiz., 2020, 5, 235–258 RSC.
- R. Garg and F. Vitale, MRS Bull., 2023, 48, 283–290 CrossRef CAS PubMed.
- S. M. George and B. Kandasubramanian, Ceram. Int., 2020, 46, 8522–8535 CrossRef CAS.
- H. Huang, R. Jiang, Y. Feng, H. Ouyang, N. Zhou, X. Zhang and Y. Wei, Nanoscale, 2020, 12, 1325–1338 RSC.
- L. Chen, Y. Li, K. Liang, K. Chen, M. Li, S. Du, Z. Chai, M. Naguib and Q. Huang, Small Methods, 2023, 7, 2300054 CrossRef CAS PubMed.
- J. Pang, S. Peng, C. Hou, X. Wang, T. Wang, Y. Cao, W. Zhou, D. Sun, K. Wang, M. H. Rümmeli, G. Cuniberti and H. Liu, Nano Res., 2022, 1–29, DOI:10.1007/s12274-12022-15272-12278.
- G. Hu, Z. Cen, Y. Xiong and K. Liang, Nanoscale, 2023, 15, 5579–5597 RSC.
- S. M. George and B. Kandasubramanian, Ceram. Int., 2020, 46, 8522–8535 CrossRef CAS.
- J. Cao, X. Li and H. Tian, Curr. Med. Chem., 2020, 27, 5949–5969 CrossRef CAS PubMed.
- M. Shyngys, J. Ren, X. Liang, J. Miao, A. Blocki and S. Beyer, Front. Bioeng. Biotechnol., 2021, 9, 603608 CrossRef PubMed.
- S. Iravani and R. S. Varma, Mater. Adv., 2021, 2, 2906–2917 RSC.
- Y. Lei, W. Zhao, Y. Zhang, Q. Jiang, J. H. He, A. J. Baeumner, O. S. Wolfbeis, Z. L. Wang, K. N. Salama and H. N. Alshareef, Small, 2019, 15, 1901190 CrossRef PubMed.
- X.-Q. Wu, Y. Liu, P.-Q. Feng, X.-H. Wei, G.-M. Yang, X.-H. Qiu and J.-G. Ma, Chem. Commun., 2019, 55, 4059–4062 RSC.
- Y. Li, S. Huang, S. Peng, H. Jia, J. Pang, B. Ibarlucea, C. Hou, Y. Cao, W. Zhou, H. Liu and G. Cuniberti, Small, 2023, 19, 2206126 CrossRef CAS PubMed.
- H.-S. Wang, Coord. Chem. Rev., 2017, 349, 139–155 CrossRef CAS.
- M. Huang, Z. Gu, J. Zhang, D. Zhang, H. Zhang, Z. Yang and J. Qu, J. Mater. Chem. B, 2021, 9, 5195–5220 RSC.
- S. Mallakpour, E. Nikkhoo and C. M. Hussain, Coord. Chem. Rev., 2022, 451, 214262 CrossRef CAS.
- G. P. Lim, C. F. Soon, N. L. Ma, M. Morsin, N. Nayan, M. K. Ahmad and K. S. Tee, Environ. Res., 2021, 201, 111592 CrossRef CAS PubMed.
- J. Huang, Z. Li, Y. Mao and Z. Li, Nano Sel., 2021, 2, 1480–1508 CrossRef CAS.
- V. Eskandari, H. Sahbafar, E. Karooby, M. H. Heris, S. Mehmandoust, D. Razmjoue and A. Hadi, Spectrochim. Acta, Part A, 2023, 298, 122762 CrossRef CAS PubMed.
- V. Eskandari, H. Sahbafar, L. Zeinalizad, R. Mahmoudi, F. Karimpour, A. Hadi and H. Bardania, Arabian J. Chem., 2022, 15, 104005 CrossRef CAS.
- J. Peng, X. Chen, W.-J. Ong, X. Zhao and N. Li, Chem, 2019, 5, 18–50 CAS.
- N. H. Solangi, R. R. Karri, N. M. Mubarak and S. A. Mazari, Adv. Ind. Eng. Polym. Res., 2023 DOI:10.1016/j.aiepr.2023.09.002.
- V. Eskandari, A. Hadi and H. Sahbafar, Adv. Nano Res., 2022, 13, 417–426 Search PubMed.
- V. Eskandari, A. Kordzadeh, L. Zeinalizad, H. Sahbafar, H. Aghanouri, A. Hadi and S. Ghaderi, Opt. Mater., 2022, 128, 112310 CrossRef CAS.
- X. Zhuang, S. Zhang, Y. Tang, F. Yu, Z. Li and H. Pang, Coord. Chem. Rev., 2023, 490, 215208 CrossRef CAS.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- S. Kumaraguru, R. Nivetha, K. Gopinath, E. Sundaravadivel, B. O. Almutairi, M. H. Almutairi, S. Mahboob, M. Kavipriya, M. Nicoletti and M. Govindarajan, J. Mater. Res. Technol., 2022, 18, 1732–1745 CrossRef CAS.
- J. A. Kumar, P. Prakash, T. Krithiga, D. J. Amarnath, J. Premkumar, N. Rajamohan, Y. Vasseghian, P. Saravanan and M. Rajasimman, Chemosphere, 2022, 286, 131607 CrossRef CAS PubMed.
- A. Rozmysłowska-Wojciechowska, A. Szuplewska, T. Wojciechowski, S. Poźniak, J. Mitrzak, M. Chudy, W. Ziemkowska, L. Chlubny, A. Olszyna and A. M. Jastrzębska, Mater. Sci. Eng. C, 2020, 111, 110790 CrossRef PubMed.
- A. Jastrzębska, A. Szuplewska, T. Wojciechowski, M. Chudy, W. Ziemkowska, L. Chlubny, A. Rozmysłowska and A. Olszyna, J. Hazard. Mater., 2017, 339, 1–8 CrossRef PubMed.
- M. Naguib, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2021, 33, 2103393 CrossRef CAS PubMed.
- J. J. Y. Lim and A. N. K. Lup, Environ. Sci.: Adv., 2022, 1, 570–583 CAS.
- S. Liu, Z. Teng, H. Liu, T. Wang, G. Wang, Q. Xu, X. Zhang, M. Jiang, C. Wang and W. Huang, Angew. Chem., 2022, 134, e202207026 CrossRef.
- N. H. Solangi, S. A. Mazari, N. M. Mubarak, R. R. Karri, N. Rajamohan and D.-V. N. Vo, Environ. Res., 2023, 222, 115337 CrossRef CAS PubMed.
- S. S. Siwal, H. Kaur, G. Chauhan and V. K. Thakur, Adv. NanoBiomed Res., 2023, 3, 2200123 CrossRef CAS.
- L. He, M. Shang, Z. Chen and Z. Yang, Chem. Rec., 2023, e202300018, DOI:10.1002/tcr.202300018.
- M. Moharramnejad, A. Ehsani, M. Shahi, S. Gharanli, H. Saremi, R. E. Malekshah, Z. S. Basmenj, S. Salmani and M. Mohammadi, J. Drug Delivery Sci. Technol., 2023, 81, 104285 CrossRef CAS.
- A. Khosla, Sonu, H. T. A. Awan, K. Singh, Gaurav, R. Walvekar, Z. Zhao, A. Kaushik, M. Khalid and V. Chaudhary, Adv. Sci., 2022, 9, 2203527 CrossRef CAS PubMed.
- S. Zhang, M. Zheng, Y. Tang, R. Zang, X. Zhang, X. Huang, Y. Chen, Y. Yamauchi, S. Kaskel and H. Pang, Adv. Funct. Mater., 2022, 32, 2204714 CrossRef CAS.
- S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- X.-T. Tian, P.-P. Cao, H. Zhang, Y.-H. Li and X.-B. Yin, Chem. Commun., 2019, 55, 6241–6244 RSC.
- W. Yu, W. Zhen, Q. Zhang, Y. Li, H. Luo, J. He and Y. Liu, ChemMedChem, 2020, 15, 1766–1775 CrossRef CAS PubMed.
- Z.-X. Chen, M.-D. Liu, M.-K. Zhang, S.-B. Wang, L. Xu, C.-X. Li, F. Gao, B.-R. Xie, Z.-L. Zhong and X.-Z. Zhang, Adv. Funct. Mater., 2018, 28, 1803498 CrossRef.
- S.-Y. Li, H. Cheng, B.-R. Xie, W.-X. Qiu, J.-Y. Zeng, C.-X. Li, S.-S. Wan, L. Zhang, W.-L. Liu and X.-Z. Zhang, ACS Nano, 2017, 11, 7006–7018 CrossRef CAS PubMed.
- W. Zhang, J. Lu, X. Gao, P. Li, W. Zhang, Y. Ma, H. Wang and B. Tang, Angew. Chem., 2018, 57, 4891–4896 CrossRef CAS PubMed.
- X. Han, W.-M. Chen, X. Han, Y.-Z. Tan and D. Sun, J. Mater. Chem. A, 2016, 4, 13040–13045 RSC.
- B. Xu, Z. Huang, Y. Liu, S. Li and H. Liu, Nano Today, 2023, 48, 101690 CrossRef CAS.
- S. F. Fatima, R. Sabouni, R. Garg and H. Gomaa, Colloids Surf., B, 2023, 113266 CrossRef CAS PubMed.
- J. Tang, C. Huang, Y. Liu, T. Wang, M. Yu, H. Hao, W. Zeng, W. Huang, J. Wang and M. Wu, Coord. Chem. Rev., 2023, 490, 215211 CrossRef CAS.
- H. Corvellec, A. F. Stowell and N. Johansson, J. Ind. Ecol., 2022, 26, 421–432 CrossRef.
- M. Geissdoerfer, P. Savaget, N. M. Bocken and E. J. Hultink, J. Cleaner Prod., 2017, 143, 757–768 CrossRef.
- W. R. Stahel, Nature, 2016, 531, 435–438 CrossRef CAS PubMed.
- J. Korhonen, A. Honkasalo and J. Seppälä, Ecol. Econ., 2018, 143, 37–46 CrossRef.
- R. Cucciniello and D. Cespi, Recycling, 2018, 3, 22 CrossRef.
- A. S. Jatoi, N. M. Mubarak, Z. Hashmi, N. H. Solangi, R. R. Karri, T. Y. Hua, S. A. Mazari, J. R. Koduru and A. Alfantazi, Chemosphere, 2022, 137497 Search PubMed.
- M. D. Firouzjaei, S. K. Nemani, M. Sadrzadeh, E. K. Wujcik, M. Elliott and B. Anasori, Adv. Mater., 2023, 35, 2300422 CrossRef PubMed.
- H. Huang, R. Jiang, Y. Feng, H. Ouyang, N. Zhou, X. Zhang and Y. Wei, Nanoscale, 2020, 12, 1325–1338 RSC.
- J. Chen, Q. Huang, H. Huang, L. Mao, M. Liu, X. Zhang and Y. Wei, Nanoscale, 2020, 12, 3574–3592 RSC.
- C. Tamames-Tabar, D. Cunha, E. Imbuluzqueta, F. Ragon, C. Serre, M. J. Blanco-Prieto and P. Horcajada, J. Mater. Chem. B, 2014, 2, 262–271 RSC.
- Q. Hu, J. Yu, M. Liu, A. Liu, Z. Dou and Y. Yang, J. Med. Chem., 2014, 57, 5679–5685 CrossRef CAS PubMed.
- J. Wu, Y. Yu and G. Su, Nanomaterials, 2022, 12, 828 CrossRef CAS PubMed.
- W. Shang, L. Peng, P. Guo, H. Hui, X. Yang and J. Tian, ACS Biomater. Sci. Eng., 2019, 6, 1008–1016 CrossRef PubMed.
- A. M. Jastrzębska, B. Scheibe, A. Szuplewska, A. Rozmysłowska-Wojciechowska, M. Chudy, C. Aparicio, M. Scheibe, I. Janica, A. Ciesielski and M. Otyepka, Mater. Sci. Eng. C, 2021, 119, 111431 CrossRef PubMed.
- S. Ahmadi, V. Jajarmi, M. Ashrafizadeh, A. Zarrabi, J. T. Haponiuk, M. R. Saeb, E. C. Lima, M. Rabiee and N. Rabiee, J. Hazard. Mater., 2022, 436, 129259 CrossRef CAS PubMed.
- N. Rabiee, M. Bagherzadeh, M. Jouyandeh, P. Zarrintaj, M. R. Saeb, M. Mozafari, M. Shokouhimehr and R. S. Varma, ACS Appl. Bio Mater., 2021, 4, 5106–5121 CrossRef CAS PubMed.
- Z.-C. Wang, Y. Zhang and Z.-Y. Li, J. Cluster Sci., 2018, 29, 1285–1290 CrossRef CAS.
- F. Hao, Z. Y. Yan and X. P. Yan, Small Sci., 2022, 2, 2200044 CrossRef CAS.
- M. Ahmadi, S. M. Ayyoubzadeh, F. Ghorbani-Bidkorbeh, S. Shahhosseini, S. Dadashzadeh, E. Asadian, M. Mosayebnia and S. Siavashy, Heliyon, 2021, 7, e06914 CrossRef CAS PubMed.
- L. Treuel, X. Jiang and G. U. Nienhaus, J. R. Soc., Interface, 2013, 10, 20120939 CrossRef PubMed.
- A. Ma, Z. Luo, C. Gu, B. Li and J. Liu, Inorg. Chem. Commun., 2017, 77, 68–71 CrossRef CAS.
- M. Ibrahim, W. H. Abuwatfa, N. S. Awad, R. Sabouni and G. A. Husseini, Pharmaceutics, 2022, 14, 254 CrossRef CAS PubMed.
- B. e. Scheibe, J. K. Wychowaniec, M. Scheibe, B. Peplinska, M. Jarek, G. Nowaczyk and Ł. Przysiecka, ACS Biomater. Sci. Eng., 2019, 5, 6557–6569 CrossRef CAS PubMed.
- V. S. Sivasankarapillai, A. K. Somakumar, J. Joseph, S. Nikazar, A. Rahdar and G. Z. Kyzas, Nano-Struct. Nano-Objects, 2020, 22, 100457 CrossRef CAS.
- S. Iravani and R. S. Varma, Nanomaterials, 2022, 12, 1200 CrossRef CAS PubMed.
- A. Jastrzębska, A. Szuplewska, A. Rozmysłowska-Wojciechowska, M. Chudy, A. Olszyna, M. Birowska, M. Popielski, J. Majewski, B. Scheibe and V. Natu, 2D Materials, 2020, 7, 025018 CrossRef.
- B. Rashid, A. Anwar, S. Shahabuddin, G. Mohan, R. Saidur, N. Aslfattahi and N. Sridewi, Materials, 2021, 14, 4370 CrossRef CAS PubMed.
- V. V. Butova, I. A. Pankin, O. A. Burachevskaya, K. S. Vetlitsyna-Novikova and A. V. Soldatov, Inorg. Chim. Acta, 2021, 514, 120025 CrossRef CAS.
- H. Shi, M. Yue, C. J. Zhang, Y. Dong, P. Lu, S. Zheng, H. Huang, J. Chen, P. Wen and Z. Xu, ACS Nano, 2020, 14, 8678–8688 CrossRef CAS PubMed.
- R. Khan and S. Andreescu, Sensors, 2020, 20, 5434 CrossRef CAS PubMed.
- B. Xu, C. Zhi and P. Shi, J. Phys.: Mater., 2020, 3, 031001 CAS.
- Z. U. D. Babar, B. D. Ventura, R. Velotta and V. Iannotti, RSC Adv., 2022, 12, 19590–19610 RSC.
- S. Alwarappan, N. Nesakumar, D. Sun, T. Y. Hu and C.-Z. Li, Biosens. Bioelectron., 2022, 205, 113943 CrossRef CAS PubMed.
- Z. Wang, Z. Zhang, Y. Zhang, X. Xu, T. Shen, H. Pan and D. Chang, Talanta, 2023, 265, 124848 CrossRef CAS PubMed.
- S. K. Bhardwaj, H. Singh, M. Khatri, K.-H. Kim and N. Bhardwaj, Biosens. Bioelectron., 2022, 202, 113995 CrossRef CAS PubMed.
- K. Deshmukh, T. Kovářík and S. K. Pasha, Coord. Chem. Rev., 2020, 424, 213514 CrossRef CAS.
- S. Kumar, Y. Lei, N. H. Alshareef, M. A. Quevedo-Lopez and K. N. Salama, Biosens. Bioelectron., 2018, 121, 243–249 CrossRef CAS PubMed.
- L. Lorencova, V. Gajdosova, S. Hroncekova, T. Bertok, J. Blahutova, A. Vikartovska, L. Parrakova, P. Gemeiner, P. Kasak and J. Tkac, Electroanalysis, 2019, 31, 1833–1844 CrossRef CAS.
- D. Lu, H. Zhao, X. Zhang, Y. Chen and L. Feng, Biosensors, 2022, 12, 820 CrossRef CAS PubMed.
- X. Zhu, Y. Zhang, M. Liu and Y. Liu, Biosens. Bioelectron., 2021, 171, 112730 CrossRef CAS PubMed.
- H. Liang, C. Chen, J. Zeng, M. Zhou, L. Wang, G. Ning, Q. Duan, R. Han, H. Liu and H. Zhao, ACS Appl. Nano Mater., 2022, 5, 16774–16783 CrossRef CAS.
- L. M. Dong, C. Ye, L. L. Zheng, Z. F. Gao and F. Xia, Nanophotonics, 2020, 9, 2125–2145 CrossRef CAS.
- S. Iravani and R. S. Varma, ACS Biomater. Sci. Eng., 2021, 7, 1900–1913 CrossRef CAS PubMed.
- X. Han, X. Jing, D. Yang, H. Lin, Z. Wang, H. Ran, P. Li and Y. Chen, Theranostics, 2018, 8, 4491–4508 CrossRef CAS PubMed.
- C. Xing, S. Chen, X. Liang, Q. Liu, M. Qu, Q. Zou, J. Li, H. Tan, L. Liu, D. Fan and H. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 27631–27643 CrossRef CAS PubMed.
- B. Zhou, H. Yin, C. Dong, L. Sun, W. Feng, Y. Pu, X. Han, X. Li, D. Du, H. Xu and Y. Chen, Advanced Science, 2021, 8, 2101043 CrossRef CAS PubMed.
- Y. Ye, Y. Zhao, Y. Sun and J. Cao, Int. J. Nanomed., 2022, 17, 2367–2395 CrossRef PubMed.
- S. F. Fatima, R. Sabouni, R. Garg and H. Gomaa, Colloids Surf., B, 2023, 225, 113266 CrossRef CAS PubMed.
- G. G. Matlou and H. Abrahamse, Front. Chem., 2022, 10, 971747 CrossRef CAS PubMed.
- H. D. Lawson, S. P. Walton and C. Chan, ACS Appl. Mater. Interfaces, 2021, 13, 7004–7020 CrossRef CAS PubMed.
- J.-Q. Liu, Z. Lin, D. Liao, C. Jiang, H. Song, A. Nezamzadeh-Ejhieh, M. Zheng, H. Yuan and C. Lu, RSC Med. Chem., 2023, 14, 1914–1933 RSC.
- M. B. Alahri, R. Arshadizadeh, M. Raeisi, M. Khatami, M. S. Sajadi, W. K. Abdelbasset, R. Akhmadeev and S. Iravani, Inorg. Chem. Commun., 2021, 134, 108997 CrossRef.
- R. B. Mokhtari, T. S. Homayouni, N. Baluch, E. Morgatskaya, S. Kumar, B. Das and H. Yeger, Oncotarget, 2017, 8, 38022–38043 CrossRef PubMed.
- Y. Zhang, X. Wang, R. Wang, Y. Chen, L. Wang, Y. Shi, Z. Wang, W. Niu and W. Shi, New J. Chem., 2023, 47, 15407–15421 RSC.
- J. Yang, D. Dai, X. Zhang, L. Teng, L. Ma and Y.-W. Yang, Theranostics, 2023, 13, 295–323 CrossRef CAS PubMed.
- X. Zhao, S. He, B. Li, B. Liu, Y. Shi, W. Cong, F. Gao, J. Li, F. Wang, K. Liu, C. Sheng, J. Su and H.-G. Hu, Nano Lett., 2023, 23, 863–871 CrossRef CAS PubMed.
- F. Mohajer, G. Mohammadi Ziarani, A. Badiei, S. Iravani and R. S. Varma, Micromachines, 2022, 13, 1773 CrossRef PubMed.
- M. R. Saeb, N. Rabiee, M. Mozafari, F. Verpoort, L. G. Voskressensky and R. Luque, Materials, 2021, 14, 7277 CrossRef CAS PubMed.
- M.-X. Wu and Y.-W. Yang, Adv. Mater., 2017, 29, 1606134 CrossRef PubMed.
- A. M. Jastrzębska, A. Szuplewska, T. Wojciechowski, M. Chudy, W. Ziemkowska, L. Chlubny, A. Rozmysłowska and A. Olszyna, J. Hazard. Mater., 2017, 339, 1–8 CrossRef PubMed.
- I. A. Vasyukova, O. V. Zakharova, D. V. Kuznetsov and A. A. Gusev, Nanomaterials, 2022, 12, 1797 CrossRef CAS PubMed.
- A. Mandal, S. Ganguly, S. Mukherjee and D. Das, Dalton Trans., 2019, 48, 13869–13879 RSC.
- M. Khatami, P. Iravani, G. Jamalipour Soufi and S. Iravani, Mater. Technol., 2022, 37, 1890–1905 CrossRef CAS.
- S. Iravani and R. S. Varma, RSC Adv., 2023, 13, 9665–9677 RSC.
- C. Guo, F. Cheng, G. Liang, S. Zhang, S. Duan, Y. Fu, F. Marchetti, Z. Zhang and M. Du, Adv. NanoBiomed Res., 2022, 2, 2200064 CrossRef CAS.
- K. Rasool, M. Helal, A. Ali, C. E. Ren, Y. Gogotsi and K. A. Mahmoud, ACS Nano, 2016, 10, 3674–3684 CrossRef CAS PubMed.
- S. S. Sana, M. Santhamoorthy, R. Haldar, C. J. Raorane, S. Iravani, R. S. Varma and S.-C. Kim, Process Biochem., 2023, 132, 200–220 CrossRef CAS.
- F. Seidi, A. A. Shamsabadi, M. D. Firouzjaei, M. Elliott, M. R. Saeb, Y. Huang, C. Li, H. Xiao and B. Anasori, Small, 2023, 2206716, DOI:10.1002/smll.202206716.
- N. Bhardwaj, S. K. Pandey, J. Mehta, S. K. Bhardwaj, K.-H. Kim and A. Deep, Toxicol. Res., 2018, 7, 931–941 CrossRef CAS PubMed.
- X. Zhang, F. Peng and D. Wang, J. Funct. Biomater., 2022, 13, 215 CrossRef CAS PubMed.
- M. Shen, F. Forghani, X. Kong, D. Liu, X. Ye, S. Chen and T. Ding, Compr. Rev. Food Sci. Food Saf., 2020, 19, 1397–1419 CrossRef CAS PubMed.
- Y. Li, X. Xia, W. Hou, H. Lv, J. Liu and X. Li, Int. J. Nanomed., 2023, 18, 1109–1128 CrossRef CAS PubMed.
- F. Seidi, A. Arabi Shamsabadi, M. Dadashi Firouzjaei, M. Elliott, M. R. Saeb, Y. Huang, C. Li, H. Xiao and B. Anasori, Small, 2023, 19, 2206716 CrossRef CAS PubMed.
- M. Mansoorianfar, K. Shahin, A. Hojjati-Najafabadi and R. Pei, Chemosphere, 2022, 290, 133383 CrossRef CAS PubMed.
- L. Chen, X. Dai, W. Feng and Y. Chen, Acc. Mater. Res., 2022, 3, 785–798 CrossRef CAS.
- H. Li, R. Fan, B. Zou, J. Yan, Q. Shi and G. Guo, J. Nanobiotechnol., 2023, 21, 73, DOI:10.1186/s12951-12023-01809-12952.
- M. Mozafari and M. Soroush, Adv. Mater., 2021, 2, 7277–7307 RSC.
- A. Iqbal, J. Hong, T. Y. Ko and C. M. Koo, Nano Convergence, 2021, 8, 1–22 CrossRef PubMed.
- M. S. Javed, A. Mateen, I. Hussain, A. Ahmad, M. Mubashir, S. Khan, M. A. Assiri, S. M. Eldin, S. S. A. Shah and W. Han, Energy Storage Mater., 2022, 53, 827–872 CrossRef.
- Y. Wang, Z. Li and Q. Hu, Nano Today, 2021, 38, 101127 CrossRef CAS.
- P. G. Moradeeya, A. Sharma, M. A. Kumar and S. Basha, Environ. Res., 2022, 204, 112384 CrossRef CAS PubMed.
- H. Lin, Y. Chen and J. Shi, Advanced Science, 2018, 5, 1800518 CrossRef PubMed.
- Z. Zhu, S. Jiang, Y. Liu, X. Gao, S. Hu, X. Zhang, C. Huang, Q. Wan, J. Wang and X. Pei, Nano Res., 2020, 13, 511–526 CrossRef CAS.
- S. Cao, Y. Li, Y. Tang, Y. Sun, W. Li, X. Guo, F. Yang, G. Zhang, H. Zhou and Z. Liu, Adv. Mater., 2023, 2301011 CrossRef CAS PubMed.
- M. Salehipour, S. Nikpour, S. Rezaei, S. Mohammadi, M. Rezaei, D. Ilbeygi, A. Hosseini-Chegeni and M. Mogharabi-Manzari, Inorg. Chem. Commun., 2023, 152, 110655 CrossRef CAS.
- S. Saxena, M. Johnson, F. Dixit, K. Zimmermann, S. Chaudhuri, F. Kaka and B. Kandasubramanian, Renewable Sustainable Energy Rev., 2023, 178, 113238 CrossRef CAS.
- S. Sagadevan and W.-C. Oh, J. Drug Delivery Sci. Technol., 2023, 104569 CrossRef CAS.
- M. Al Sharabati, R. Sabouni and G. A. Husseini, Nanomaterials, 2022, 12, 277 CrossRef CAS PubMed.
- P. Kumar, B. Anand, Y. F. Tsang, K.-H. Kim, S. Khullar and B. Wang, Environ. Res., 2019, 176, 108488 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |