Supramolecular biomaterials for enhanced cancer immunotherapy
Han
Zhang
a,
Jiafei
Zhu
a,
Tianxu
Fang
b,
Meng
Li
*c,
Guojun
Chen
*b and
Qian
Chen
 *a
*a
aInstitute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, Suzhou 215123, China. E-mail: chenqian@suda.edu.cn
bDepartment of Biomedical Engineering, and the Rosalind & Morris Goodman Cancer Institute, McGill University, Montreal, QC H3G 0B1, Canada. E-mail: guojun.chen@mcgill.ca
cDepartment of Dermatology, Shanghai 9th Peoples’ Hospital, Shanghai Jiaotong University, Shanghai, China. E-mail: lemonlives_dr@163.com
First published on 9th March 2022
Abstract
Cancer immunotherapy has achieved promising clinical results. However, many limitations associated with current cancer immunotherapy still exist, including low response rates and severe adverse effects in patients. Engineering biomaterials for the delivery of immunotherapeutic reagents has been suggested to be an effective strategy to improve cancer immunotherapy. Among different biomaterials, supramolecular biomaterials with flexible and versatile structures and functions have exhibited unparalleled advantages in promoting cancer immunotherapy. In recent years, various supramolecular formulations have been extensively explored as immunotherapeutic delivery platforms due to their high cargo-loading capacity/feasibility, facile immunization function, and excellent biocompatibility, which make them possible candidates for modular and personalized cancer immunotherapy. These nanoarchitectures with unique topologies possess distinguishing advantages in cancer immunotherapy, incarnating a structure–property relationship. Based on extensive state-of-the-art research, this minireview highlights recent advances in supramolecular biomaterials for cancer immunotherapy and discusses the possible mechanisms underlying how supramolecular biomaterials promote the development of cancer immunotherapy together with their potential for clinical translation.
1. Introduction
Cancer immunotherapy is a revolutionary cancer treatment aiming to activate or boost the inherent immune system to recognize and kill cancer cells and has achieved promising therapeutic responses in the clinic.1–3 Adaptive immune responses are essential for killing tumor cells, which are predominantly cell-mediated with the following sequential steps: release of antigens from cancer cells, delivery of cancer antigens to antigen-presenting cells (APCs), presentation of antigens to T cells, priming and activation of T cells, trafficking and infiltrating of cytotoxic T lymphocytes (CTLs) into tumors, recognizing and killing of tumors by CTLs with cytokine secretion, and overcoming immunosuppression in the tumor microenvironment.4,5 In recent years, various cancer immunotherapies, including cancer vaccines,6,7 cytokine therapy,8 immune checkpoint blockade and adoptive T cell therapy,9–11 have shown promising clinical outcomes. Despite great promise in certain scenarios, immune reaction cascades are often thwarted by inefficient and unendurable immune responses, which result in immune escape of tumor cells.12–14 Furthermore, these immunotherapies induce severe and sometimes lethal side effects in cancer patients, such as colitis, hepatitis, and endocrinopathies.15–17 To tackle these limitations of immunotherapy, novel materials spurred by the rapid development of materials science in past decades have been investigated.18 Nano/microscale biomaterials have been developed to encapsulate various immunotherapeutic agents and effectively deliver them into tumor tissue, which could boost multiple stages of immune responses and reduce off-target side effects.19–22 However, these delivery systems also bring additional challenges to clinical translation. Most of these biomaterials are often hindered by tedious organic synthesis, complicated fabrication and inefficient cargo loading.23,24 Biomaterials with uncontrollable physiochemical properties, such as size, charge and morphology, may induce uncertainty regarding the efficacy of cancer immunotherapy.25,26 Thus, it is of great importance to develop facile, flexible and versatile strategies to fabricate intelligent drug delivery systems to achieve efficient and controllable cancer immunotherapy.Nature guides rational methods of supramolecular material construction, as exemplified by protein folding, the DNA double helix and phospholipid bilayer membrane formation, in harnessing the power of molecular self-assembly.27,28 Supramolecular self-assembly is a “bottom-up” organization tactic to spontaneously fabricate well-ordered nano/microscale architectures, which are driven by noncovalent interactions, including hydrophobic interactions, π–π stacking, hydrogen bonding, electrostatic attractions, and coordination interactions.29,30 Over the past few decades, self-assembly of building blocks, such as small molecules, biomacromolecules, and polymers, to form multitudinous nanoarchitectures with various topologies and dimensions from 0 dimensional (0D) to 3D in physiological environments has received increasing attention.31–33 It was found that the distinctive structures have induced significant influences on many properties of these nanoarchitectures, including specific surface area,34 stability,35 softness,36 responsiveness,37 encapsulation capability,38 cellular uptake,39 and pharmacokinetics.40 Recently, supramolecular materials have been investigated as delivery platforms for various immunotherapeutic agents, including small molecule adjuvants,41 macromolecular antibodies,42 cytokines and immune cells.43,44 They have exhibited a high cargo-loading capacity/feasibility, controllable payload release behaviors, simple immune functionalization and excellent biocompatibility.45,46 It is worth noting that by modulating the structural properties, supramolecular materials exert significant influences on the activities of various immune cells.47,48 Specifically, supramolecular materials with suitable size, shape, charge, stiffness and surface pattern have been proven essential in stimulating immune responses, including activation of APCs and T cells and intratumoral infiltration of cytotoxic T lymphocytes (CTLs) to recognize and kill cancer cells.49,50 Additionally, “self-delivery” supramolecular biomaterials based on immune stimulants have attracted wide attention due to their minimized excipient contents, high drug loading capacity, and synergistic immune functions with cargoes.51,52 Thus, supramolecular biomaterials present great advantages in promoting cancer immunotherapy.
In this minireview, we focus on the state-of-the-art developments of supramolecular biomaterials for enhanced cancer immunotherapy. This review is composed of two main sections (Scheme 1). First, noncovalent driving forces are introduced for the fabrication and physicochemical property modulation of supramolecular biomaterials. Second, we will discuss the supramolecular architectures with different topological morphologies and dimensions to improve cancer immunotherapy. In this section, self-assembly to control the biophysical properties of supramolecular architectures provides a better understanding of the relationship between biophysical properties and immune responses. The main goal of this review is to unveil the emerging opportunities and challenges of supramolecular biomaterials for cancer immunotherapy. Given the currently extensive investigations on supramolecular biomaterials for cancer immunotherapy, this review is expected to motivate and inspire further exploration in this field.
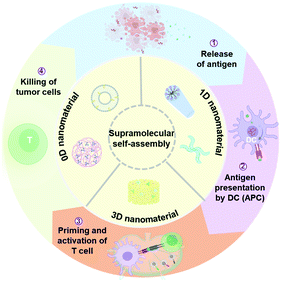 | ||
| Scheme 1 Supramolecular self-assembly offers the possibility for modulating nanostructures and reprogramming immunological properties, paving the way for enhanced cancer immunotherapy. | ||
2. Modulating self-assembly of supramolecular biomaterials
Supramolecular biomaterials are usually prepared through a bottom-up process, which is the self-assembly of building blocks via noncovalent interactions, including hydrophobic interactions, π–π stacking, hydrogen bonding, electrostatic interactions, and coordination interactions.53 Supramolecular biomaterials with a tunable structure and composition and formed under environmental cues often exhibit exquisitely tailored physicochemical properties.54 Importantly, understanding the self-assembly process of supramolecular biomaterials is crucial for the design and construction of biomaterials with desired biophysical properties and biofunctions.The hydrophobic effect plays a vital role in regulating the self-aggregation behaviors of apolar molecules, especially amphiphiles.55 According to the principle of least entropy production, hydrophobic groups tend to aggregate to form hydrophobic cores, while hydrophilic segments are arranged on the outside to contact water. Driven by hydrophobic interactions, amphiphiles tend to form ordered spherical aggregates.56 The most representative example is liposomes, which exhibit amphiphilic phospholipid packing into vesicles.57 Considering their unique architecture, liposomes are able to entrap hydrophobic substances in their membranes and load hydrophilic cargoes into the central aqueous hollow. π–π stacking interactions usually coexist with hydrophobic interactions, which can also induce directional growth of assemblies. These interactions are also robust in water because of the limited solubility of molecules containing aromatic groups.58 Zheng and coworkers fabricated vesicles self-assembled by single phospholipid-conjugated porphyrin derivatives via hydrophobic and π–π stacking interactions, which have been proven to be ultrastable in vivo (Fig. 1a).59
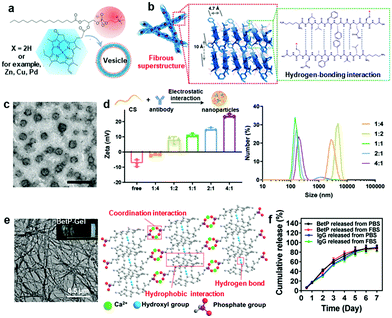 | ||
Fig. 1 (a) Schematic representation of pyropheophorbide–lipid self-assembly into nanovesicles driven by hydrophobic and π–π stacking interactions.59 Copyright 2011, Springer Nature. (b) The fibrous superstructure self-assembled by the β-sheet-forming peptide with multiple hydrogen bonds forming between amide bonds.63 Copyright 2019, Springer Nature. (c) Morphology of CS/antibody nanoparticles. (d) Zeta potential and size distribution of electrostatic assemblies with different feeding ratios of CS and antibody ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 4 4 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mass 1 (mass![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mass).65 Copyright 2021, John Wiley and Sons. (e) Morphology of BetP-Gel self-assembled by hydrogen-bonding, hydrophobic and coordination interactions. (f) Cumulative release curves of BetP and IgG from BetP-Gel.73 Copyright 2020, American Chemical Society. mass).65 Copyright 2021, John Wiley and Sons. (e) Morphology of BetP-Gel self-assembled by hydrogen-bonding, hydrophobic and coordination interactions. (f) Cumulative release curves of BetP and IgG from BetP-Gel.73 Copyright 2020, American Chemical Society. | ||
In addition to hydrophobic and π–π stacking interactions, hydrogen bonding with high selectivity and directionality is also a commonly used force for supramolecular biomaterial fabrication.60 Typically, many biomolecules, such as nucleic acids, peptides and proteins, have multiple hydrogen bond formation sites, including amide groups, amino groups, hydroxyl groups, and carboxyl groups, which can facilitate hydrogen bond-modulated nanostructures.60–62 Wang and Zhao designed a variety of peptide-assembled nanoparticles and nanofibers based on β-sheet-forming peptides (Fig. 1b).63 These nanostructures with different morphologies showed different tumor permeability and retention behaviors. Electrostatic interactions between opposite charges are another non-directional noncovalent interaction used for self-assembly. Electrostatic interactions are largely dependent on the ratio of opposite charges, pH value, concentration and ionic strength.64 Recently, our group fabricated chitosan (CS)–antibody self-assembled nanoparticles, which were driven by electrostatic interactions. The size and charge of the nanoparticles varied with the ratios of the two components, demonstrating that electrostatic interactions played a crucial role during self-assembly (Fig. 1c and d).65 Coordination bonds are formed spontaneously between metal ions and organic ligands mainly through Lewis acid/base interactions, which is a special intermolecular force with an intermediate strength that is equivalent to weak interactions and exhibits a dynamic nature in certain circumstances.66 For instance, Tezcan et al. engineered a monomeric protein with Zn2+-binding sites, which could self-assemble to form 1D, 2D or 3D nanostructures by adjusting the metal ion/protein ratios or pH values.67
Due to the adaptive or dynamic nature of noncovalent interactions, supramolecular biomaterials are more sensitive to external stimuli, indicating that structural destruction or deformation could occur in response to external stimuli, including pH, enzymes, solvents, and temperature, which could be leveraged for on-demand payload release.68,69 Stupp and coworkers reported a pH-responsive self-assembled nanofiber using histidine peptide amphiphiles, which can encapsulate camptothecin (CPT) with an encapsulation efficiency of up to 60%. In the acidic tumor microenvironment, most histidine residues are protonated, leading to electrostatic repulsion, disassembly of nanoparticles, and release of CPT.70 In another work, a nanoplatform formed by self-assembly of amphiphilic amino acids (Fmoc-L-Lys) and chlorin e6 (Ce6) was obtained via multiple weak intermolecular interactions, including electrostatic forces, π–π stacking, and hydrophobic interactions. These self-assembled nanodrugs exhibited multiple favorable therapeutic features, including tunable size, high loading efficiency, controllable drug release in response to pH, surfactant, and enzyme stimuli, as well as preferable cellular uptake and biodistribution.71 Ding et al. fabricated an acid-sensitive PEG-decorated calcium carbonate (CaCO3) nanoparticle incorporating curcumin (CUR; a Ca2+ enhancer) (PEGCaCUR). PEGCaCUR released Ca2+ and CUR in an acid tumor microenvironment (TME) inducing mitochondrial Ca2+ overload and immunogenic cell death (ICD) for improved cancer therapy.72 Recently, our group engineered an injectable anti-inflammatory steroid drug-based supramolecular hydrogel (BetP-Gel) for the local delivery of antibodies. The nanofiber hydrogel was formed by hydrogen-bonding, hydrophobic and coordination interactions (Fig. 1e).73 The multiple noncovalent interactions permitted its injectable properties (quick gel–sol phase transition) for minimally invasive administration. Due to the competitive interaction between phosphate under physiological conditions and calcium ions in BetP-Gel, this hydrogel was gradually degraded to release the encapsulated drugs (Fig. 1f).
In addition to the modulation of the disassembly process, the in situ self-assembly process could also be modulated by these noncovalent interactions.74 Yin et al. prepared a size-reducible nanodrug using dye-chemodrug conjugates, which were synthesized by covalently attaching pentamethine indocyanine (ICy5) dye with cyclic Arg-Gly-Asp (RGD) peptide and camptothecin (CPT), via molecular self-assembly. Upon red light irradiation, the degradation of ICy5 through the C–C cleavage of polyene chains reduced the size of the nanodrug from 90 to 10 nm, which facilitated deep tumor penetration of the nanodrug and release of the chemodrug.75 Wang and coworkers reported in situ-formed nanofibers of enzyme-responsive purpurin18-peptide conjugates.76 The peptide precursors could be cleaved by gelatinase overexpressed in tumors to increase the hydrophobicity and reduce the steric hindrance of peptide molecules, resulting in the self-assembly of peptides into nanofibers in situ and enhancing the retention of peptides in tumors. Moreover, pH, reactive oxygen species (ROS) and light can also induce the morphological transformation of self-assembled structures.77 Therefore, supramolecular biomaterials exhibit many unique advantages for drug delivery.
3. Supramolecular biomaterials for enhanced cancer immunotherapy
Supramolecular biomaterials with flexible and accurate tailored physicochemical properties have shown versatility in modulating their biological performance related to drug encapsulation efficiency, immunogenicity, multivalency, immune cell behaviors and immunotherapy responses, which could contribute to efficient and safe cancer immunotherapy.77,78 Here, we will classify the supramolecular system for immunotherapy according to the dimensions of topological structures, which mainly include 0D, 1D and 3D structures, and introduce their application for cancer immunotherapy enhancement.3.1 0D nanoparticles for cancer immunotherapy
0D nanobiomaterials are defined as nanoparticles with three dimensions confined to the nanoscale.79 Supramolecular 0D nanoparticles exhibit many unique properties, such as tunable surface properties, versatile loading capacity, fast internalization rate, prone to deformability, and flexible administration routes.47 Therefore, supramolecular 0D nanoparticles have been extensively explored for cancer immunotherapy. Here, we will mainly introduce two main types of 0D architectures, namely, nanocapsules with core–shell structures and nanoparticles with solid structures.Micelles, liposomes or some polymeric particles are formed by self-assembly of amphiphilic materials.80 These nanocapsules can be used to encapsulate water-soluble immunotherapeutic agents in aqueous cores and hydrophobic drugs within hydrophobic interiors.81 Furthermore, the surface of nanocapsules can be engineered with suitable charge, softness and multivalency to interact with immune cells to boost immune responses.82 Seder et al. developed a vaccine platform (SNP-7/8a) based on charge-modified peptide-TLR-7/8a conjugates that were chemically programmed to self-assemble into micelles with a uniform size of approximately 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm (Fig. 2a).83 This approach realized precise loading of diverse peptide neoantigens after linking to the TLR-7/8a adjuvant, which exhibited increased APC uptake and T cell mediated immune responses (Fig. 2b). Li et al. constructed nanovesicles using an oxaliplatin (OXA) prodrug and PEGylated photosensitizer (PS) through hydrophobic interactions.84 This nanovesicle with a size of 80 nm showed high tumor accumulation after intravenous (i.v.) injection into mice, which could further elicit antitumor immune responses by inducing immunogenic cell death (ICD) of tumor cells. Recently, Mooney and coworkers also developed a cationic liposome based on 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)/cholesterol encapsulated with hydrophilic STING agonists for treating lung metastasis of melanoma.85 The cationic liposome could bind to the anionic cell membrane, leading to enhanced cell association and cytosolic delivery of cGAMP, effectively activating the STING pathway and inhibiting the growth of tumors. In another work, Chen et al. developed size-transformable artificial antigen-presenting cells (aAPCs), which were self-assembled by copolymer biotin-PEG-PHPMA(-SH)-PDMA, loaded with IL-2 in the inner core and decorated with a peptide-loaded major histocompatibility complex (MHC) monomer and CD28 on the surface.86 When aAPCs encountered preactivated antigen-specific T cells, they transformed from nanosized to microsized with disulfide bond cleavage into thiols. aAPCs with microsizes exhibited obviously prolonged retention in tumors, achieving potent T cell-mediated immune responses.
nm (Fig. 2a).83 This approach realized precise loading of diverse peptide neoantigens after linking to the TLR-7/8a adjuvant, which exhibited increased APC uptake and T cell mediated immune responses (Fig. 2b). Li et al. constructed nanovesicles using an oxaliplatin (OXA) prodrug and PEGylated photosensitizer (PS) through hydrophobic interactions.84 This nanovesicle with a size of 80 nm showed high tumor accumulation after intravenous (i.v.) injection into mice, which could further elicit antitumor immune responses by inducing immunogenic cell death (ICD) of tumor cells. Recently, Mooney and coworkers also developed a cationic liposome based on 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)/cholesterol encapsulated with hydrophilic STING agonists for treating lung metastasis of melanoma.85 The cationic liposome could bind to the anionic cell membrane, leading to enhanced cell association and cytosolic delivery of cGAMP, effectively activating the STING pathway and inhibiting the growth of tumors. In another work, Chen et al. developed size-transformable artificial antigen-presenting cells (aAPCs), which were self-assembled by copolymer biotin-PEG-PHPMA(-SH)-PDMA, loaded with IL-2 in the inner core and decorated with a peptide-loaded major histocompatibility complex (MHC) monomer and CD28 on the surface.86 When aAPCs encountered preactivated antigen-specific T cells, they transformed from nanosized to microsized with disulfide bond cleavage into thiols. aAPCs with microsizes exhibited obviously prolonged retention in tumors, achieving potent T cell-mediated immune responses.
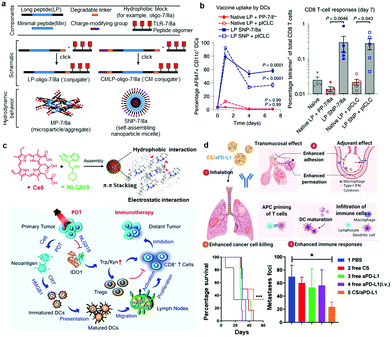 | ||
| Fig. 2 (a) A nanovaccine platform (SNP-7/8a) based on peptide–TLR-7/8a conjugates that are chemically programmed to self-assemble into micelles. (b) The percentage of total CD11+ DCs that had taken up vaccine (left) and CD8+ T cell responses from blood assessed by tetramer staining (right).82 Copyright 2020, Springer Nature. (c) Ce6 and NLG919 self-assembled into uniform nanosized particles through hydrophobic, π–π stacking, and electrostatic interactions and the proposed mechanism for photodynamical sensitized immunotherapy.88 Copyright 2020, American Chemical Society. (d) Schematic depiction of inhaled CS/aPD-L1 nanoparticles to suppress lung metastases (top), survival curves and numbers of lung metastatic foci after different treatments (bottom).65 Copyright 2021, John Wiley and Sons. | ||
In addition to the above-mentioned vesicles composed of amphiphiles, nanoparticles with solid structures that are made from substances such as drugs, pigments, polysaccharides, proteins, and even cell membranes have also been investigated for enhanced cancer immunotherapy.87 These substances can not only act as building blocks of nanoparticles but are also able to activate antitumor immune responses. Li and coworkers constructed self-assembled nanoparticles by optimizing the noncovalent interactions between chlorine e6 (Ce6) and an inhibitor of idoleamine 2,3-dioxygenase (NLG919) for photodynamic immunotherapy (Fig. 2c).88 These self-assembled nanodrugs exhibited improved solubility and stability, achieving relatively high drug loading capability and evading the risk of possible immunogenicity induced by accessory structures in core–shell nanoparticle compositions. In another work, Liu and Wang prepared a cancer vaccine using Mn2+ ions and the nucleotide oligomerization binding domain 1 (Nod1) agonist meso-2,6-diaminopimelic acid (DAP) via coordination interactions with encapsulated ovalbumin (OVA).89 The formed OVA@Mn-DAP nanoparticles exhibited a strong protective effect against cancer cells. In addition, Seder et al. attached hydrophobic Toll-like receptor agonists (TLR-7/8a) to hydrophilic HPMA-based polymers (polymer–TLR-7/8a) and evaluated the size influences of assemblies of polymer–TLR-7/8a on the location, magnitude and duration of the innate immune system.90 More recently, our group reported self-assembled nanoparticles of chitosan (CS) and anti-programmed cell death protein ligand 1 (aPD-L1) via electrostatic interactions to treat lung metastasis of melanoma tumors (Fig. 2d).65 CS not only temporarily opened the tight junctions of epithelial cells to promote the pulmonary delivery of aPD-L1 but also exhibited adjuvant effects by activating the STING pathway. Interestingly, noninvasive aerosol inhalation of CS/aPD-L1 nanoparticles could effectively activate different kinds of immune cells, especially cytotoxic T lymphocytes (CTLs), and prolonged the survival of mice (Fig. 2d). Very recently, Ding et al. reviewed the role of nanoparticle-mediated ICD in cancer immunotherapy. These nanoparticles delivered ICD-inducing drugs and antibodies to the tumors and improved the activity of reagents, regulating TME and boosting the immune response.91
3.2 1D fibrous biomaterials for cancer immunotherapy
1D nanomaterials, including filamentous micelles and nanofibers, with elongated structures, usually exhibit a number of unique physicochemical properties in terms of stability, tolerability and multivalency, which are closely associated with many biological processes, including phagocytosis, biodistribution, and bioavailability.92 Thus, 1D fibrous biomaterials also possess some unique advantages in enhancing cancer immunotherapy.Filamentous micelles with monolayers are usually formed by single amphiphilic components such as diblock copolymers and peptide amphiphiles through hydrophobic and/or hydrogen interactions.93 However, while filamentous micelles have been studied for a long time, their foray into immunotherapy has only been reported in recent years.47 Collier and coworkers developed a series of filomicelles formed by self-assembly of β-sheet peptide Q11-linked T cell epitopes and/or B cell epitopes (Q11 epitopes), which exhibited many advantageous immune effects.94–96 For instance, Q11-OVA323–339 (O-Q11) peptide self-assembled filomicelles with high-density antigens displayed on the surface could effectively deliver antigens to APCs and elicit strong antibody responses without any additional adjuvants (Fig. 3a).94 In another work, they further demonstrated that the length of fibers could be optimized to improve the internalization, processing, and presentation of antigens.97 Moreover, Rudra et al. linked the model antigenic peptide OVA to L- or D-amino acids to produce enantiomeric filamentous micelles through a self-assembly strategy.98 Compared to filamentous micelles based on L-amino acids, D-amino acid peptide nanofibers elicited stronger antibody responses and long-term antigen presentation in vivo, indicating that the stereochemistry of biomaterials could be used to program adaptive immune responses. Filomicelles assembled by polymers rather than peptides have also been investigated.99 For example, Scott and coworkers developed filomicelles assembled from PEG-PPS block copolymers, which were capable of targeting dendritic cells (DCs) in vivo.100 Recently, they further demonstrated that filomicelles can reassemble into micelles with hydrophilic group-modified propylene sulfide under oxidative conditions.101 This cylinder-to-sphere transition under physiological oxidative conditions allowed the sustained delivery of immunotherapeutic agents for one month without inflammatory bioresorption, providing a new tool for efficient and safe immunotherapy.
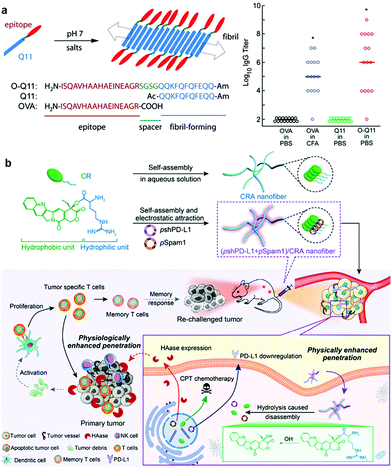 | ||
| Fig. 3 (a) Schematic depiction of the assembly process and molecular composition of the Q11 epitope peptide (left) and IgG titer results suggesting that O-Q11 elicited high IgG titers without the addition of adjuvant (right).94 Copyright 2010, PNAS National Academy of Sciences, American Institute of Physics. (b) Cationic spiral nanofibers assembled with a CPT prodrug for plasmid (pshPD-L1 and pSpam1) delivery and the immune response mechanism triggered by chemoimmunotherapy.105 Copyright 2021, American Chemical Society. | ||
Apart from the monolayer micelles self-assembled from the amphiphilic building block, self-assembled nanofibers also exhibited diverse advantages for immunotherapy. In a recent work, dual functional coordination polymer nanofibers based on zoledronic acid and gadolinium were constructed via the ordered self-assembly process.102 Notably, compared with the coordination nanoparticles, nanofibers were more conducive to endocytosis by macrophages. Moreover, these nanofibers could deposit X-rays for improved reactive oxygen species production to induce potent immunogenic cell death (ICD), synergistically improving DC maturation, promoting T cell infiltration, and inhibiting the growth of primary, distant, and metastatic tumors.102 In another work, Guler and coworkers investigated the ability of 0D and 1D self-assembled peptide nanostructures encapsulating unmethylated CpG motifs to activate the immune response.103 The nanofibrous structures were found to directly induce Th1 immune responses and obviously promote uptake by DCs, whereas the nanospheres mainly induced the Th2-associated immune response. Furthermore, Yan et al. fabricated supramolecular nanofibrils through coassembly of clinically approved immunomodulatory thymopentin (TPS) and near-infrared indocyanine green (ICG) for localized photothermal immunotherapy of pancreatic tumors.104 It was found that nanofibrils with long-range ordered structures show improved photophysical capabilities for photothermal conversion. More interestingly, compared to nanospheres, fibrous nanodrugs showed obviously improved retention in tumor tissue, which could significantly promote the proliferation and differentiation of both CD8+ T cells and CD4+ T cells to kill pancreatic tumors. In another work, Chen et al. constructed camptothecin (CPT) prodrug-assembled nanofibers to deliver two plasmids, pshPD-L1 and pSpam1, to enhance cancer chemoimmunotherapy (Fig. 3b).105 Compared with the spherical form, the nanofibers exhibited better blood circulation ability and enhanced tumor penetration, which could effectively inhibit the growth of both primary and distant tumors while working together with immune checkpoint inhibitors. Hence, the integration of supramolecular nanofibers for immunotherapy is promising to inhibit tumor growth, metastasis and recurrence.
3.3 3D hydrogel for cancer immunotherapy
Supramolecular hydrogels, including injectable hydrogels and in situ formed hydrogels, with 3D networks are also ideal local delivery systems for cancer immunotherapy.106 Moreover, supramolecular hydrogels assembled by dynamic and reversible noncovalent interactions could usually act as an “intelligent” drug delivery system with stimulus responsiveness, desirable biodegradability and high biosafety.107 Owing to the simple preparation and administration process, supramolecular hydrogels have attracted extensive attention in encapsulating immune therapeutics, including small molecules, macromolecules or cells. More interestingly, hydrogels can achieve excellent spatial and temporal control of drug release by precisely adjusting the pore and mesh sizes of hydrogel scaffolds, together with the interactions between drugs and networks or the degradation behavior of hydrogels.108 Thus, supramolecular hydrogels may play an important role in cancer immunotherapy.Injectable supramolecular hydrogels with quick gel–sol phase transition properties could usually maintain their geometry and architecture at the injection site, achieving high local drug concentration, prolonged drug retention, and minimal invasiveness.109 Wang and coworkers designed a self-assembled supramolecular hydrogel encapsulating DCs based on the RADA16 peptide to prepare DC-based vaccines.110 The injectable RADA16 peptide hydrogel could effectively deliver exogenous DCs, antigens, and aPD-L1 antibody simultaneously in a minimally invasive manner, significantly enhancing the proliferation of antigen-specific T cells and inducing potent cellular immune responses. In another example, Song et al. engineered an injectable PEG-b-poly(L-alanine) hydrogel for sustained local codelivery of tumor cell lysate, granulocyte-macrophage colony stimulating factor, and immune checkpoint inhibitors, achieving significantly enhanced tumor-specific immune responses.111 More recently, self-delivery hydrogels that are directly self-assembled by bioactive gelators for delivery to target sites have become popular. In a recent work by our group, an anti-inflammatory nanofiber hydrogel self-assembled by steroid drugs was developed for the local delivery of aPDL1 to achieve systemic cancer immunotherapy (Fig. 4a).73 Interestingly, such a carrier-free system based on steroid drugs could not only reprogram the immunosuppressive TME to an antitumoral microenvironment but also serve as a reservoir for sustained release of aPDL1, effectively inhibiting the growth of both local and abscopal tumors (Fig. 4b). In another study, Yang et al. developed a D- or L-peptide self-assembled supramolecular hydrogel with OVA entrapped in the cavity or physically adsorbed on the surface of the nanofibers.112 Compared with L-gel, D-gel was capable of serving as a promising vaccine adjuvant to evoke both humoral and cellular immune responses. Jiang et al. also demonstrated that right-handed fiber hydrogels could induce stronger humoral and cellular immune responses than left-handed hydrogels.113
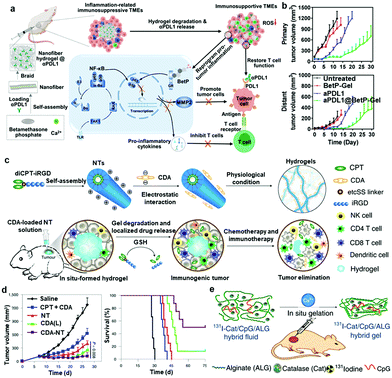 | ||
| Fig. 4 (a) Schematic showing the formation of nanofiber hydrogels by cross-linking filamentous assemblies via physical interaction between betamethasone phosphate and calcium ions. This nanofiber hydrogel could reprogram the protumoral immunosuppressive TME by inhibiting the NF-κB signaling pathway and sustainably release aPDL1 to activate T cells. (b) Growth kinetics of primary and distant CT26 tumors in different groups.73 Copyright 2021, American Chemical Society. (c) Schematics of localized CPT and CDA delivery using a bioresponsive CPT-based nanotube hydrogel for TME regulation and chemoimmunotherapy. (d) Tumour-growth kinetics and survival curve of GL-261 tumour-bearing mice in different groups.116 Copyright 2020, Springer Nature. (e) Scheme illustrating in situ gelation of a 131I-Cat/ALG hybrid fluid after local injection into tumours.117 Copyright 2018, Springer Nature. | ||
Apart from injectable supramolecular hydrogels, supramolecular hydrogels formed in situ are also a useful delivery system for cancer immunotherapy, which could entrap bioactive molecules or cells by simple injection at the targeted sites.114 These supramolecular hydrogels usually have the ability to immediately undergo morphological changes to external stimuli, realizing sustained and controlled release of encapsulated therapeutics.115 Cui et al. developed a supramolecular hydrogel based on peptide–drug conjugates to intratumorally deliver a STING agonist to activate cancer immunotherapy (Fig. 4c).116 In aqueous solution, the synthesized drug amphiphile (diCPT–iRGD), consisting of a peptide moiety iRGD and camptothecin (CPT), could spontaneously assemble into supramolecular nanotubes. The negatively charged STING agonist (CDA) could be absorbed on the surface of positively charged nanotubes through electrostatic complexations. After injection into the tumor site, the nanotubes immediately formed hydrogels upon response to counterions, functioning as the local reservoir for extended local release of CDA and CPT to awake both innate and adaptive immune systems (Fig. 4d). They also demonstrated that aPD1 could be effectively delivered into tumors using such matrix metalloproteinase-responsive supramolecular hydrogels.42 In another study, Liu and coworkers designed an in situ formed sodium alginate (ALG) gel containing radioisotope-labeled catalase (131I-Cat) and CpG oligonucleotides.117 Upon injection, the ALG fluid containing different drugs rapidly transformed into a gel by coordination with endogenous Ca2+ ions within the tumor (Fig. 4e). When combined with an immune checkpoint blockade, this gel could induce strong antitumor immune responses to attack distant cancer cells and strong immunological memory effects to inhibit cancer recurrence. In addition, Ma and coworkers developed thermosensitive hydrogels based on triblock copolymers (PLGA–PEG–PLGA) for the sustained release of IL2.118 With the appropriate gelation temperature at 29.5–30 °C, thermosensitive hydrogels containing IL2 could be injected into any site of the body in a minimally invasive and highly efficient manner.
4. Challenges and future outlook
In this review, we summarized the recent significant research advancements of supramolecular biomaterials for cancer immunotherapy. By modulating multiple noncovalent interactions (hydrophobic, hydrogen bonding, electrostatic and coordination interactions), the physicochemical properties (size, morphology, charge, and specific surface area) and responsiveness of supramolecular biomaterials could be elaborately refined, facilitating the construction of functional adjustable platforms for cancer immunotherapy. The dynamic and adaptive nature of self-assembled nanoarchitectures affords enhanced sensitivity to changes in environmental conditions, favoring the spatiotemporal modulation of payload encapsulation and liberation. These nanoarchitectures with various dimensions and different topologies, mainly including nanocapsules, nanoparticles, filamentous micelles, nanofibers and hydrogels, possess distinguishing advantages in applications for cancer immunotherapy, incarnating a structure–property relationship. Thus, supramolecular biomaterials could target multiple vulnerabilities of cancer to boost the antitumor immune response effectively and safely.Compared with the existing immunotherapy systems, supramolecular biomaterials exhibit many unique advantages for cancer immunotherapy. First, self-assembly is a simple, flexible and green fabrication strategy. Supramolecular biomaterials integrate multifunctionality into immunotherapy systems without time consumption, expensive synthesis processes and toxic reagents, making these products more clinically translatable. Second, supramolecular biomaterials could achieve high drug encapsulation efficiency by coassembly via noncovalent interactions, and the physical interaction could finely retain the activity of immunotherapeutics. Third, due to their dynamic and adaptive nature, supramolecular biomaterials afford enhanced sensitivity to circumstance cues, favoring more rational and controllable therapeutic encapsulation or liberation. Finally, physiochemical properties, such as topological structure, surface charge and antigen density of supramolecular biomaterials, are flexibly modulated, which could exert synergistic functions with immune therapeutics to amplify immune responses and improve cancer immunotherapy.
Despite these unique advantages of supramolecular biomaterials for cancer immunotherapy, successful clinical translations of these nanoformulations remain challenging. First of all, nanomedicines administered intravenously face many extracellular and intracellular barriers in vivo. Due to the weak noncovalent properties, attention should be paid to the stability of supramolecular biomaterials during blood circulation in cancer immunotherapy. Synergism and cooperativity of various non-covalent interactions could be considered in the design and fabrication of supramolecular nanomedicines to improve their stability and immunotherapy performance. Second, although supramolecular immunotherapeutics could co-deliver different immunotherapeutic agents to achieve combination therapy, controllable release of multiple therapeutic agents spatiotemporally remains challenging and needs improvements. Multiple responsive release modalities and noncovelent bonds with different strengths could be involved in the supramolecular nanomedicines to construct elegant responsive nanoplatforms. Last but not least, the long-term safety and toxicity profiles of supramolecular nanomedicine still remain obstacles for clinical cancer immunotherapy. The non-biocompatible carriers and untargeted drug delivery are the two main reasons for adverse effects caused in immunotherapy. On the one hand, employment of non-immunogenic constituents and the use of a drug itself as a building block may reduce many adverse interactions with immune systems. Additionally, judicious investigation of the surface physiochemical properties of supramolecular nanoplatforms and modification of targeting groups could be valuable for achieving precise and safe immunotherapy.
As stated, engineering elegant nanoplatforms in the past decade provides potential strategies to improve cancer immunotherapy. It is hoped that continuous advances in this field will soon overcome the existing difficulties for further development of supramolecular immunotherapeutics. As interest in this research field continues to evolve, it is also anticipated that supramolecular nanotechnology will inspire the development of many novel and powerful approaches for cancer immunotherapy and act as one of the key drivers for successful clinical transformation in the near future.
Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (91959104, 21927803, 51903182, 51525203 and 52103347), the Natural Science Foundation of Jiangsu Province (BK20190826), the China Postdoctoral Science Foundation (2020M671583), the Collaborative Innovation Center of Suzhou Nano Science and Technology, the 111 Program from the Ministry of Education of China, the Start-Up Package of McGill University (G. C.), NSERC Discovery Grant (G. C.), and GCI studentship (T. F.).Notes and references
- R. D. Schreiber, L. J. Old and M. J. Smyth, Science, 2011, 331, 1565–1570 CrossRef CAS PubMed.
- I. Mellman, G. Coukos and G. Dranoff, Nature, 2011, 480, 480–489 CrossRef CAS PubMed.
- D. F. Quail and J. A. Joyce, Nat. Med., 2013, 19, 1423–1437 CrossRef CAS PubMed.
- D. S. Chen and I. Mellman, Immunity, 2013, 39, 1–10 CrossRef CAS PubMed.
- A. Stoddart, Nat. Rev. Mater., 2017, 2, 17027 CrossRef.
- O. J. Finn, Nat. Rev. Immunol., 2003, 3, 630–641 CrossRef CAS PubMed.
- M. S. Gebre, L. A. Brito, L. H. Tostanoski, D. K. Edwards, A. Carfi and D. H. Barouch, Cell, 2021, 184, 1589–1603 CrossRef CAS PubMed.
- M. B. Howren, D. M. Lamkin and J. Suls, Psychosom. Med., 2009, 71, 171–186 CrossRef CAS PubMed.
- A. Ribas and J. D. Wolchok, Science, 2018, 359, 1350–1355 CrossRef CAS PubMed.
- P. C. Tumeh, C. L. Harview, J. H. Yearley, I. P. Shintaku, E. J. M. Taylor, L. Robert, B. Chmielowski, M. Spasic, G. Henry, V. Ciobanu, A. N. West, M. Carmona, C. Kivork, E. Seja, G. Cherry, A. J. Gutierrez, T. R. Grogan, C. Mateus, G. Tomasic, J. A. Glaspy, R. O. Emerson, H. Robins, R. H. Pierce, D. A. Elashoff, C. Robert and A. Ribas, Nature, 2014, 515, 568–571 CrossRef CAS PubMed.
- C. H. June, J. Clin. Invest., 2007, 117, 1466–1476 CrossRef CAS PubMed.
- S. A. Rosenberg, J. C. Yang and N. P. Restifo, Nat. Med., 2004, 10, 909–915 CrossRef CAS PubMed.
- A. York, Nat. Rev. Microbiol., 2021, 19, 222–223 CrossRef CAS PubMed.
- R. Kuai, L. J. Ochyl, K. S. Bahjat, A. Schwendeman and J. J. Moon, Nat. Mater., 2017, 16, 489–496 CrossRef CAS PubMed.
- Y. Jing, J. Liu, Y. Ye, L. Pan, H. Deng, Y. Wang, Y. Yang, L. Diao, S. H. Lin, G. B. Mills, G. Zhuang, X. Xue and L. Han, Nat. Commun., 2020, 11, 4946 CrossRef CAS PubMed.
- M. S. Goldberg, Nat. Rev. Cancer, 2019, 19, 587–602 CrossRef CAS PubMed.
- G. Dranoff, Nat. Rev. Cancer, 2004, 4, 11–22 CrossRef CAS PubMed.
- R. S. Riley, C. H. June, R. Langer and M. J. Mitchell, Nat. Rev. Drug Discovery, 2019, 18, 175–196 CrossRef CAS PubMed.
- G. Liu, M. Zhu, X. Zhao and G. Nie, Adv. Drug Delivery Rev., 2021, 176, 113889 CrossRef CAS PubMed.
- N. Gong, Y. Zhang, X. Teng, Y. Wang, S. Huo, G. Qing, Q. Ni, X. Li, J. Wang, X. Ye, T. Zhang, S. Chen, Y. Wang, J. Yu, P. C. Wang, Y. Gan, J. Zhang, M. J. Mitchell, J. Li and X.-J. Liang, Nat. Nanotechnol., 2020, 15, 1053–1064 CrossRef CAS PubMed.
- C.-T. Jiang, K.-G. Chen, A. Liu, H. Huang, Y.-N. Fan, D.-K. Zhao, Q.-N. Ye, H.-B. Zhang, C.-F. Xu, S. Shen, M.-H. Xiong, J.-Z. Du, X.-Z. Yang and J. Wang, Nat. Commun., 2021, 12, 1359 CrossRef CAS PubMed.
- Y. Xia, T. Song, Y. Hu and G. Ma, Acc. Chem. Res., 2020, 53, 2068–2080 CrossRef CAS PubMed.
- J. B. A. G. Haanen, F. Carbonnel, C. Robert, K. M. Kerr, S. Peters, J. Larkin and K. Jordan, Ann. Oncol., 2017, 28, 119–142 CrossRef PubMed.
- P. Xing and Y. Zhao, Small Methods, 2018, 2, 1700364 CrossRef.
- J. Wang, Y. Li and G. Nie, Nat. Rev. Mater., 2021, 6, 766–783 CrossRef CAS PubMed.
- Q. Chen, M. Chen and Z. Liu, Chem. Soc. Rev., 2019, 48, 5506–5526 RSC.
- J. H. van Esch, Nature, 2010, 466, 193–194 CrossRef CAS PubMed.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- T. Fukino, H. Joo, Y. Hisada, M. Obana, H. Yamagishi, T. Hikima, M. Takata, N. Fujita and T. Aida, Science, 2014, 344, 499–504 CrossRef CAS PubMed.
- J. Wang, K. Liu, R. Xing and X. Yan, Chem. Soc. Rev., 2016, 45, 5589–5604 RSC.
- S. Zhang, Nat. Biotechnol., 2003, 21, 1171–1178 CrossRef CAS PubMed.
- Q. Chen, C. Wang, X. Zhang, G. Chen, Q. Hu, H. Li, J. Wang, D. Wen, Y. Zhang, Y. Lu, G. Yang, C. Jiang, J. Wang, G. Dotti and Z. Gu, Nat. Nanotechnol., 2019, 14, 89–97 CrossRef CAS PubMed.
- Y. Zhang, S. Ma, X. Liu, Y. Xu, J. Zhao, X. Si, H. Li, Z. Huang, Z. Wang, Z. Tang, W. Song and X. Chen, Adv. Mater., 2021, 33, 2007293 CrossRef CAS PubMed.
- D. P. Patterson, A. Rynda-Apple, A. L. Harmsen, A. G. Harmsen and T. Douglas, ACS Nano, 2013, 7, 3036–3044 CrossRef CAS PubMed.
- Z. Zhou, C. Du, Q. Zhang, G. Yu, F. Zhang and X. Chen, Angew. Chem., Int. Ed., 2021, 60, 21033–21039 CrossRef CAS PubMed.
- Y. Xia, J. Wu, W. Wei, Y. Du, T. Wan, X. Ma, W. An, A. Guo, C. Miao, H. Yue, S. Li, X. Cao, Z. Su and G. Ma, Nat. Mater., 2018, 17, 187–194 CrossRef CAS PubMed.
- H. Zhang, K. Liu, S. Li, X. Xin, S. Yuan, G. Ma and X. Yan, ACS Nano, 2018, 12, 8266–8276 CrossRef CAS PubMed.
- J. Zheng, R. Fan, H. Wu, H. Yao, Y. Yan, J. Liu, L. Ran, Z. Sun, L. Yi, L. Dang, P. Gan, P. Zheng, T. Yang, Y. Zhang, T. Tang and Y. Wang, Nat. Commun., 2019, 10, 1604 CrossRef CAS PubMed.
- E. N. Chin, C. Yu, V. F. Vartabedian, Y. Jia, M. Kumar, A. M. Gamo, W. Vernier, S. H. Ali, M. Kissai, D. C. Lazar, N. Nguyen, L. E. Pereira, B. Benish, A. K. Woods, S. B. Joseph, A. Chu, K. A. Johnson, P. N. Sander, F. Martínez-Peña, E. N. Hampton, T. S. Young, D. W. Wolan, A. K. Chatterjee, P. G. Schultz, H. M. Petrassi, J. R. Teijaro and L. L. Lairson, Science, 2020, 369, 993–999 CrossRef CAS PubMed.
- L. Zhang, D. Jing, N. Jiang, T. Rojalin, C. M. Baehr, D. Zhang, W. Xiao, Y. Wu, Z. Cong, J. J. Li, Y. Li, L. Wang and K. S. Lam, Nat. Nanotechnol., 2020, 15, 145–153 CrossRef CAS PubMed.
- X. Li, Y. Wang, Y. Zhang, C. Liang, Z. Zhang, Y. Chen, Z. Hu and Z. Yang, Adv. Funct. Mater., 2021, 31, 2100729 CrossRef CAS.
- F. Wang, D. Xu, H. Su, W. Zhang, X. Sun, M. K. Monroe, R. W. Chakroun, Z. Wang, W. Dai, R. Oh, H. Wang, Q. Fan, F. Wan and H. Cui, Sci. Adv., 2020, 6, eaaz8985 CrossRef PubMed.
- N. Guziewicz, A. Best, B. Perez-Ramirez and D. L. Kaplan, Biomaterials, 2011, 32, 2642–2650 CrossRef CAS PubMed.
- S. B. Stephan, A. M. Taber, I. Jileaeva, E. P. Pegues, C. L. Sentman and M. T. Stephan, Nat. Biotechnol., 2015, 33, 97–101 CrossRef CAS PubMed.
- J. Zhou, L. Rao, G. Yu, T. R. Cook, X. Chen and F. Huang, Chem. Soc. Rev., 2021, 50, 2839–2891 RSC.
- Z. Shen, H. Ye, X. Yi and Y. Li, ACS Nano, 2019, 13, 215–228 CrossRef CAS PubMed.
- C. W. Shields, L. L. Wang, M. A. Evans and S. Mitragotri, Adv. Mater., 2020, 32, 1901633 CrossRef CAS PubMed.
- X. Feng, W. Xu, Z. Li, W. Song, J. Ding and X. Chen, Adv. Sci., 2019, 6, 1900101 CrossRef PubMed.
- E. Froimchuk, S. T. Carey, C. Edwards and C. M. Jewell, Acc. Chem. Res., 2020, 53, 2534–2545 CrossRef CAS PubMed.
- H. B. Eppler and C. M. Jewell, Adv. Mater., 2020, 32, 1903367 CrossRef CAS PubMed.
- T. Bhattacharyya, P. Saha and J. Dash, ACS Omega, 2018, 3, 2230–2241 CrossRef CAS PubMed.
- X. Xu, Y. Jian, Y. Li, X. Zhang, Z. Tu and Z. Gu, ACS Nano, 2014, 8, 9255–9264 CrossRef CAS PubMed.
- M. J. Webber, E. A. Appel, E. W. Meijer and R. Langer, Nat. Mater., 2016, 15, 13–26 CrossRef CAS PubMed.
- M. J. Webber and R. Langer, Chem. Soc. Rev., 2017, 46, 6600–6620 RSC.
- S. Toksoz, H. Acar and M. O. Guler, Soft Matter, 2010, 6, 5839 RSC.
- J. Voskuhl and B. J. Ravoo, Chem. Soc. Rev., 2009, 38, 495–505 RSC.
- H. Pick, A. C. Alves and H. Vogel, Chem. Rev., 2018, 118, 8598–8654 CrossRef CAS PubMed.
- W. Song, J. Kuang, C.-X. Li, M. Zhang, D. Zheng, X. Zeng, C. Liu and X.-Z. Zhang, ACS Nano, 2018, 12, 1978–1989 CrossRef CAS PubMed.
- J. F. Lovell, C. S. Jin, E. Huynh, H. Jin, C. Kim, J. L. Rubinstein, W. C. W. Chan, W. Cao, L. V. Wang and G. Zheng, Nat. Mater., 2011, 10, 324–332 CrossRef CAS PubMed.
- M. J. Sailor and J.-H. Park, Adv. Mater., 2012, 24, 3779–3802 CrossRef CAS PubMed.
- T. P. Knowles, A. W. Fitzpatrick, S. Meehan, H. R. Mott, M. Vendruscolo, C. M. Dobson and M. E. Welland, Science, 2007, 318, 1900–1903 CrossRef CAS PubMed.
- L. Zhou, T. Qiu, F. Lv, L. Liu, J. Ying and S. Wang, Adv. Healthcare Mater., 2018, 7, 1800670 CrossRef PubMed.
- H.-W. An, L.-L. Li, Y. Wang, Z. Wang, D. Hou, Y.-X. Lin, S.-L. Qiao, M.-D. Wang, C. Yang, Y. Cong, Y. Ma, X.-X. Zhao, Q. Cai, W.-T. Chen, C.-Q. Lu, W. Xu, H. Wang and Y. Zhao, Nat. Commun., 2019, 10, 1–15 CrossRef PubMed.
- J. J. Richardson, M. Björnmalm and F. Caruso, Science, 2015, 348, aaa2491 CrossRef PubMed.
- Q. Jin, W. Zhu, J. Zhu, J. Zhu, J. Shen, Z. Liu, Y. Yang and Q. Chen, Adv. Mater., 2021, 33, 2007557 CrossRef CAS PubMed.
- H. Zhang, L. Kang, Q. Zou, X. Xin and X. Yan, Curr. Opin. Biotechnol, 2019, 58, 45–52 CrossRef CAS PubMed.
- J. D. Brodin, X. I. Ambroggio, C. Tang, K. N. Parent, T. S. Baker and F. A. Tezcan, Nat. Chem., 2012, 4, 375–382 CrossRef CAS PubMed.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- M. Grzelczak, L. M. Liz-Marzán and R. Klajn, Chem. Soc. Rev., 2019, 48, 1342–1361 RSC.
- T. J. Moyer, J. A. Finbloom, F. Chen, D. J. Toft, V. L. Cryns and S. I. Stupp, J. Am. Chem. Soc., 2014, 136, 14746–14752 CrossRef CAS PubMed.
- K. Liu, R. Xing, Q. Zou, G. Ma, H. Möhwald and X. Yan, Angew. Chem., Int. Ed., 2016, 55, 3036–3039 CrossRef CAS PubMed.
- P. Zheng, B. B. Ding, Z. Y. Jiang, W. G. Xu, G. Li, J. X. Ding and X. Chen, Nano Lett., 2021, 21, 2088–2093 CrossRef CAS PubMed.
- M. Chen, Y. Tan, Z. Dong, J. Lu, X. Han, Q. Jin, W. Zhu, J. Shen, L. Cheng, Z. Liu and Q. Chen, Nano Lett., 2020, 20, 6763–6773 CrossRef CAS PubMed.
- G.-B. Qi, Y.-J. Gao, L. Wang and H. Wang, Adv. Mater., 2018, 30, 1703444 CrossRef PubMed.
- C. Ji, Q. Gao, X. Dong, W. Yin, Z. Gu, Z. Gan, Y. Zhao and M. Yin, Angew. Chem., Int. Ed., 2018, 57, 11384–11388 CrossRef CAS PubMed.
- D. Zhang, G.-B. Qi, Y.-X. Zhao, S.-L. Qiao, C. Yang and H. Wang, Adv. Mater., 2015, 27, 6125–6130 CrossRef CAS PubMed.
- J. Yang, H.-W. An and H. Wang, ACS Appl. Bio Mater., 2021, 4, 24–46 CrossRef CAS PubMed.
- Z. Huang, W. Song and X. Chen, Front. Chem., 2020, 8(380), 1 Search PubMed.
- K. Panneerselvam, M. E. Lynge, C. F. Riber, S. Mena-Hernando, A. A. A. Smith, K. N. Goldie, A. N. Zelikin and B. Städler, Biomicrofluidics, 2015, 9, 052610 CrossRef PubMed.
- M. Ramanathan, L. K. Shrestha, T. Mori, Q. Ji, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2013, 15, 10580 RSC.
- S. Wu, Y. Xia, Y. Hu and G. Ma, Adv. Drug Delivery Rev., 2021, 176, 113871 CrossRef CAS PubMed.
- G. M. Lynn, C. Sedlik, F. Baharom, Y. Zhu, R. A. Ramirez-Valdez, V. L. Coble, K. Tobin, S. R. Nichols, Y. Itzkowitz, N. Zaidi, J. M. Gammon, N. J. Blobel, J. Denizeau, P. de la Rochere, B. J. Francica, B. Decker, M. Maciejewski, J. Cheung, H. Yamane, M. G. Smelkinson, J. R. Francica, R. Laga, J. D. Bernstock, L. W. Seymour, C. G. Drake, C. M. Jewell, O. Lantz, E. Piaggio, A. S. Ishizuka and R. A. Seder, Nat. Biotechnol., 2020, 38, 320–332 CrossRef CAS PubMed.
- Y. Wang, H. Xu and X. Zhang, Adv. Mater., 2009, 21, 2849–2864 CrossRef CAS.
- F. Zhou, B. Feng, H. Yu, D. Wang, T. Wang, Y. Ma, S. Wang and Y. Li, Adv. Mater., 2019, 31, 1805888 CrossRef PubMed.
- S. T. Koshy, A. S. Cheung, L. Gu, A. R. Graveline and D. J. Mooney, Adv. Biosyst., 2017, 1, 1600013 CrossRef PubMed.
- W. Yang, H. Deng, S. Zhu, J. Lau, R. Tian, S. Wang, Z. Zhou, G. Yu, L. Rao, L. He, Y. Ma and X. Chen, Sci. Adv., 2020, 6, eabd1631 CrossRef CAS PubMed.
- S. Naahidi, M. Jafari, F. Edalat, K. Raymond, A. Khademhosseini and P. Chen, J. Controlled Release, 2013, 166, 182–194 CrossRef CAS PubMed.
- L.-P. Zhao, R.-R. Zheng, J.-Q. Huang, X.-Y. Chen, F.-A. Deng, Y.-B. Liu, C.-Y. Huang, X.-Y. Yu, H. Cheng and S.-Y. Li, ACS Nano, 2020, 14, 17100–17113 CrossRef CAS PubMed.
- H. Zhao, J. Xu, Y. Li, X. Guan, X. Han, Y. Xu, H. Zhou, R. Peng, J. Wang and Z. Liu, ACS Nano, 2019, 13, 13127–13135 CrossRef CAS PubMed.
- G. M. Lynn, R. Laga, P. A. Darrah, A. S. Ishizuka, A. J. Balaci, A. E. Dulcey, M. Pechar, R. Pola, M. Y. Gerner, A. Yamamoto, C. R. Buechler, K. M. Quinn, M. G. Smelkinson, O. Vanek, R. Cawood, T. Hills, O. Vasalatiy, K. Kastenmüller, J. R. Francica, L. Stutts, J. K. Tom, K. A. Ryu, A. P. Esser-Kahn, T. Etrych, K. D. Fisher, L. W. Seymour and R. A. Seder, Nat. Biotechnol., 2015, 33, 1201–1210 CrossRef CAS PubMed.
- Y. Sun, X. Feng, C. Wan, J. F. Lovell, H. Jin and J. Ding, Asian J. Pharm., 2021, 16, 129–132 Search PubMed.
- Q. Sun, M. Barz, B. G. De Geest, M. Diken, W. E. Hennink, F. Kiessling, T. Lammers and Y. Shi, Chem. Soc. Rev., 2019, 48, 351–381 RSC.
- K. Ariga, M. Nishikawa, T. Mori, J. Takeya, L. K. Shrestha and J. P. Hill, Sci. Technol. Adv. Mater., 2019, 20, 51–95 CrossRef CAS PubMed.
- J. S. Rudra, Y. F. Tian, J. P. Jung and J. H. Collier, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 622–627 CrossRef CAS PubMed.
- C. Mora-Solano, Y. Wen, H. Han, J. Chen, A. S. Chong, M. L. Miller, R. R. Pompano and J. H. Collier, Biomaterials, 2017, 149, 1–11 CrossRef CAS PubMed.
- Y. Si, Y. Wen, S. H. Kelly, A. S. Chong and J. H. Collier, J. Controlled Release, 2018, 282, 120–130 CrossRef CAS PubMed.
- Y. Si, Q. Tian, F. Zhao, S. H. Kelly, L. S. Shores, D. F. Camacho, A. I. Sperling, M. S. Andrade, J. H. Collier and A. S. Chong, Sci. Adv., 2020, 6, eaba0995 CrossRef CAS PubMed.
- R. Appavu, C. B. Chesson, A. Y. Koyfman, J. D. Snook, F. J. Kohlhapp, A. Zloza and J. S. Rudra, ACS Biomater. Sci. Eng., 2015, 1, 601–609 CrossRef CAS PubMed.
- J. Mougin, C. Bourgaux and P. Couvreur, Adv. Drug Delivery Rev., 2021, 172, 127–147 CrossRef CAS PubMed.
- S. Yi, S. D. Allen, Y.-G. Liu, B. Z. Ouyang, X. Li, P. Augsornworawat, E. B. Thorp and E. A. Scott, ACS Nano, 2016, 10, 11290–11303 CrossRef CAS PubMed.
- N. B. Karabin, S. Allen, H.-K. Kwon, S. Bobbala, E. Firlar, T. Shokuhfar, K. R. Shull and E. A. Scott, Nat. Commun., 2018, 9, 1–13 CrossRef CAS PubMed.
- Z. Huang, D. Yao, Q. Ye, H. Jiang, R. Gu, C. Ji, J. Wu, Y. Hu and A. Yuan, ACS Nano, 2021, 15, 8450–8465 CrossRef CAS PubMed.
- R. Mammadov, G. Cinar, N. Gunduz, M. Goktas, H. Kayhan, S. Tohumeken, A. E. Topal, I. Orujalipoor, T. Delibasi, A. Dana, S. Ide, A. B. Tekinay and M. O. Guler, Sci. Rep., 2015, 5, 16728 CrossRef CAS PubMed.
- S. Li, W. Zhang, R. Xing, C. Yuan, H. Xue and X. Yan, Adv. Mater., 2021, 33, 2100595 CrossRef CAS.
- Z. Guo, Y. Hu, M. Zhao, K. Hao, P. He, H. Tian, X. Chen and M. Chen, Nano Lett., 2021, 21, 3721–3730 CrossRef CAS PubMed.
- M. Norouzi, B. Nazari and D. W. Miller, Drug Discovery Today, 2016, 21, 1835–1849 CrossRef CAS PubMed.
- Z. Li, N. Song and Y.-W. Yang, Matter, 2019, 1, 345–368 CrossRef.
- R. Narayanaswamy and V. P. Torchilin, Molecules, 2019, 24, 603 CrossRef PubMed.
- D. Fan, Y. Tian and Z. Liu, Front. Chem., 2019, 675, 1–11 Search PubMed.
- P. Yang, H. Song, Y. Qin, P. Huang, C. Zhang, D. Kong and W. Wang, Nano Lett., 2018, 18, 4377–4385 CrossRef CAS PubMed.
- H. Song, P. Yang, P. Huang, C. Zhang, D. Kong and W. Wang, Theranostics, 2019, 9, 2299–2314 CrossRef CAS PubMed.
- Z. Luo, Q. Wu, C. Yang, H. Wang, T. He, Y. Wang, Z. Wang, H. Chen, X. Li, C. Gong and Z. Yang, Adv. Mater., 2017, 29, 1601776 CrossRef PubMed.
- Y. Tian, H. Wang, Y. Liu, L. Mao, W. Chen, Z. Zhu, W. Liu, W. Zheng, Y. Zhao, D. Kong, Z. Yang, W. Zhang, Y. Shao and X. Jiang, Nano Lett., 2014, 14, 1439–1445 CrossRef CAS PubMed.
- Y. Chao, Q. Chen and Z. Liu, Adv. Funct. Mater., 2020, 30, 1902785 CrossRef CAS.
- R. Dong, Y. Zhou, X. Huang, X. Zhu, Y. Lu and J. Shen, Adv. Mater., 2015, 27, 498–526 CrossRef CAS PubMed.
- F. Wang, H. Su, D. Xu, W. Dai, W. Zhang, Z. Wang, C. F. Anderson, M. Zheng, R. Oh, F. Wan and H. Cui, Nat. Biomed. Eng., 2020, 4, 1090–1101 CrossRef CAS PubMed.
- Y. Chao, L. Xu, C. Liang, L. Feng, J. Xu, Z. Dong, L. Tian, X. Yi, K. Yang and Z. Liu, Nat. Biomed. Eng., 2018, 2, 611–621 CrossRef CAS PubMed.
- Q. Lv, C. He, F. Quan, S. Yu and X. Chen, Bioact. Mater., 2018, 3, 118–128 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |


