 Open Access Article
Open Access ArticleUiO-66 metal organic frameworks with high contents of flexible adipic acid co-linkers†
Tristan T. Y.
Tan
 a,
Xin
Li
a,
Xin
Li
 a,
Ken-ichi
Otake
a,
Ken-ichi
Otake
 ab,
Ying Chuan
Tan
ab,
Ying Chuan
Tan
 c,
Xian Jun
Loh
c,
Xian Jun
Loh
 ad,
Susumu
Kitagawa
ad,
Susumu
Kitagawa
 *ab and
Jason Y. C.
Lim
*ab and
Jason Y. C.
Lim
 *ad
*ad
aLaboratory for Green Porous Materials, Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Singapore 138634, Singapore. E-mail: kitagawa@icems.kyoto-u.ac.jp; jason_lim@imre.a-star.edu.sg
bInstitute for Integrated Cell-Material Sciences (iCeMS), Institute for Advanced Study, Kyoto University (KUIAS), Yoshida Ushinomiyacho, Sakyo-ku, Kyoto 606-8501, Japan
cInstitute of Sustainability for Chemicals, Energy and Environment (ISCE2), A*STAR, 2 Fusionopolis Way, Singapore 138634, Singapore
dDepartment of Materials Science and Engineering, National University of Singapore (NUS), 9 Engineering Drive 1, Singapore 117576, Singapore
First published on 13th September 2022
Abstract
Adipic acid, an industrially-important chemical that can be sustainably derived from biomass and post-consumer nylon, is traditionally overlooked as a linker for MOFs. Herein, we report the first direct one-pot method for synthesising UiO-66 MOFs with an unprecedented 69 mol% adipate content, as well as the feasibility of these materials for MOF defect engineering by rapid and selective adipate thermolysis.
As the field of metal–organic framework (MOF) chemistry has matured over the years, so has the interest in the development of large-scale sustainable syntheses and applications.1 Organic ligands are a major component of MOFs, and the material and energy costs of synthesizing such ligands are important considerations for scale-up.2 In this regard, terephthalic acid, a core organic component of many MOFs, is especially suitable, as it is a widely available industrial chemical,3 and can be derived from biomass4 or post-consumer polyethylene terephthalate.5 Similarly, adipic acid is another industrially-important diacid as a component of nylon-66,6 with an annual production of nearly 3 million tonnes.7 Furthermore, adipic acid's sustainable production has been extensively studied,8 and it can also be obtained from post-consumer plastic or biomass.9 Adipic acid would thus be an attractive sustainably producible ligand to synthesize MOFs. However, synthesizing MOFs with aliphatic linkers is inherently challenging, due to the flexibility of the alkyl backbones.10 Indeed, adipate can take different conformers even within one unit cell,11 and to date, there have only been a few reports of using adipic acid as a linker in MOFs.11,12 We thus sought to investigate the behaviour of adipate as a co-linker, together with terephthalate to provide rigidity to the framework.
We selected UiO-6613 as the platform for our study, as it is a prototypical MOF with many varied applications, such as catalysis14 and drug delivery15 amongst others.16 The wide utility of UiO-66 stems in part from its excellent hydrolytic and thermal stabilities,17 owing to the strength of the Zr–O bonds. In addition, the crystal nucleation and growth mechanisms of UiO-66 are well understood,18 which is advantageous for tailoring the synthetic conditions. Incorporation of adipic acid into UiO-66 would be of particular interest, as incorporation of aliphatic diacids can result in an increased CO2 uptake.19 Furthermore, it is possible to selectively introduce defects by controlled thermolysis of adipic acid.20 It has been reported that crystalline MOFs can be synthesized from Zr precursors and adipic acid;21 however, better crystallinity was obtained when using 3-methyl adipic acid,21 due to stronger non-covalent interactions, or trans-1,4-cyclohexanedicarboxylic acid, which is more rigid.20a,22 Unlike pure adipic acid, 3-methyl adipic acid and trans-1,4-cyclohexanedicarboxylic acid cannot be sustainably sourced on an industrial scale. In addition, the post synthetic ligand introduction of adipate into UiO-66 via ligand exchange has also been reported, allowing for 17.3% adipate content concentrated on the exterior of the crystals.19
We herein report a highly convenient, one-pot synthetic method for synthesizing UiO-66 with adipate content as high as 69% while still retaining the crystallinity (Fig. 1). This allows us to study the structural stability and gas uptake properties with unprecedented content of the highly flexible adipate linker. We also show that adipate can be selectively thermolyzed at lower temperatures than terephthalate within only 5 minutes, allowing for the generation of missing linker defects.20 Other than being much more facile than other thermolabile linkers previously reported, which require sustained heating for ≥2 hours for defect generation, this also allows considerable energy savings for sustainable MOF synthesis.
 | ||
| Fig. 1 Schematic illustration of the synthesis of UiO-66 containing mixed adipate and terephthalate linkers. Adipate in the MOF is highlighted in orange. | ||
Initial attempts to synthesize UiO-66 containing adipic acid were met with some unexpected observations. Using ZrOCl2·8H2O and an acid modulator (100 equiv. of formic or acetic acid), a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mol
1 (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) mix of terephthalic acid and adipic acid in the reaction mixture was found to completely suppress any crystal nucleation, under conditions which otherwise typically furnish crystalline UiO-66 in good yield.18a,20b We hypothesized that the presence of flexible adipic acids on the surfaces of precursor clusters would inhibit their ability to form nanocrystal nuclei.18b Decreasing the amount of modulator used did allow for solids to form, however all materials formed under these conditions were amorphous. Conditions that promoted faster nucleation, while still allowing for crystal growth slow enough to afford good quality crystals, were thus required.18c,d
mol) mix of terephthalic acid and adipic acid in the reaction mixture was found to completely suppress any crystal nucleation, under conditions which otherwise typically furnish crystalline UiO-66 in good yield.18a,20b We hypothesized that the presence of flexible adipic acids on the surfaces of precursor clusters would inhibit their ability to form nanocrystal nuclei.18b Decreasing the amount of modulator used did allow for solids to form, however all materials formed under these conditions were amorphous. Conditions that promoted faster nucleation, while still allowing for crystal growth slow enough to afford good quality crystals, were thus required.18c,d
The use of aqueous HCl and ZrCl4 has been shown to speed up nucleation,29,30 and monotopic carboxylic acids could be avoided under these conditions. Under these conditions, a series of ten samples with an increasing mole ratio of adipate to terephthalate were successfully obtained (Fig. 2a, see ESI† for experimental details). We herein will refer to our synthesized materials as UiO-66-x, where x is the mole fraction of adipate that replaces terephthalate in the material, following previously reported conventions.20a,23 The ratio of the two ligands in the final products was determined by 1H NMR spectroscopy of the samples digested in K3PO4/D2O solution (ESI,† Section 4), as well as by TGA.24 The materials were also characterized by scanning electron microscopy (SEM), powder X-ray diffraction (XRD) and solid-state nuclear magnetic resonance (NMR) spectroscopy (see ESI,† Sections S2–S5).
Compared to previously reported syntheses of mixed linker MOFs,20,23,25 where the ratio of the two linkers in the final product was comparable to the ratio used as starting material, adipic acid was found to be much more reluctant to be incorporated into the framework. Indeed, when a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of terephthalic acid and adipic acid was used in the reaction mixture, hardly any adipate could be detected in the final product (Fig. 2b, see Table S3, ESI,† for mole ratio determined by 1H NMR and TGA, calculated by previously reported methods).26 This would be expected, given the larger loss of conformation entropy when a free adipate is fixed between two nodes compared to rigid diacid incorporation. However, measurable quantities of adipate start being incorporated into the MOFs when adipic to terephthalic acid ratios beyond 1
1 ratio of terephthalic acid and adipic acid was used in the reaction mixture, hardly any adipate could be detected in the final product (Fig. 2b, see Table S3, ESI,† for mole ratio determined by 1H NMR and TGA, calculated by previously reported methods).26 This would be expected, given the larger loss of conformation entropy when a free adipate is fixed between two nodes compared to rigid diacid incorporation. However, measurable quantities of adipate start being incorporated into the MOFs when adipic to terephthalic acid ratios beyond 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were used in the synthesis. The MOFs are all crystalline except for UiO-66-87, for which the powder XRD data showed only broad diffraction, indicative of an amorphous product.
1 were used in the synthesis. The MOFs are all crystalline except for UiO-66-87, for which the powder XRD data showed only broad diffraction, indicative of an amorphous product.
We were then interested in the conformations adopted by the adipate linkers within UiO-66. Using computational modelling with ORCA,27 we probed the relative energies of different conformers that adipate can adopt while bridging two Zr6O4(OH)4 nodes, while retaining the same unit cell size of UiO-66 bridged by terephthalate. The MOF was modelled using a cluster model,28 using two truncated Zr6O4(OH)4 nodes which were extracted from the crystal structure reported by Lillerud and coworkers,29 and the bridging terephthalate replaced with adipate. An initial conformer search using semiempirical methods was done,30 followed by reoptimizing the conformers at the DFT level. Fig. 3 shows the most stable conformer found, where the adipate backbone is folded into a chair-like structure. The next most stable conformer, where the adipate backbone was in a linear conformation, was found to be 6.8 kcal mol−1 higher in energy (see ESI,† Section 8 for additional computational details).
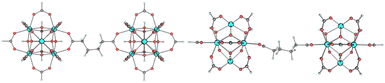 | ||
| Fig. 3 DFT optimized structure of the most stable conformer of adipate bridging two Zr6O4(OH)4 nodes, viewed from two directions. | ||
MOFs built from flexible aliphatic ligands are prone to lose crystallinity upon evacuation.20a,22 Indeed, upon studying the gas uptake of our materials, our mixed ligand materials showed an almost identical gas uptake trend to those of the mixed terephthalate/cyclohexane dicarboxylate UiO-66 reported previously,20a where increasing quantities of adipic acid resulted in decreased gas uptake by the MOF (Fig. 4a). We surmise that observation can be explained by the collapse of pores upon evacuation, due to ligand flexibility. The instability of the adipic acid-containing MOFs towards evacuation was further investigated by powder XRD analysis. As shown in Fig. 4b, the XRD pattern of a sample UiO-66-40 treated by vacuum in a Schlenk tube (labelled as UiO-66-40-vac) for 12 hours revealed significant peak broadening. Analysis of the peak width before and after evacuation using the Scherrer equation31 indicated that the crystallite size decreased from about 50 nm to 25 nm after evacuation (see ESI,† Table S5), which could be due to the collapse of pores and fragmentation of the crystallites into smaller pieces. This agrees with the observed low N2 sorption measurements. Furthermore, UiO-66-51 was found to lose all crystallinity under the same vacuum treatment (Fig. 4c).
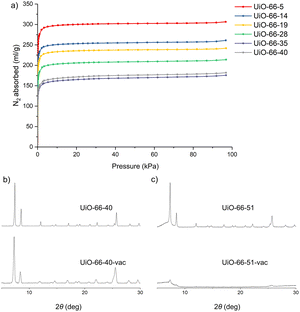 | ||
| Fig. 4 (a) N2 sorption isotherms of UiO-66-x at 77k, (b) XRD pattern of UiO-66-40 and UiO-66-40-vac, and (c) XRD pattern of UiO-66-51 and UiO-66-51-vac. | ||
The CO2 uptake of our materials was also found to decrease with increasing adipate mol% (Fig. S59, ESI†). This contrasts with previously reported findings,19 and could be due to some of the adipate introduced not completely bridging the Zr6O4(OH)4 nodes. With only one COOH group attached to a node, the other pendant COOH within the pores could enhance CO2 interaction and adsorption (see ESI,† Fig. S74–S81 for infrared spectra, which indicate that free COOH groups are not present in our materials).32
Having found that high adipate contents affected the stability of UiO-66 to evacuation, we next investigated if the materials were stable towards thermolysis. Given that adipate, containing aliphatic C-H bonds, should combust in air at a lower temperature than terephthalate, it would be possible to thermally remove the adipate while leaving the remaining framework intact, while also generating missing linker defects. Similar selective thermolysis of mixed ligand MOFs has been shown with UiO-66 containing 2-aminoterephthalic acid and 1,4-cyclohexanedicarboxylic acid.20
Thermogravimetric analysis (TGA) of the UiO-66-x samples under air clearly displayed a drop in weight at about 250 °C, with the magnitude of the drop being proportional to the adipate content of the MOFs ((Fig. 5), see Fig. S48–S58, ESI† for all TGA plots). We chose 300 °C as the thermolysis temperature to ensure complete removal of adipate. Heating the UiO-66-x samples at 300 °C for only 5 minutes in a furnace under air was sufficient to oxidize all adipate from the samples, as indicated by solid state 13C NMR spectroscopy (Fig. S35–S47, ESI†) and liquid state 1H NMR spectroscopy of the digested samples (Fig. S14–S34, ESI†).
Powder XRD of the thermolyzed materials, herein referred to as UiO-66-x-th, showed that MOFs with up to 40 mol% adipate retained their crystallinity after thermolysis, while thermolysis of samples containing higher adipate contents yielded amorphous materials (Fig. S11, ESI†). Interestingly, analysis of the XRD pattern of UiO-66-40-th indicated that the crystallite sizes had decreased less upon thermolysis than upon evacuation (Fig. S12 and Table S5, ESI†). In addition, SEM images of all samples showed that the crystal morphologies did not change noticeably after thermolysis (Fig. S1–S9, ESI†). Gas sorption measurements of the UiO-66-x-th materials were conducted and revealed a general increase in gas uptake after thermolysis for adipate content of less than 30%, which is consistent with previous reports where defects are created post-synthetically.20 The pore size distribution analysis (Fig. S60–S65, ESI†) revealed that no macropores were formed after thermolysis, indicating that the increase in gas uptake is due to missing linker defects rather than missing cluster defects, again in agreement with the previous reports of defects generated by thermolysis.20a
To test the general compatibility between aliphatic diacids with terephthalic acid within UiO-66, we extended our study to 3-methyladipic acid, which has been previously shown to be a suitable linker in Zr MOFs.21 We found that 3-methyladipic acid was more readily incorporated into UiO-66, where a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 3-methyladipic acid to terephthalic acid in the starting solution yielded UiO-66 containing 11 mol% 3-methyladipate, compared to 0 mol% when adipic acid was used (see ESI† for details).21 Finally, we attempted to extend our synthetic method to the shorter succinic acid, considering that Zr MOFs have been shown to accommodate linkers of varying lengths.25 However, no succinate incorporation was detected in the product by 1H NMR and TGA, suggesting that while Zr MOFs can accommodate a length mismatch between fumarate and terephthalate, the length and flexibility mismatch between succinate and terephthalate makes them incompatible with each other.
1 ratio of 3-methyladipic acid to terephthalic acid in the starting solution yielded UiO-66 containing 11 mol% 3-methyladipate, compared to 0 mol% when adipic acid was used (see ESI† for details).21 Finally, we attempted to extend our synthetic method to the shorter succinic acid, considering that Zr MOFs have been shown to accommodate linkers of varying lengths.25 However, no succinate incorporation was detected in the product by 1H NMR and TGA, suggesting that while Zr MOFs can accommodate a length mismatch between fumarate and terephthalate, the length and flexibility mismatch between succinate and terephthalate makes them incompatible with each other.
In summary, we have overcome the challenge of building crystalline MOFs using the flexible adipate linker under one-pot synthesis conditions by using terephthalate as a co-linker to provide rigidity to the framework. Adipate is a very attractive ligand as a MOF building block, as it is very inexpensive and can be sourced sustainably, and can be used for engineering defects in UiO-66 at 300 °C quickly. We also found that large quantities of adipate reduce the stability of UiO-66 towards evacuation, which has important implications for using this ligand in MOFs for gas storage. Numerous other applications for incorporating adipate into MOFs are still open for exploration, such as the tuning of electronic structure33 or hydrophobicity.10b
S. K. would like to thank the support from Kyoto University's Overseas On-site Laboratory Program, and the financial support of KAKENHI, Grant-in-Aid for Scientific Research (S) (JP22H05005); J. Y. C. L. acknowledges the A*STAR Central Research Fund for generous financial support of this project. iCeMS analysis center is acknowledged for access to the solid-state NMR instrument. This work was supported by the A*STAR Computational Resource Centre through the use of its high performance computing facilities.
Conflicts of interest
There are no conflicts of interest to declare.References
- (a) P. A. Julien, C. Mottillo and T. Friščić, Green Chem., 2017, 19, 2729–2747 RSC; (b) M. Rubio-Martinez, C. Avci-Camur, A. W. Thornton, I. Imaz, D. Maspoch and M. R. Hill, Chem. Soc. Rev., 2017, 46, 3453–3480 RSC; (c) M. I. Severino, E. Gkaniatsou, F. Nouar, M. L. Pinto and C. Serre, Faraday Discuss., 2021, 231, 326–341 RSC.
- E.-S. M. El-Sayed and D. Yuan, Green Chem., 2020, 22, 4082–4104 RSC.
- R. A. F. Tomás, J. C. M. Bordado and J. F. P. Gomes, Chem. Rev., 2013, 113, 7421–7469 CrossRef PubMed.
- J. Pang, M. Zheng, R. Sun, A. Wang, X. Wang and T. Zhang, Green Chem., 2016, 18, 342–359 RSC.
- (a) W. P. R. Deleu, I. Stassen, D. Jonckheere, R. Ameloot and D. E. de Vos, J. Mater. Chem. A, 2016, 4, 9519–9525 RSC; (b) S.-H. Lo, D. Senthil Raja, C.-W. Chen, Y.-H. Kang, J.-J. Chen and C.-H. Lin, Dalton Trans., 2016, 45, 9565–9573 RSC.
- S. van de Vyver and Y. Román-Leshkov, Catal. Sci. Technol., 2013, 3, 1465–1479 RSC.
- E. Skoog, J. H. Shin, V. Saez-Jimenez, V. Mapelli and L. Olsson, Biotechnol. Adv., 2018, 36, 2248–2263 CrossRef CAS PubMed.
- J. C. J. Bart and S. Cavallaro, Ind. Eng. Chem. Res., 2015, 54, 1–46 CrossRef CAS.
- (a) R. Beerthuis, G. Rothenberg and N. R. Shiju, Green Chem., 2015, 17, 1341–1361 RSC; (b) J. Rios, J. Lebeau, T. Yang, S. Li and M. D. Lynch, Green Chem., 2021, 23, 3172–3190 RSC.
- (a) Z.-J. Lin, J. Lü, M. Hong and R. Cao, Chem. Soc. Rev., 2014, 43, 5867–5895 RSC; (b) V. D. Slyusarchuk, P. E. Kruger and C. S. Hawes, ChemPlusChem, 2020, 85, 845–854 CrossRef CAS PubMed.
- (a) T. K. Kim, K. J. Lee, M. Choi, N. Park, D. Moon and H. R. Moon, New J. Chem., 2013, 37, 4130 RSC; (b) H. Reinsch, R. S. Pillai, R. Siegel, J. Senker, A. Lieb, G. Maurin and N. Stock, Dalton Trans., 2016, 45, 4179–4186 RSC.
- (a) D. T. de Lill, A. de Bettencourt-Dias and C. L. Cahill, Inorg. Chem., 2007, 46, 3960–3965 CrossRef CAS; (b) D. T. de Lill and C. L. Cahill, Cryst. Growth Des., 2007, 7, 2390–2393 CrossRef CAS; (c) J. A. Ridenour, R. G. Surbella, A. V. Gelis, D. Koury, F. Poineau, K. R. Czerwinski and C. L. Cahill, Angew. Chem., Int. Ed., 2019, 58, 16508–16511 CrossRef CAS; (d) P. J. Saines, P. T. Barton, M. Jura, K. S. Knight and A. K. Cheetham, Mater. Horiz., 2014, 1, 332–337 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- A. Dhakshinamoorthy, A. Santiago-Portillo, A. M. Asiri and H. Garcia, ChemCatChem, 2019, 11, 899–923 CrossRef CAS.
- I. Abánades Lázaro and R. S. Forgan, Coord. Chem. Rev., 2019, 380, 230–259 CrossRef.
- J. Winarta, B. Shan, S. M. Mcintyre, L. Ye, C. Wang, J. Liu and B. Mu, Cryst. Growth Des., 2020, 20, 1347–1362 CrossRef CAS.
- A. J. Howarth, Y. Liu, P. Li, Z. Li, T. C. Wang, J. T. Hupp and O. K. Farha, Nat. Rev. Mater., 2016, 1, 1–15 Search PubMed.
- (a) W. Morris, S. Wang, D. Cho, E. Auyeung, P. Li, O. K. Farha and C. A. Mirkin, ACS Appl. Mater. Interfaces, 2017, 9, 33413–33418 CrossRef CAS; (b) H. Xu, S. Sommer, N. L. N. Broge, J. Gao and B. B. Iversen, Chem. – Eur. J., 2019, 25, 2051–2058 CrossRef CAS PubMed; (c) M. J. Katz, Z. J. Brown, Y. J. Colón, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449–9451 RSC; (d) F. Ragon, P. Horcajada, H. Chevreau, Y. K. Hwang, U.-H. Lee, S. R. Miller, T. Devic, J.-S. Chang and C. Serre, Inorg. Chem., 2014, 53, 2491–2500 CrossRef CAS PubMed.
- D. H. Hong and M. P. Suh, Chem. – Eur. J., 2014, 20, 426–434 CrossRef CAS PubMed.
- (a) B. Bueken, N. van Velthoven, A. Krajnc, S. Smolders, F. Taulelle, C. Mellot-Draznieks, G. Mali, T. D. Bennett and D. de Vos, Chem. Mater., 2017, 29, 10478–10486 CrossRef CAS; (b) L. Feng, S. Yuan, L.-L. Zhang, K. Tan, J.-L. Li, A. Kirchon, L.-M. Liu, P. Zhang, Y. Han, Y. J. Chabal and H.-C. Zhou, J. Am. Chem. Soc., 2018, 140, 2363–2372 CrossRef CAS PubMed.
- H. Reinsch, I. Stassen, B. Bueken, A. Lieb, R. Ameloot and D. de Vos, CrystEngComm, 2015, 17, 331–337 RSC.
- B. Bueken, F. Vermoortele, M. J. Cliffe, M. T. Wharmby, D. Foucher, J. Wieme, L. Vanduyfhuys, C. Martineau, N. Stock, F. Taulelle, V. van Speybroeck, A. L. Goodwin and D. de Vos, Chem. – Eur. J., 2016, 22, 3264–3267 CrossRef CAS PubMed.
- S. M. Chavan, G. C. Shearer, S. Svelle, U. Olsbye, F. Bonino, J. Ethiraj, K. P. Lillerud and S. Bordiga, Inorg. Chem., 2014, 53, 9509–9515 CrossRef CAS PubMed.
- G. C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye and K. P. Lillerud, Chem. Mater., 2016, 28, 3749–3761 CrossRef CAS.
- S. Yuan, L. Huang, Z. Huang, D. Sun, J.-S. Qin, L. Feng, J. Li, X. Zou, T. Cagin and H.-C. Zhou, J. Am. Chem. Soc., 2020, 142, 4732–4738 CrossRef CAS.
- G. C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye and K. P. Lillerud, Chem. Mater., 2016, 28, 3749–3761 CrossRef CAS.
- (a) F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS; (b) F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1606 Search PubMed; (c) F. Neese, F. Wennmohs, U. Becker and C. Riplinger, J. Chem. Phys., 2020, 152, 224108 CrossRef CAS PubMed.
- V. Bernales, M. A. Ortuño, D. G. Truhlar, C. J. Cramer and L. Gagliardi, ACS Cent. Sci., 2018, 4, 5–19 CrossRef CAS PubMed.
- S. Øien, D. Wragg, H. Reinsch, S. Svelle, S. Bordiga, C. Lamberti and K. P. Lillerud, Cryst. Growth Des., 2014, 14, 5370–5372 CrossRef.
- C. Bannwarth, S. Ehlert and S. Grimme, J. Chem. Theory Comput., 2019, 15, 1652–1671 CrossRef CAS PubMed.
- P. Scherrer, Göttinger Nachrichten Math. Phys., 1918, 2, 98–100 Search PubMed.
- F. Zhou, J. Zhou, X. Gao, C. Kong and L. Chen, RSC Adv., 2017, 7, 3713–3719 RSC.
- A. de Vos, K. Hendrickx, P. van der Voort, V. van Speybroeck and K. Lejaeghere, Chem. Mater., 2017, 29, 3006–3019 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc03285f |
| This journal is © The Royal Society of Chemistry 2022 |


