Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
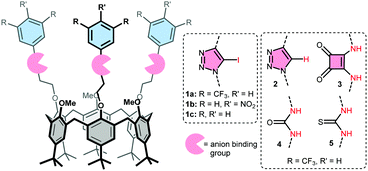
Calix[6]arenes with halogen bond donor groups as selective and efficient anion transporters†
Anurag
Singh
 a,
Aaron
Torres-Huerta
a,
Aaron
Torres-Huerta
 a,
Tom
Vanderlinden
ab,
Nathan
Renier
a,
Tom
Vanderlinden
ab,
Nathan
Renier
 a,
Luis
Martínez-Crespo
a,
Luis
Martínez-Crespo
 a,
Nikolay
Tumanov
a,
Nikolay
Tumanov
 c,
Johan
Wouters
c,
Johan
Wouters
 c,
Kristin
Bartik
c,
Kristin
Bartik
 a,
Ivan
Jabin
a,
Ivan
Jabin
 b and
Hennie
Valkenier
b and
Hennie
Valkenier
 *a
*a
aUniversité libre de Bruxelles (ULB), Ecole polytechnique de Bruxelles, Engineering Molecular NanoSystems, Avenue Franklin Roosevelt 50, 1050 Brussels, Belgium. E-mail: hennie.valkenier@ulb.be
bUniversité libre de Bruxelles (ULB), Faculty of science, Laboratoire de Chimie Organique, Avenue Franklin Roosevelt 50, 1050 Brussels, Belgium
cNamur Institute of Structured Matter and Namur Research Institute for Life Sciences, Department of Chemistry, University of Namur, 61 rue de Bruxelles, B-5000 Namur, Belgium
First published on 6th May 2022
Abstract
Here we present the anion binding and anion transport properties of a series of calix[6]arenes decorated on their small rim with either halogen bond or hydrogen bond donating groups. We show that the halogen bond donating iodotriazole groups enable highly selective transport of chloride and nitrate anions, without transport of protons or hydroxide, at rates similar to those observed with thiourea or squaramide groups.
Synthetic ion transporters have potential for the treatment of channelopathies, such as cystic fibrosis, linked to deficient ion transport.1,2 Most anion transporters described in the literature rely on hydrogen bonding (HB) between relatively acidic hydrogen atoms and the anion.3 However, deprotonation of these acidic HB donors can lead to a net transport of protons along with the anions.4,5 Proton transport is linked to an increasing risk of toxicity,6 which is interesting in the context of anti-cancer activity, but undesirable for treatment of channelopathies. Selectivity for (i) anion transport without dissipation of pH gradients and (ii) certain anions over others represent major challenges in anion transport.7
A strategy to achieve selective transport of anions is the use of less acidic H-bond donors,4,8,9 or interactions based on Sigma holes.10 A Sigma hole is an electron deficient region on a bound halogen, chalcogen, pnictogen, or tetrel atom, which can interact with electron rich entities, such as anions.11–13 This interaction is referred to as halogen bond (XB) if it involves a halogen atom, which are typically less toxic than the other atoms which can bear a Sigma hole. The use of XBs in transport has been pioneered by Matile and co-workers.14 Various XB-based transporters have been studied15–18 and a 5-fold selectivity for transport of chloride over HCl transport by an XB donor was recently reported by Langton, Beer, and co-workers.19 However, published studies compare transporters with XB donors to those with an H atom in the place of the halogen atom, rather than comparing XB donors to powerful HB donating motifs, such as (thio)ureas and squaramides.20–22
Here we present a comparison between XB and HB donating compounds in terms of anion binding, transport activity, and selectivity, using receptors with (Fig. 1): iodotriazoles as XB donors (1a), regular triazoles as control (2), and squaramides or (thio)ureas as strong HB donors (3–5). Three of these groups were attached to the small rim of a calix[6]arene to effectively bind the anion and shield its charge from the apolar interior of the membrane. The value of this calix[6]arene-based design was demonstrated previously by the activity of tris-urea 4 and tris-thiourea 5 as anion receptors23 and carriers.24 The larger iodotriazole binding groups are also readily accommodated by the calix[6]arene platform.25 Electron-poor bis(CF3)phenyl rings were used to increase the binding strength of the various motifs.20–24 These groups and the calix[6]arene skeleton also ensure that the overall lipophilicity and lipophilic balance26 of the studied receptors is similar (see Table 1 for c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values). Two additional calix[6]arenes bearing iodotriazole units with pNO2phenyl (1b) and phenyl groups (1c) were prepared to evaluate the effect of the electron withdrawing substituents on binding and transport by XB donors.
P values). Two additional calix[6]arenes bearing iodotriazole units with pNO2phenyl (1b) and phenyl groups (1c) were prepared to evaluate the effect of the electron withdrawing substituents on binding and transport by XB donors.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, affinity constants for Cl−, and anion transport results for compounds 1–5
P values, affinity constants for Cl−, and anion transport results for compounds 1–5
| Receptor | c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa Pa |
K a in acetone (M−1) | K a in CHCl3 (M−1) | Transport rate [I] (s−1) c | Transport selectivityd |
|---|---|---|---|---|---|
| a Calculated using MarvinSketch 19.25. b Not determined due the low solubility. c Initial rate of Cl−/NO3− antiport in the lucigenin assay divided by the transporter to lipid ratio. d Selectivity of Cl− uniport/HCl symport, based on results from the HPTS assay in NMDGHCl. | |||||
| 1a | 34 | >105 | 7.7·102 | 34 | >100 |
| 1b | 29 | n.d.b | 1.3·102 | < 1 | — |
| 1c | 29 | 8.9·103 | <10 | < 1 | — |
| 2 | 32 | 58 | <10 | < 1 | — |
| 3 | 29 | 8.7·102 | n.d.b | 45 | 1 |
| 4 | 29 | 1.4·103 | 1.8·104 | 12 | 1 |
| 5 | 32 | 1.4·103 | 2.9·104 | 25 | 1 |
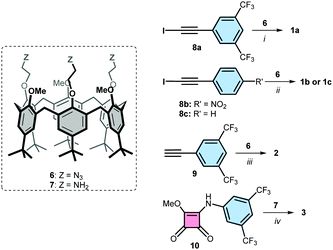
Iodotriazole-based receptors 1a-c were prepared in 70–80% isolated yield from the reported calix[6]arene tris-azide 6,27via a cycloaddition reaction with the corresponding iodoalkynes 8a-c, in the presence of copper(I) and tris(benzyltriazolyl-methyl)amine (TBTA) as catalyst in THF18 (Scheme 1, see ESI† for details28). Triazole-based receptor 2 was prepared from calix[6]arene tris-azide 6 in 97% yield via a cycloaddition with alkyne 9 in the presence of 2,6-lutidine and a catalytic amount of copper(I) in DCM.27 The squaramide containing receptor 3 was isolated in 45% yield from the reaction of calix[6]arene tris-amine 727 and compound 10 in methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM (9
DCM (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), using DIPEA as base.29 Receptors 4 and 5 were prepared following previously reported procedures.23,24
1), using DIPEA as base.29 Receptors 4 and 5 were prepared following previously reported procedures.23,24
The anion binding properties of receptors 1–5 were studied via1H-NMR titrations with tetrabutylammonium chloride (TBACl) in acetone-d6 or CDCl3. Binding constants were determined by fitting the shift of the signals of the protons closest to the binding site to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model30 (see Table 1 and ESI†).28 For receptor 1a in acetone, the signals show a linear shift during the addition of a first eq. of TBACl, while no further changes are observed when adding up to 5 eq. TBACl, supporting a 1
1 model30 (see Table 1 and ESI†).28 For receptor 1a in acetone, the signals show a linear shift during the addition of a first eq. of TBACl, while no further changes are observed when adding up to 5 eq. TBACl, supporting a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode in solution and indicating that the affinity is too high to be quantified. A Ka of 770 M−1 was found for 1a in chloroform and lower affinities were found for receptors 1b and 1c, which have fewer or no electron withdrawing groups on the aryl rings. Compound 2, which only has triazole groups, showed the weakest binding of Cl−. When comparing XB-based receptor 1a to HB-based receptors 3–5, which have the same aryl groups, we observe that, in acetone, 1a has an affinity which is at least two orders of magnitude higher than those of 3–5 (Table 1).31 This trend is inverted in CDCl3, in line with previous observations that XB donors show lower anion affinities in chloroform than in acetone.32 Titrations of 1a with various other anions revealed the following trend in affinities: Cl− > Br− ≫ H2PO4− > NO3−, AcO− (Table S1, ESI†), indicating selectivity for halides over oxoanions.
1 binding mode in solution and indicating that the affinity is too high to be quantified. A Ka of 770 M−1 was found for 1a in chloroform and lower affinities were found for receptors 1b and 1c, which have fewer or no electron withdrawing groups on the aryl rings. Compound 2, which only has triazole groups, showed the weakest binding of Cl−. When comparing XB-based receptor 1a to HB-based receptors 3–5, which have the same aryl groups, we observe that, in acetone, 1a has an affinity which is at least two orders of magnitude higher than those of 3–5 (Table 1).31 This trend is inverted in CDCl3, in line with previous observations that XB donors show lower anion affinities in chloroform than in acetone.32 Titrations of 1a with various other anions revealed the following trend in affinities: Cl− > Br− ≫ H2PO4− > NO3−, AcO− (Table S1, ESI†), indicating selectivity for halides over oxoanions.
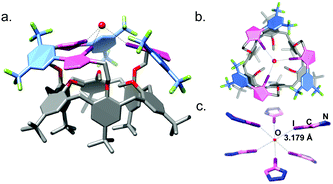
The molecular structure of receptor 1a determined by single-crystal X-ray diffraction (SCXRD, Fig. 2) shows that the methoxy groups of the calix[6]arene are oriented into the cavity and the arms oriented outward. The three XB donor iodotriazole groups coordinate one molecule of water by O⋯I interactions with a distance of 3.179(2) Å. The coordination sphere of the water molecule is completed by a second molecule of 1a to afford an octahedral geometry around the oxygen atom (Fig. 2c and Fig. S63, ESI†). This structural analysis shows that the three XB donor groups of 1a can indeed cooperate to act as an efficient receptor towards electronegative atoms, in agreement with the high affinity observed for chloride.
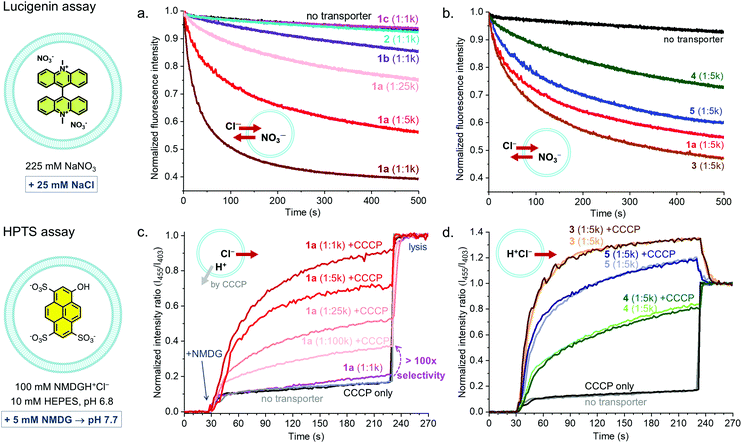
Receptors 1–5 were studied as transmembrane transporters for Cl− and NO3− using the lucigenin assay.20 They were preincorporated in the lipid bilayer of large unilamellar vesicles (LUVs) that contained the chloride sensitive dye lucigenin in a NaNO3 solution. A NaCl pulse was added to create a chloride concentration gradient and the quenching of the fluorescence of lucigenin was monitored to assess Cl− influx. The transport of Cl− into the vesicles is compensated by the transport of NO3− out of the vesicles, ensuring the charge balance (Cl−/NO3− antiport). XB-based receptor 1a shows clear transport activity at concentrations down to 1 transporter per 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lipid molecules (0.004 mol%, 16 nM), 1b showed poor activity (too low to quantify), and 1c and 2 were inactive at 1
000 lipid molecules (0.004 mol%, 16 nM), 1b showed poor activity (too low to quantify), and 1c and 2 were inactive at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 transporter to lipid ratio (Fig. 3a). The decrease in activity with a decreasing number of electron withdrawing substituents on the aryl group and the absence of transport by 2 confirm the role of the Sigma hole in anion transport.
1000 transporter to lipid ratio (Fig. 3a). The decrease in activity with a decreasing number of electron withdrawing substituents on the aryl group and the absence of transport by 2 confirm the role of the Sigma hole in anion transport.
When comparing XB-based receptor 1a to HB-based receptors 3–5, all with bisCF3phenyl groups, the highest transport rates were observed with tris-squaramide 3, followed by 1a and previously reported tris-thiourea 5, and the lowest rate was given by tris-urea 4 (Fig. 3b and Table 1). This demonstrates that receptors with XB donor groups can achieve similar transport activities as related receptors with powerful HB donor groups.
Having established the good activity of 1a, the transport selectivity of this compound in comparison to transporters 3-5 was studied. For this, the pH sensitive dye 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium (HPTS) was encapsulated and a buffer solution with N-methyl-D-glucosamine hydrochloride (NMDGH+Cl−) and HEPES at pH 6.8 was used inside and outside the vesicles. Transport was initiated by addition of a base pulse (NMDG) giving an exterior pH of 7.7. In this assay, the dissipation of the pH gradient (by transport of H+ or OH−) can only be balanced by the transport of Cl−, as NMDGH+ is a membrane impermeable cation.4
Receptors 3–5 gave efficient transport, while no significant activity was observed with 1a even at a transporter:lipid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (Fig. 3c and d). This shows that 1a is incapable of dissipating a pH gradient via either H+Cl− symport or OH−/Cl− antiport. Transport of HCl by 3–5 follows the same order as for Cl−/NO3− antiport, with tris-squaramide 3 giving the highest rates, followed by tris-thiourea 5 and tris-urea 4. The experiments were also run in the presence of the H+ transporter carbonyl cyanide 3-chlorophenylhydrazone (CCCP). No impact was observed on the rates of transport by 3–5, indicating that transport of H+ is not rate limiting for these transporters with acidic H-bond donors. In contrast, the addition of CCCP clearly switched on the transport of 1a, even when present at concentrations as low as 1
1000 (Fig. 3c and d). This shows that 1a is incapable of dissipating a pH gradient via either H+Cl− symport or OH−/Cl− antiport. Transport of HCl by 3–5 follows the same order as for Cl−/NO3− antiport, with tris-squaramide 3 giving the highest rates, followed by tris-thiourea 5 and tris-urea 4. The experiments were also run in the presence of the H+ transporter carbonyl cyanide 3-chlorophenylhydrazone (CCCP). No impact was observed on the rates of transport by 3–5, indicating that transport of H+ is not rate limiting for these transporters with acidic H-bond donors. In contrast, the addition of CCCP clearly switched on the transport of 1a, even when present at concentrations as low as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. This can be attributed to Cl− uniport by 1a coupled to H+ transport by CCCP and shows that 1a has >100-fold selectivity for electrogenic Cl− transport over H+Cl− symport or OH−/Cl− antiport. As 1a has no acidic protons, net transport of HCl by a mechanism involving deprotonation of a binding group upon anion release4 is indeed impossible. Furthermore, 1a has ∼20-fold higher selectivity compared to the recently reported transporter with two XB donor groups,19 which is likely to originate from the efficient encapsulation of Cl− anions by the three XB donor groups on the calix[6]arene. These experiments demonstrate that transporter 1a has an unprecedented selectivity in combination with a good activity (EC50 of ∼0.007 mol%).
000. This can be attributed to Cl− uniport by 1a coupled to H+ transport by CCCP and shows that 1a has >100-fold selectivity for electrogenic Cl− transport over H+Cl− symport or OH−/Cl− antiport. As 1a has no acidic protons, net transport of HCl by a mechanism involving deprotonation of a binding group upon anion release4 is indeed impossible. Furthermore, 1a has ∼20-fold higher selectivity compared to the recently reported transporter with two XB donor groups,19 which is likely to originate from the efficient encapsulation of Cl− anions by the three XB donor groups on the calix[6]arene. These experiments demonstrate that transporter 1a has an unprecedented selectivity in combination with a good activity (EC50 of ∼0.007 mol%).
Similar experiments were performed, replacing Cl− by NO3− (NMDGH+NO3− buffer), and selectivity of 1a for electrogenic NO3− transport over H+NO3− symport (or OH−/NO3− antiport) was observed (Fig. S60, ESI†). The rate of NO3− uniport by 1a was lower than that of Cl− uniport. HB-based transporters 3–5 showed again hardly any selectivity. In contrast to 3–5, 1a was also found incapable of transporting OH− or H+ in absence of transportable anions such as Cl− and NO3− (Fig. S61, ESI†). This remarkable selectivity of 1a was further highlighted using the lucigenin assay in NaHCO3 or NaOAc solutions to test for Cl−/HCO3− and Cl−/AcO− antiport (Fig. S57 and S58, ESI†). In both experiments clear activity was observed for transporter 3, but not for 1a. This shows that 1a is not only highly selective for Cl− compared to HCl transport, but also for Cl− compared to other anions. The anion transport selectivity of 1a follows the order: Cl− > NO3− ⋙ HCO3−, AcO−, OH− and is correlated to its selectivity in anion binding.
In contrast, HB donors commonly show anion binding selectivities that correlate with the hydration energy or hydrogen bond accepting ability of the anions.33,34 As a result, HB-based receptors are very general transporters and only a slight selectivity of 5 for transport of NO3− > Cl− was observed.35 We note that, despite the poor selectivity, tris-squaramide 3 showed the highest transport activity of the series of compounds tested in all the transport assays used. This is in agreement with previous reports on squaramides with a single binding unit which outperformed ureas and thioureas,21 while attempts to achieve similar results in structures with two or three binding units often failed due to too strong binding or poor solubility of the squaramides.29,36
In conclusion, this study of transport activity and selectivity of lipophilic calix[6]arenes with different anion binding groups has revealed the remarkable selectivity of halogen bond donor 1a to transport Cl− and NO3−, compared to the transport of other anions and to the dissipation of pH gradients. This high selectivity is accompanied by a transport activity on par with the analogous tris-thiourea 5 and close to tris-squaramide 3. This demonstrates that XB donor groups connected to a platform can achieve transport rates similar to those of powerful HB donors, while providing a superior selectivity for Cl−, which opens the way to therapeutic applications of XB donors for treatment of channelopathies such as cystic fibrosis, where Cl− transport is required but dissipation of pH gradients could lead to undesired side effects.
We thank Koen Robeyns for his help with crystal structure refinement and Frank Meyer for discussions. The results reported here are part of a project that has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant agreement No. 802727). HV is a research associate of the Fonds de la Recherche Scientifique – FNRS. We thank the PC2 technological platform at the University of Namur for access to the single-crystal X-ray diffractometer.
Conflicts of interest
There are no conflicts to declare.References
- H. Li, H. Valkenier, L. W. Judd, P. R. Brotherhood, S. Hussain, J. A. Cooper, O. Jurček, H. A. Sparkes, D. N. Sheppard and A. P. Davis, Nat. Chem., 2016, 8, 24–32 CrossRef CAS PubMed.
- A. Roy and P. Talukdar, ChemBioChem, 2021, 22, 2925–2940 CrossRef CAS PubMed.
- J. T. Davis, P. A. Gale and R. Quesada, Chem. Soc. Rev., 2020, 49, 6056–6086 RSC.
- X. Wu, L. W. Judd, E. N. W. Howe, A. M. Withecombe, V. Soto-Cerrato, H. Li, N. Busschaert, H. Valkenier, R. Pérez-Tomás, D. N. Sheppard, Y.-B. Jiang, A. P. Davis and P. A. Gale, Chem, 2016, 1, 127–146 CAS.
- S. V. Shinde and P. Talukdar, Chem. Commun., 2018, 54, 10351–10354 RSC.
- P. A. Gale, R. Pérez-Tomás and R. Quesada, Acc. Chem. Res., 2013, 46, 2801–2813 CrossRef CAS PubMed.
- X. Wu, A. M. Gilchrist and P. A. Gale, Chem, 2020, 6, 1296–1309 CAS.
- J. Shang, W. Si, W. Zhao, Y. Che, J.-L. Hou and H. Jiang, Org. Lett., 2014, 16, 4008–4011 CrossRef CAS PubMed.
- H. Valkenier, O. Akrawi, P. Jurček, K. Sleziaková, T. Lízal, K. Bartik and V. Šindelář, Chem, 2019, 5, 429–444 CAS.
- L. M. Lee, M. Tsemperouli, A. I. Poblador-Bahamonde, S. Benz, N. Sakai, K. Sugihara and S. Matile, J. Am. Chem. Soc., 2019, 141, 810–814 CrossRef CAS PubMed.
- Halogen Bonding in Solution, ed. S. Huber, Wiley, 1st edn, 2021 Search PubMed.
- M. S. Taylor, Coord. Chem. Rev., 2020, 413, 213270 CrossRef CAS.
- H. V. Humeniuk, A. Gini, X. Hao, F. Coelho, N. Sakai and S. Matile, JACS Au, 2021, 1, 1588–1593 CrossRef CAS PubMed.
- A. Vargas Jentzsch, A. Hennig, J. Mareda and S. Matile, Acc. Chem. Res., 2013, 46, 2791–2800 CrossRef CAS PubMed.
- A. Vargas Jentzsch, D. Emery, J. Mareda, S. K. Nayak, P. Metrangolo, G. Resnati, N. Sakai and S. Matile, Nat. Commun., 2012, 3, 905 CrossRef PubMed.
- C. Ren, X. Ding, A. Roy, J. Shen, S. Zhou, F. Chen, S. F. Y. Li, H. Ren, Y. Y. Yang and H. Zeng, Chem. Sci., 2018, 9, 4044–4051 RSC.
- W.-L. Huang, X.-D. Wang, S. Li, R. Zhang, Y.-F. Ao, J. Tang, Q.-Q. Wang and D.-X. Wang, J. Org. Chem., 2019, 84, 8859–8869 CrossRef CAS PubMed.
- L. E. Bickerton, A. J. Sterling, P. D. Beer, F. Duarte and M. J. Langton, Chem. Sci., 2020, 11, 4722–4729 RSC.
- L. E. Bickerton, A. Docker, A. J. Sterling, H. Kuhn, F. Duarte, P. D. Beer and M. Langton, Chem. – Eur. J., 2021, 27, 11738–11745 CrossRef CAS PubMed.
- H. Valkenier, L. W. Judd, H. Li, S. Hussain, D. N. Sheppard and A. P. Davis, J. Am. Chem. Soc., 2014, 136, 12507–12512 CrossRef CAS PubMed.
- N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2012, 51, 4426–4430 CrossRef CAS PubMed.
- H. Li, H. Valkenier, A. G. Thorne, C. M. Dias, J. A. Cooper, M. Kieffer, N. Busschaert, P. A. Gale, D. N. Sheppard and A. P. Davis, Chem. Sci., 2019, 10, 9663–9672 RSC.
- M. Hamon, M. Ménand, S. Le Gac, M. Luhmer, V. Dalla and I. Jabin, J. Org. Chem., 2008, 73, 7067–7071 CrossRef CAS PubMed.
- G. Grauwels, H. Valkenier, A. P. Davis, I. Jabin and K. Bartik, Angew. Chem., Int. Ed., 2019, 58, 6921–6925 CrossRef CAS PubMed.
- A. Ikeda and S. Shinkai, Chem. Rev., 1997, 97, 1713–1734 CrossRef CAS PubMed.
- H. Valkenier, C. J. E. Haynes, J. Herniman, P. A. Gale and A. P. Davis, Chem. Sci., 2014, 5, 1128–1134 RSC.
- M. Ménand and I. Jabin, Org. Lett., 2009, 11, 673–676 CrossRef PubMed.
- Datasets (NMR spectra and transport curves) are available at Zenodo DOI:10.5281/zenodo.6010342.
- S. J. Edwards, H. Valkenier, N. Busschaert, P. A. Gale and A. P. Davis, Angew. Chem., Int. Ed., 2015, 54, 4592–4596 CrossRef CAS PubMed.
- https://supramolecular.org .
- See ESI† for chloride biding data of 3 in DMSO-d6/0.5%H2O and a comparison between 3–5.
- M. G. Sarwar, B. Dragisić, E. Dimitrijević and M. S. Taylor, Chem. – Eur. J., 2013, 19, 2050–2058 CrossRef CAS PubMed.
- S. J. Pike, J. J. Hutchinson and C. A. Hunter, J. Am. Chem. Soc., 2017, 139, 6700–6706 CrossRef CAS PubMed.
- O. Jurček, H. Valkenier, R. Puttreddy, M. Novák, H. A. Sparkes, R. Marek, K. Rissanen and A. P. Davis, Chem. – Eur. J., 2018, 24, 8178–8185 CrossRef PubMed.
- Y. Yang, X. Wu, N. Busschaert, H. Furuta and P. A. Gale, Chem. Commun., 2017, 53, 9230–9233 RSC.
- I. Marques, P. M. R. Costa, M. Q. Miranda, N. Busschaert, E. N. W. Howe, H. J. Clarke, C. J. E. Haynes, I. L. Kirby, A. M. Rodilla, R. Pérez-Tomás, P. A. Gale and V. Félix, Phys. Chem. Chem. Phys., 2018, 20, 20796–20811 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and full characterization of 1-3, details for the binding and transport studies, and the XRD structure of 1a. CCDC 2125602. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc00847e |
| This journal is © The Royal Society of Chemistry 2022 |