A gas-pressure-assisted ratiometric atomic flame assay for the point-of-care testing of tumor-cell-derived exosomes†
Shengqiang
Hu
 *,
Xueting
Fang
*,
Xueting
Fang
 ,
Guijing
Liu
,
Guixiang
Ma
,
Fanggui
Ye
,
Guijing
Liu
,
Guixiang
Ma
,
Fanggui
Ye
 * and
Shulin
Zhao
* and
Shulin
Zhao

State Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin 541004, P. R. China. E-mail: fangguiye@163.com
First published on 2nd November 2021
Abstract
The multicolor-based point-of-care testing (POCT) of tumor cell-derived exosomes is of vital importance for understanding tumor growth and metastasis. Multicolor-based ratiometric signals most often rely on molecular optics, such as fluorescence resonance energy transfer (FRET)-dependent molecular fluorescence and localized surface plasmon resonance (LSPR)-related molecular colorimetry. However, finding acceptable FRET donor–acceptor fluorophore pairs and the kinetically slow color responses during size-related molecular colorimetry have greatly impeded POCT applications. Herein, an atomic flame was used to develop a visual sensing platform for the POCT of tumor-cell-derived exosomes. In comparison with common molecular optics, the atomic flame possessed the advantages of providing both a variety of ratiometric flame signals and fast response sensitivity. The integration of a gas-pressure-assisted flame reaction and dual-aptamer recognition guaranteed the sensitive and selective analysis of exosomes with a low limit of detection (LOD) of 7.6 × 102 particles per mL. Such a novel optical signal will inspire the development of more user-friendly POCT approaches.
1. Introduction
Exosomes are nanosized vesicles with a size of about 30 to 150 nm, which play a vital function in cell-to-cell communication.1,2 Both normal and tumor cells have been demonstrated to secrete exosomes,3,4 whereas tumor cells secrete more exosomes for tumor growth and metastasis.5 The released exosomes carry abundant biological information from their parent cells, which showed the potential for their use in non-invasive liquid biopsies.6,7 For the detection of exosomes, most of the reported methods rely on surface plasma resonance imaging,8 nanoparticle counting,9 electrochemistry,10 molecular fluorescence and colorimetry,11,12 and thermal and acoustic signals.13,14 Among them, color-based point-of-care testing (POCT) was greatly preferred due to its naked eye recognition, simple operation and portable instruments.15–18 Because human eyes are sensitive to the color change rather than the brightness variation of the same color, a ratiometric signal is thus adopted for POCT because of its higher sensitivity.19So far, multicolor signal focused on molecular optics such as molecular fluorescence and molecular colorimetry.20,21 To obtain ratiometric fluorescence signals, a fluorescence resonance energy transfer (FRET) system is always designed with two different chromophores (i.e., one donor and one acceptor).22 In spite of the capability of the self-calibrated internal standard, the FRET needs to be activated depending on the extent of spectral overlap and intermolecular distance between the donor and the acceptor,23 which causes a more complex conformational arrangement. Relative to molecular fluorescence, ratiometric molecular colorimetry could be performed with single noble metal nanomaterials, which can simplify the design of the optical probe. Taking advantage of the size-related localized surface plasmon resonance (LSPR), the etching of gold nanorods and aggregation of gold nanoparticles (AuNPs) are capable of being used to generate multicolor signals.24–26 However, most of the reported quantitative analyses are carried out at one time point before completing the etching and aggregation process because these processes always last for a long period of time.27,28 Therefore, such a colorimetric POCT saves testing time at the expense of sensitivity.
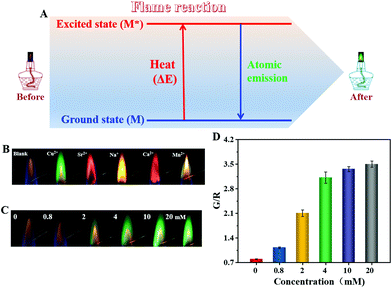
When placed in a burning flame, metal atoms could adsorb heat and cause their electrons to be excited from the low-energy ground state to a high-energy excited state (Fig. 1A).29 These excited electrons are always unstable and tend to emit energy in the form of photons to return to the ground state.30 In most cases, the wavelength range of the emitted photons is located at the visual region. As a result, a colorful flame is observed due to atomic emission and the process is termed as a flame reaction. Because burning ethanol has an intact flame, which is different to that of metal ions, it provides an excellent alternative for the readout of ratiometric optical signals.31,32
In this work, an atomic flame-dependent ratiometric POCT platform for visual analysis of tumor cell-derived exosomes is described. Relative to the ratiometric molecular signals based on FRET-dependent molecular fluorescence and size-related molecular colorimetry, atomic flame-based optical signal showed the advantages of providing both a variety of ratiometric colors and fast optical response (within 1 min). By virtue of the generated gas pressure from the decomposition of hydrogen dioxide (H2O2) catalyzed by aptamer functionalized platinum nanoparticles (PtNPs), the volume spilled from the sealed system of a high concentration of Cu2+ was proportional to the added exosomes, which enabled the atomic flame-based POCT of tumor cell-derived exosomes. Efficient magnetic separation and dual-aptamer recognition allowed selective quantitative analysis of the target exosomes.
2. Experimental section
2.1 Flame reaction of metal ions
Typically, 10 μL of metal ion (20 mM) solution was dropped on a twisted iron wire, followed by exposure to the burning flame of an alcohol lamp. Meanwhile, an image of the flame was recorded with a smartphone. After the flame reaction, the twisted iron wire was cleaned with 3 M hydrochloric acid. For the optimization experiment, the volume and concentration of Cu2+ solution were varied.2.2 Comparison between atomic flame and FRET-dependent molecular fluorescence, AuNPs-related molecular colorimetry
Firstly, ratiometric optical signals were compared with FRET-dependent molecular fluorescence and the atomic flame. For the system of ratiometric fluorescence, a Cy3-labeled hairpin DNA probe (H1) and Cy5-labeled hairpin DNA probe (H2) were mixed and then further incubated with the catalytic probe for 2.0 h. Then, the solution was photographed with a smartphone under light irradiation of 365 nm. For the ratiometric flame system, the flame of the metal ion mixture (Na+, Cu2+ and Sr2+) with different molar ratios was recorded. The concentrations of Na+, Sr2+ and Cu2+ were 5, 10 and 20 mM, respectively.Then, the sensitivity of optical response was compared between AuNP-related molecular colorimetry and the atomic flame. For the molecular colorimetry, AuNPs (15 nM) were mixed with 11.8 mM NaCl at 37 °C. The time-dependent color change of the AuNP solution was recorded with a smartphone. For the atomic flame, the flame of Cu2+ at different concentrations (20 mM and 4.35 M) was recorded with a smartphone.
2.3 The detection of exosome concentration
Firstly, 200 μL of CD63 aptamer functionalized magnetic nanoparticles (AptCD63-MNPs) was incubated with exosomes at 4 °C for 1 h. After magnetic separation, 20 μL of AptEpCAM functionalized platinum nanoparticles (AptEpCAM-PtNPs) were added for further binding. The formed sandwich structure was collected and transferred to a home-made sensing device, followed by the addition of 10 M H2O2. Finally, the Cu2+ solution spilled from the sealed system was collected and the solution volume was made up to 500 μL with deionized water, and 10 μL of the diluted Cu2+ solution was utilized for the subsequent flame reaction.3. Results and discussion
3.1 Flame-reaction-based ratiometric atomic flame
As shown in Fig. 1B, the alcohol burnt with a yellow-orange flame.31 Upon the addition of Cu2+, the flame color turned to green, demonstrating a distinctive flame reaction. For Sr2+ and Ca2+, the flame colors were magenta and brick red respectively, whereas the samples of Na+ and Mn2+ displayed yellow and olivine colored flames, respectively. Different energy level differences between the ground state and excited state could explain the generation of the different characteristic flame colors of the metal atoms. Obviously, a significant flame color change presented in the absence and presence of metal ions, which meant that it would be possible to output ratiometric flame signals.Next, the Cu2+ was used as a model metal ion to further investigate the flame reaction, wherein the strategy of the red-green-blue (RGB) value was employed for quantitative analysis. According to the digital photographs in Fig. 1C, the intensity of the green flame was directly proportional to the increased concentration of Cu2+, and was accompanied by the gradually disappearing yellow-orange background flame. An obvious green flame could only be observed if the concentration used was more than 2 mM. The concentration-dependent green intensity was further demonstrated by determining the G/R value (the maximal ratio of the G value over R value) of the digital photograph (Fig. 1D). The blank control displayed a low G/R value. A significant increase in the G/R value was obtained for 0.8 mM of Cu2+ in spite of the negligible switch of flame color in Fig. 1C when observed with the naked eye, which proved the advantage of an RGB value-based strategy in response sensitivity.
3.2 Comparison between atomic flame, molecular fluorescence, and molecular colorimetry-based ratiometric signals
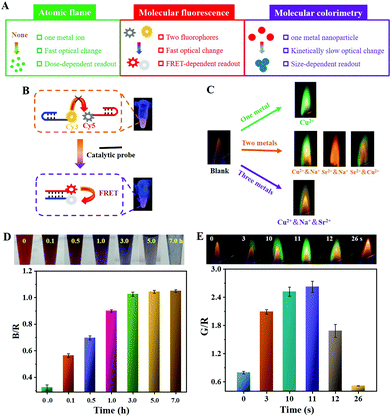
At present, most of the reported ratiometric optical signals have been dependent on molecular fluorescence and molecular colorimetry. With regard to the molecular fluorescence system, the FRET design is one of the most used, in which the decreased fluorescence of the donors is accompanied by the increased fluorescence of the acceptors. For the classic example of Cy3–Cy5 donor–acceptor pairs, the yellow fluorescence emission of Cy3 under irradiation with 365 nm light suggested a low FRET efficiency between Cy3 and Cy5 chromophores (the top photograph in Fig. 2B), although the fluorescence emission spectrum of Cy3 was largely overlapped with the absorption spectrum of Cy5 (Fig. S1, ESI†). This could be explained by the fact that the FRET efficiency is largely related to not only the optical properties but also to the relative distance between the donor and the acceptor molecules.23 Due to the catalytic hairpin assembly (CHA) reaction initiated by the catalytic probe, duplex DNA formed which ensured that Cy3 was close to Cy5 and thus activated the FRET, as shown by the red fluorescence emission of Cy5 (the bottom photograph in Fig. 2B). The need for two specific fluorophores has greatly reduced the candidates for the FRET probe and the distance-dependent efficiency caused a more complex structure arrangement. By contrast, a ratiometric atomic flame could be achieved with only one type of metal ion (Fig. 1B). Furthermore, different ratiometric signals could be achieved with a particular metal ion. Because the flame reaction happens independently, the resultant flame of co-existing metal ions was ascribed to the joint effect of the multiple flame reaction processes. Therefore, such ratiometric flame signals could be further expanded by mixing different metal ions (the bottom two panels in Fig. 2C) and adjusting their composition ratio (Fig. S2, ESI†).After comparing the atomic flame with molecular fluorescence, the use of molecular colorimetry was then studied. The AuNPs have been extensively used for developing colorimetric sensors due to the solution color change mediated by aggregation. As shown in Fig. 2D and S3A (ESI),† the solution of citrate-stabilized AuNPs with a diameter of 13 ± 3.2 nm was red colored. The low stability was readily broken with the addition of NaCl (11.8 mM) by screening the charge repulsion, and the negatively charged AuNPs tended to aggregate (Fig. S3B, ESI†). It should be noted that a low concentration of NaCl was used with the aim of obtaining high repeatability. Meanwhile, the solution color was gradually changed from red to purple, and then blue with an increasing incubation time (the top photographs in Fig. 2D). The time-dependent B/R value kept increasing, which demonstrated that the salt-induced AuNPs aggregation was a complex and kinetically slow process (Fig. 2D).28 To achieve rapid POCT, the operation time has to balance against the sensitivity. For the atomic flame-based ratiometric system, Cu2+ solution could rapidly evaporate for the following flame reaction due to the high thermal energy supply from the burning of alcohol, as shown by the appearance of a green flame within 3 s (Fig. 2E). The green flame lasted less than 30 s, and demonstrated the fast response of the flame color. Indeed, higher concentrations of Cu2+ required a longer time for the flame reaction. However, the saturated Cu2+ (about 4.35 M) completed the whole flame reaction within 1 min (Fig. S4, ESI†).
Consequently, the atomic flame showed some significant advantages in outputting the ratiometric signal (Fig. 2A). Firstly, the ratiometric signal could be obtained by simply using one type of metal ion and the flame color could be further expanded by mixing different metal ions or adjusting the ratio. Secondly, a sensitive response of flame reaction towards metal ions guaranteed a fast signal output. Finally, the ratiometric signal intensity largely depended on the added concentration of the metal ion.
3.3 Gas-pressure-assisted atomic flame assays
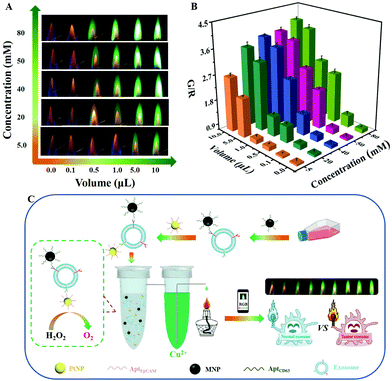
After demonstrating the merits in outputting ratiometric signals, an attempt was made to evolve the atomic flame into a visual POCT platform. As a proof-of-concept assay, tumor cell-derived exosomes were analyzed visually. As discussed previously (Fig. 1C), the flame reaction required a high concentration of metal ions (in the mM range). Therefore, the volume of concentrated metal ion solution was considered to be an indicator of the target amount. Because the flame intensity was positively correlated with the added concentration of metal ions, the solution volume was firstly optimized by changing the concentration of the metal ions (Fig. 3A). For 5.0 mM Cu2+, the green flame was emitted only when the added solution volume was more than 5.0 μL. However, an obvious green flame could be seen with 0.5 μL of Cu2+ when the concentration was over 50 mM. There is no doubt that the volume used could even be further reduced by increasing the ionic concentration. A similar trend was also obtained with the R/G value-based method (Fig. 3B), further proving the possibility of improving the response sensitivity of the atomic flame by concentrating the metal ions. To obtain a wide response range, an optimal concentration of 20 mM Cu2+ was selected for the subsequent studies.To accomplish the goal of a volume-indicated analysis, a home-made sealed device was designed, consisting of two spatially separated but connected tubes in which one tube was used to generate oxygen gas from the decomposition of H2O2 catalyzed by AptEpCAM-PtNPs, while the other one contained a high concentration of Cu2+ (Fig. 3C). Therefore, the increased gas pressure caused the metal ion solution to spill from the sealed system for the subsequent flame reaction. Considering that a variety of metal ions might co-exist in biological samples, AptCD63-MNPs were successfully prepared for recognizing the exosomes and removing the matrix interference via magnetic isolation (Fig. S5A and S5B, ESI†). Such a lab-on-tube design and dual-aptamer recognition guaranteed a high response sensitivity and selectivity of the atomic flame assay.
In order to verify the feasibility of the gas pressure-mediated spill of the ionic solution, nanoflower-like PtNPs with a size of 54 ± 14.0 nm were synthesized (Fig. S5C, ESI†). After the addition of PtNPs (4.9 μg mL−1), a number of bubbles were visually seen to rise to the surface of the H2O2 solution (photograph not shown), leading to a strong green flame originating from the Cu2+ spilled by the increased gas pressure of the sealed system (the upper-right photograph in Fig. S5E, ESI†). More PtNPs meant a higher G/R value (Fig. S5F, ESI†), which confirmed the possibility of a volume-indicated atomic flame assay. In addition, the functional modification with AptEpCAM molecules did not change the catalytic performance of the PtNPs (Fig. S5B and S5F, ESI†). Taken together, the gas pressure-assisted atomic flame assay shows potential for the volume-indicated bioanalysis of tumor cell-derived exosomes.
3.4 Feasibility of exosome POCT
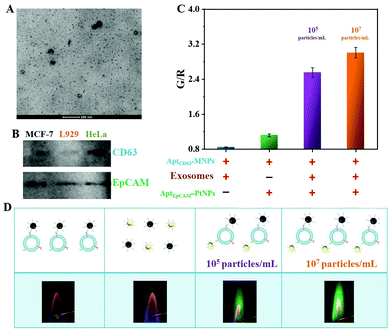
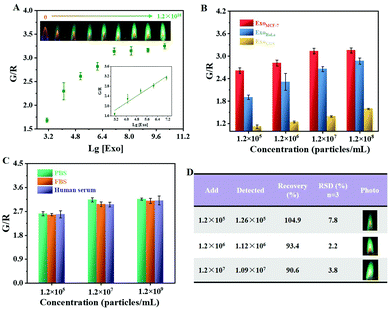
By virtue of a commercial ExoQuick-TC Isolation Kit, exosomes were collected from the supernatant of a cell culture. Transmission electron microscopy (TEM) characterization demonstrated that the MCF-7 breast cancer cell-derived exosomes (ExoMCF-7) possessed a typical cup-shaped structure with a size ranging from 43 to 74 nm (Fig. 4A), and this was consistent with a previous report in the literature.33 The isolated exosomes with an intact concentration of 1.2 × 1010 particles per mL were measured using nanoparticle tracking analysis (NTA).After an efficient capture and isolation using AptCD63-MNPs, the ExoMCF-7 were incubated with AptEpCAM-PtNPs, followed by the addition of H2O2 to initiate the gas pressure-assisted flame reaction. Due to the dual expression of CD63 and EpCAM surface proteins (Fig. 4B), both AptEpCAM-PtNPs and AptCD63-MNPs could be tightly bound to the ExoMCF-7, which was validated by the green flame of Cu2+ (the third and fourth photographs in Fig. 4D). In contrast, the G/R value for the sample without exosomes was nearly 2.5 orders lower than that in the presence of 105 particles per mL exosomes, indicating that the free AptEpCAM-PtNPs were almost magnetically washed away, although weak nonspecific binding of AptCD63-MNPs and AptEpCAM-PtNPs existed (violet and green bars in Fig. 4C). No significant flame color change was observed for the sample of AptCD63-MNPs attached on the surface of exosome (the first photo in Fig. 4D), which suggested the importance of AptEpCAM-PtNPs in gas pressure-assisted atomic flame assay. Atomic flame intensity was positively correlated with the concentration of exosomes (the violet- and yellow-colored bars in Fig. 4C), which verified the feasibility of atomic flame-based visual analysis of tumor cell-derived exosomes.
3.5 Performance testing
As expected, the yellow-orange flame gradually turned to a green flame when the concentration of ExoMCF-7 increased from 0 to 1.2 × 1010 particles per mL (see the inset in Fig. 5A). The exosome concentration-dependent ratiometric signal change was further quantitatively determined with the G/R value (Fig. 5A). The increased G/R value was linearly scaled with the concentration of exosomes from 1.2 × 103 to 1.2 × 107 particles per mL (see the inset in Fig. 5A). Because of the limited loading amount of the twisted iron wire (≤10 μL), the saturation point emerged when the concentration of exosomes exceeding 1.2 × 107 particles per mL. The LOD was estimated to be 7.6 × 102 particles per mL based on 3σ/slope, wherein σ represents the standard deviation of the blank control. The strong catalytic activity of PtNPs coupled with a high concentration of Cu2+ volume-based indicator contributed to highly sensitive analysis, and the LOD was lower than that relied on molecular fluorescence and colorimetry (Table 1).| Detection mode | Response range (particles per mL) | LOD | Ref. |
|---|---|---|---|
| Fluorescence | 8.0 × 107–1.6 × 1010 | 5.0 × 107 | 34 |
| Fluorescence | 1.7 × 107–4.2 × 1010 | 7.6 × 106 | 35 |
| Fluorescence | 1.0 × 108–1.0 × 1012 | 1.0 × 108 | 36 |
| Fluorescence | 5.5 × 106–1.1 × 1010 | 1.3 × 106 | 11 |
| Fluorescence | 1.0 × 104–1.0 × 109 | 1.4 × 103 | 37 |
| Colorimetry | 5.6 × 107–8.9 × 108 | 4.5 × 106 | 38 |
| Colorimetry | 1.8 × 109–2.2 × 1010 | 5.2 × 108 | 12 |
| Colorimetry | 2.3 × 106–2.3 × 108 | 2.2 × 106 | 39 |
| Colorimetry | 0.2 × 1010–3.4 × 1010 | 1.4 × 107 | 40 |
| Colorimetry | 4.0 × 107–6.0 × 108 | 7.0 × 106 | 41 |
| Atomic flame | 1.2 × 103–1.2 × 107 | 7.6 × 102 | This work |
Because the expression level of the two markers (CD63 and EpCAM) differed in different cell-derived exosomes, atomic flame-based visual analysis was then applied to distinguish between the different exosomes. Despite the fact that the G/R value increased as a function of exosome concentration, the G/R value for the L929 cell-derived exosomes was obviously lower than those from breast cancer cells and cervical cancer cells (MCF-7 and HeLa, respectively) (Fig. 5B). These results were in accordance with the results of the western blotting analysis (Fig. 4B), in which two clear bands of CD63 and EpCAM proteins were observed on the gel for the exosomes secreted by MCF-7 and HeLa cells, whereas the expression amount of the CD63 protein was relatively low for the sample of ExoL929. The ExoMCF-7 possessed a higher G/R value than that of ExoHeLa, which might be originated from the higher expression of EpCAM protein on the ExoMCF-7. Therefore, such a dual-aptamer recognition strategy could allow exosome phenotyping based on parent cells.
To test the analysis sensitivity under the conditions of the complex system, ExoMCF-7 was spiked into healthy human serum diluted 500-fold to mimic real samples. A reliable detection stability of the proposed method was firstly demonstrated by obtaining almost the same G/R value in the presence of different working environments including phosphate buffered saline (PBS), exosome-free fetal bovine serum (FBS) and healthy human serum (Fig. 5C). Because there was only a few exosomes in the healthy human serum and a low expression amount of both CD63 and EpCAM proteins, a good recovery of 90.6%–104.9% was achieved for the assay of the spiked exosomes (Fig. 5D). All the relative standard deviations (RSDs) of three independent measurements were below 8.0%, which suggested the excellent reproducibility of the proposed atomic flame-based visual sensing platform.
4. Conclusions
In summary, a ratiometric atomic flame was explored for the POCT of tumor-cell-derived exosomes. Relative to common molecular optics, the atomic flame has some unique advantages in relation to ratiometric signals. Firstly, various types of ratiometric flame could be provided. Secondly, a less than 1-min reaction process allowed a fast flame response. In combination with a gas-pressure-assisted atomic flame assay and dual-aptamer recognition, the POCT of exosomes was achieved with an excellent linear relationship ranging from 1.2 × 103 to 1.2 × 107 particles per mL. The strong catalytic activity of PtNPs coupled with a high concentration of Cu2+ volume-based indicator contributed to a low LOD of 7.6 × 102 particles per mL. The proposed atomic-flame-based visual sensing platform is a general approach for the sensitive bioanalysis of disease-related biomolecules and metabolites.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22064004), the Guangxi Provincial Science and Technology Bases and Special Fund for Talented Persons (No. 2019AC20288), the Natural Science Foundation of Guangxi Province (No. 2019JJB120037) and the BAGUI Scholar Program.All experiments were performed in accordance with the relevant laws or guidelines of China, and followed institutional guidelines of China, and were approved by the Institutional Ethical Committee (IEC) of Guangxi Normal University. Informed consent was obtained from all human subjects.
References
- F. Song, C. Wang, C. Wang, J. Gao, H. Liu, Y. Zhang and L. Han, Anal. Chem., 2021, 93, 4697–4706 CrossRef CAS.
- J. Li, K. Peng, Y. Li, J. Wang, J. Huang, Y. Yan, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 21510–21514 CrossRef CAS PubMed.
- K. Mori, M. Hirase, T. Morishige, E. Takano, H. Sunayama, Y. Kitayama, S. Inubushi, R. Sasaki, M. Yashiro and T. Takeuchi, Angew. Chem., Int. Ed., 2019, 58, 1612–1615 CrossRef CAS PubMed.
- X. Wu, H. Zhao, A. Natalia, C. Z. J. Lim, N. R. Y. Ho, C.-A. J. Ong, M. C. C. Teo, J. B. Y. So and H. Shao, Sci. Adv., 2020, 6, eaba2556 CrossRef CAS PubMed.
- A. Bagheri Hashkavayi, B. S. Cha, E. S. Lee, S. Kim and K. S. Park, Anal. Chem., 2020, 92, 12733–12740 CrossRef CAS PubMed.
- R. Xu, A. Rai, M. Chen, W. Suwakulsiri, D. W. Greening and R. J. Simpson, Nat. Rev. Clin. Oncol., 2018, 15, 617–638 CrossRef CAS.
- Y. Lyu, D. Cui, J. Huang, W. Fan, Y. Miao and K. Pu, Angew. Chem., Int. Ed., 2019, 58, 4983–4987 CrossRef CAS.
- S. Picciolini, A. Gualerzi, R. Vanna, A. Sguassero, F. Gramatica, M. Bedoni, M. Masserini and C. Morasso, Anal. Chem., 2018, 90, 8873–8880 CrossRef CAS PubMed.
- Q. Tian, C. He, G. Liu, Y. Zhao, L. Hui, Y. Mu, R. Tang, Y. Luo, S. Zheng and B. Wang, Anal. Chem., 2018, 90, 6556–6562 CrossRef CAS.
- H. Zhang, Z. Wang, F. Wang, Y. Zhang, H. Wang and Y. Liu, Anal. Chem., 2020, 92, 5546–5553 CrossRef CAS PubMed.
- X. Wang, H. Shang, C. Ma and L. Chen, Anal. Chem., 2021, 93, 8493–8500 CrossRef CAS PubMed.
- Y. Xia, M. Liu, L. Wang, A. Yan, W. He, M. Chen, J. Lan, J. Xu, L. Guan and J. Chen, Biosens. Bioelectron., 2017, 92, 8–15 CrossRef CAS PubMed.
- N. Cheng, Y. Song, Q. Shi, D. Du, D. Liu, Y. Luo, W. Xu and Y. Lin, Anal. Chem., 2019, 91, 13986–13993 CrossRef CAS PubMed.
- J. Suthar, E. S. Parsons, B. W. Hoogenboom, G. R. Williams and S. Guldin, Anal. Chem., 2020, 92, 4082–4093 CrossRef CAS PubMed.
- J. Sun, Y. Xianyu and X. Jiang, Chem. Soc. Rev., 2014, 43, 6239–6253 RSC.
- A. Malekjahani, S. Sindhwani, A. M. Syed and W. C. W. Chan, Acc. Chem. Res., 2019, 52, 2406–2414 CrossRef CAS PubMed.
- S. Hu, J. Wang and J. Liu, J. Phys. Chem. C, 2019, 123, 12015–12022 CrossRef CAS.
- Z. Wei, Y. Yu, S. Hu, X. Yi and J. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 16801–16811 CrossRef CAS PubMed.
- A. Bigdeli, F. Ghasemi, S. Abbasi-Moayed, M. Shahrajabian, N. Fahimi-Kashani, S. Jafarinejad, M. A. Farahmand Nejad and M. R. Hormozi-Nezhad, Anal. Chim. Acta, 2019, 1079, 30–58 CrossRef CAS PubMed.
- C.-C. Fang, C.-C. Chou, Y.-Q. Yang, T. Wei-Kai, Y.-T. Wang and Y.-H. Chan, Anal. Chem., 2018, 90, 2134–2140 CrossRef CAS PubMed.
- J. Yudkin, Nature, 1945, 155, 50–50 CrossRef CAS.
- K. E. Sapsford, L. Berti and I. L. Medintz, Angew. Chem., Int. Ed., 2006, 45, 4562–4589 CrossRef CAS PubMed.
- I. Rasnik, S. A. McKinney and T. Ha, Acc. Chem. Res., 2005, 38, 542–548 CrossRef CAS PubMed.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS PubMed.
- J. Liu and Y. Lu, J. Am. Chem. Soc., 2003, 125, 6642–6643 CrossRef CAS PubMed.
- S. Hu, P.-J. J. Huang, J. Wang and J. Liu, Anal. Chem., 2020, 92, 13354–13360 CrossRef CAS PubMed.
- S. Christau, T. Moeller, J. Genzer, R. Koehler and R. von Klitzing, Macromolecules, 2017, 50, 7333–7343 CrossRef CAS.
- X. Zhang, X. Fan, Y. Wang, F. Lei, L. Li, J. Liu and P. Wu, Anal. Chem., 2020, 92, 1455–1462 CrossRef CAS PubMed.
- M. J. Sanger, J. Chem. Educ., 2004, 81, 1776A CrossRef CAS.
- S. L. Bretz and A. V. Murata Mayo, J. Chem. Educ., 2018, 95, 17–27 CrossRef CAS.
- R. O. Ragsdale and J. A. Driscoll, J. Chem. Educ., 1992, 69, 828 CrossRef CAS.
- X. Fang, X. Zhang, Z. Zhang, S. Hu, F. Ye and S. Zhao, Chem. Commun., 2021, 57, 3327–3330 RSC.
- H. Zheng, S. Guan, X. Wang, J. Zhao, M. Gao and X. Zhang, Anal. Chem., 2020, 92, 9239–9246 CrossRef CAS PubMed.
- F. He, J. Wang, B.-C. Yin and B.-C. Ye, Anal. Chem., 2018, 90, 8072–8079 CrossRef CAS PubMed.
- L. Wang, Y. Yang, Y. Liu, L. Ning, Y. Xiang and G. Li, Chem. Commun., 2019, 55, 2708–2711 RSC.
- X. Yu, L. He, M. Pentok, H. Yang, Y. Yang, Z. Li, N. He, Y. Deng, S. Li, T. Liu, X. Chen and H. Luo, Nanoscale, 2019, 11, 15589–15595 RSC.
- Q. Zhang, F. Wang, H. Zhang, Y. Zhang, M. Liu and Y. Liu, Anal. Chem., 2018, 90, 12737–12744 CrossRef CAS.
- Y. Zhang, Q. Su, D. Song, J. Fan and Z. Xu, Anal. Chim. Acta, 2021, 1145, 9–16 CrossRef CAS.
- F. He, H. Liu, X. Guo, B.-C. Yin and B.-C. Ye, Anal. Chem., 2017, 89, 12968–12975 CrossRef CAS.
- Y.-M. Wang, J.-W. Liu, G. B. Adkins, W. Shen, M. P. Trinh, L.-Y. Duan, J.-H. Jiang and W. Zhong, Anal. Chem., 2017, 89, 12327–12333 CrossRef CAS.
- J. Chen, Y. Xu, Y. Lu and W. Xing, Anal. Chem., 2018, 90, 14207–14215 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1an01825f |
| This journal is © The Royal Society of Chemistry 2022 |