Open Access Article
Open Access ArticleEnabling large-scale hydrogen storage in porous media – the scientific challenges
Niklas
Heinemann
*a,
Juan
Alcalde
 b,
Johannes M.
Miocic
cd,
Suzanne J. T.
Hangx
e,
Jens
Kallmeyer
f,
Christian
Ostertag-Henning
g,
Aliakbar
Hassanpouryouzband
b,
Johannes M.
Miocic
cd,
Suzanne J. T.
Hangx
e,
Jens
Kallmeyer
f,
Christian
Ostertag-Henning
g,
Aliakbar
Hassanpouryouzband
 a,
Eike M.
Thaysen
a,
Gion J.
Strobel
h,
Cornelia
Schmidt-Hattenberger
f,
Katriona
Edlmann
a,
Mark
Wilkinson
a,
Michelle
Bentham
i,
R.
Stuart Haszeldine
a,
Ramon
Carbonell
b and
Alexander
Rudloff
a,
Eike M.
Thaysen
a,
Gion J.
Strobel
h,
Cornelia
Schmidt-Hattenberger
f,
Katriona
Edlmann
a,
Mark
Wilkinson
a,
Michelle
Bentham
i,
R.
Stuart Haszeldine
a,
Ramon
Carbonell
b and
Alexander
Rudloff
 f
f
aSchool of Geosciences, University of Edinburgh, UK. E-mail: n.heinemann@ed.ac.uk
bGeosciences Barcelona, CSIC, Barcelona, Spain
cInstitute of Earth and Environmental Science, University of Freiburg, Germany
dEnergy and Sustainability Research Institute Groningen (ESRIG), University of Groningen, The Netherlands
eDepartment of Earth Sciences, Utrecht University, The Netherlands
fGFZ German Research Centre for Geosciences, Potsdam, Germany
gFederal Institute for Geosciences and Natural Resources, Germany
hInstitute of Subsurface Energy Systems, Clausthal University of Technology, Germany
iBritish Geological Survey, Keyworth, Nottingham, UK
First published on 5th January 2021
Abstract
Expectations for energy storage are high but large-scale underground hydrogen storage in porous media (UHSP) remains largely untested. This article identifies and discusses the scientific challenges of hydrogen storage in porous media for safe and efficient large-scale energy storage to enable a global hydrogen economy. To facilitate hydrogen supply on the scales required for a zero-carbon future, it must be stored in porous geological formations, such as saline aquifers and depleted hydrocarbon reservoirs. Large-scale UHSP offers the much-needed capacity to balance inter-seasonal discrepancies between demand and supply, decouple energy generation from demand and decarbonise heating and transport, supporting decarbonisation of the entire energy system. Despite the vast opportunity provided by UHSP, the maturity is considered low and as such UHSP is associated with several uncertainties and challenges. Here, the safety and economic impacts triggered by poorly understood key processes are identified, such as the formation of corrosive hydrogen sulfide gas, hydrogen loss due to the activity of microbes or permeability changes due to geochemical interactions impacting on the predictability of hydrogen flow through porous media. The wide range of scientific challenges facing UHSP are outlined to improve procedures and workflows for the hydrogen storage cycle, from site selection to storage site operation. Multidisciplinary research, including reservoir engineering, chemistry, geology and microbiology, more complex than required for CH4 or CO2 storage is required in order to implement the safe, efficient and much needed large-scale commercial deployment of UHSP.
1. Introduction
Hydrogen is attracting global attention as a key future low-carbon energy carrier, for the decarbonisation of transport, power and heating, and of fuel-energy intensive industries, such as the chemical and steel industries.1–5 The United Nations Industrial Development Organisation6 has defined hydrogen as “a true paradigm shift in the area of more efficient energy storage, especially for renewable energy on industrial scale” and the IPCC's 1.5 °C Report7 states that hydrogen must play a significant role as a fuel substitute to limit global warming and that it will lead to emission reductions in energy-intensive industries.Large-scale hydrogen storage can help alleviate the main drawbacks of renewable energy generation, their intermittency and their seasonal and geographical constraints. Renewable energy sources are greatly dependent on seasonally fluctuating atmospheric events (e.g. sunlight level and intensity, wind force8,9), which when combined with annually varying, but steady, energy demand, results in renewable energy excesses or deficits. Therefore, renewable energy without energy storage is unable to satisfy the whole system energy demand.10,11 Excess renewable energy can be converted to hydrogen through electrolysis (“green hydrogen”) and stored to be used during periods of high energy demand (Fig. 1). Even hydrogen generated from hydrocarbons, in combination with Carbon Capture and Storage, (“blue hydrogen”) can help to reduce emissions in the energy sector while transitioning towards low-carbon industry.12 This has prompted national and international research and development efforts focussing on the potential of large-scale hydrogen technologies (e.g. 7000 MEUR in Germany,13 70 M$ in Australia,14 H2020-FCH15). These initiatives are aimed at accelerating the research and deployment of hydrogen technologies through feasibility, demonstration or commercial-scale projects.
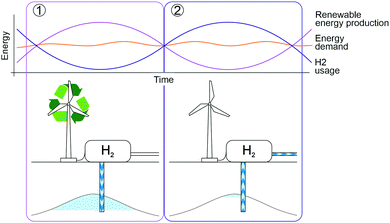 | ||
| Fig. 1 Hydrogen from renewable energy is stored during periods of high renewable energy production (1) to satisfy demand during times of high energy demand and low renewable energy production (2). | ||
Surface hydrogen storage facilities, such as pipelines or tanks have limited storage and discharge capacity (MW h; hours-days). By contrast, to supply energy in the GW h/TW h-range (weeks-months), subsurface storage of hydrogen in salt caverns, depleted hydrocarbon reservoir and/or deep saline aquifers is needed. Salt caverns are frequently used to store natural gas,16,17 and hydrogen storage has been commercially implemented for over 30 years at Teeside (UK) and at the US Gulf Coast.18 Cavern storage is ideally suited to short- to medium-term energy demand fluctuations, as they allow for multiple injection-reproduction cycles per year and very rapid production rates. However, they are geographically constrained to the presence of evaporitic formations with suitable thickness and extent, offering storage capacities of a few 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s to up to 1
000 s to up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 with an energy content of up to several 100 s GW h.19
000 m3 with an energy content of up to several 100 s GW h.19
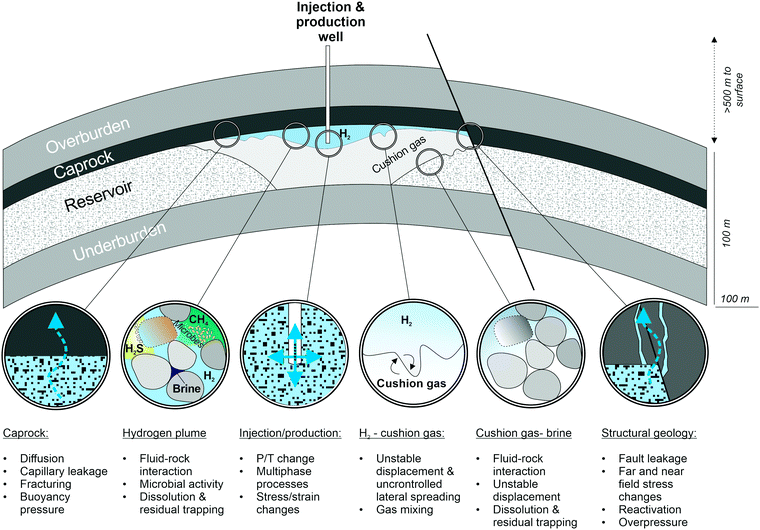
For storage over longer periods of time (months), for example to supply energy to domestic homes during the winter season, porous saline aquifers and depleted hydrocarbon fields offer storage capacities several orders of magnitude larger than salt caverns, and provide a geographically more independent and flexible solution for large-scale hydrogen storage.20,21 Such geological hydrogen stores feature a porous and permeable reservoir formation, a caprock and a trap structure.18,22 The injected hydrogen will displace the in situ pore fluids, usually brine and/or residual hydrocarbons, and spread out underneath a low-permeable caprock capable of retaining the fluid. A trap structure will prevent the hydrogen from escaping laterally and will keep the hydrogen in place to allow reproduction (Fig. 2). In order to maintain sufficient operational pressure, typically a share of the injected gas, referred to as cushion gas, will remain in the reservoir, compared to the reproducible working gas. The storage of hydrogen has been debated since the 1980s,23 and it was determined that the physical and chemical challenges associated with hydrogen storage in sedimentary formations were manageable.24 So why is large-scale UHSP in porous formations still a controversy? After all, the geology of the target formations, such as brine-filled sandstone aquifers or depleted gas fields, is generally well known. Furthermore, selected examples of both depleted fields and saline aquifer anticlines have been targets for current or future gas storage operations, hence there is compelling evidence that they have retained and will retain injected gas.
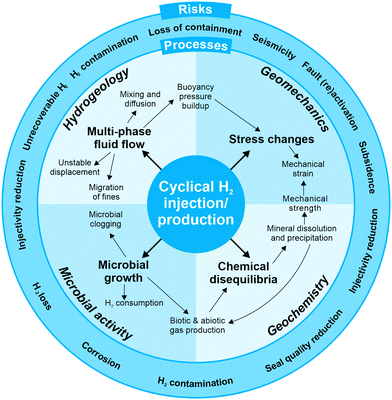
However, experience with underground hydrogen storage in porous geological formations is very limited and practical applications are restricted to the storage of town gas, i.e. gas mixtures with 25–60% hydrogen, and smaller amounts of CH4 (10–33%), CO and CO2 (12–20%) and <30% N2. Town gas storage has been utilised in aquifers in France (Beynes), Czechoslovakia (Lobodice) and Germany (Engelborstel, Bad Lauchstaedt, Kiel).18,24–27 Additionally, scientists and engineers can utilise experiences from other gas storage operations facing similar technical, geological and hydraulic challenges, such as the underground storage of natural gas (UGS), compressed air (CAES), and, to a lesser degree, CO2 subsurface storage (UCS). However, several aspects unique to hydrogen must be taken into consideration (Fig. 3). Firstly, hydrogen has very different physical and chemical properties compared to other geologically stored fluids such as CH4, air or CO2. Secondly, hydrogen may react with the subsurface minerals and fluids, potentially affecting the storage operations. Thirdly, the presence of hydrogen in the subsurface can trigger growth of hydrogen consuming microbes; and fourthly, the stress field in hydrogen storage sites will change during repeated injection-reproduction cycles and hence containment may be compromised. Therefore, within the context of these complex processes, suitable UHSP sites need specific characterisation in order to guarantee secure and economic hydrogen injection and reproduction. Uncertainties related to potential leakage, as well as other risks such as induced seismicity and the loss of hydrogen due to microbial activity need to be investigated and quantified, and new monitoring programs require investigation and calibration. This perspective outlines the scientific challenges of hydrogen storage in deep saline aquifers and depleted hydrocarbon fields, in order to spark a discussion within the multidisciplinary energy research community. In addition to the technical and socioeconomic challenges, the underlying scientific questions outlined below need to be addressed in order to provide the basis to accurately assess the opportunities and challenges associated with UHSP. Only then can industry, regulators and the public implement policies for large-scale hydrogen storage in porous media and determine how this technology can contribute to the energy transition.
2. Hydrogen fluid properties
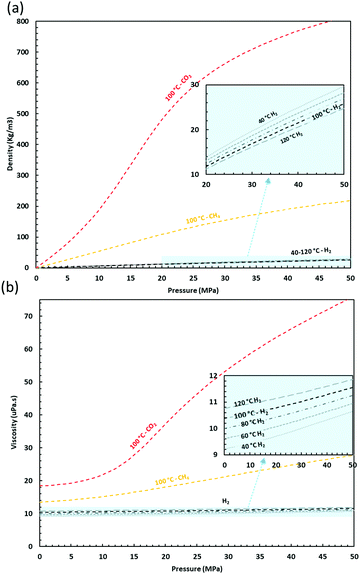
Hydrogen has a higher energy density per mass (∼120 MJ kg−1) than hydrocarbons.28 However, its low density (0.084 kg m−3 at 20 °C and 0.1 MPa – see Fig. 4) means it will require a greater volumetric storage capacity compared to natural gas to deliver the same energy output.29 Injection of hydrogen into porous storage reservoirs displaces the formation fluids, leading to complex multiphase displacement patterns, controlled by the fluid and rock properties (e.g. fluid phase viscosity, density, compressibility, porosity and intrinsic permeability of the porous media) and the functional relationships between fluid saturation and relative permeability. Hydrogen storage operations will rely on the accurate prediction of multi-phase fluid displacement in porous media. Pure hydrogen properties are well established, but the multi-phase properties in porous media, essential for hydrogen subsurface storage, are still uncertain. Given the critical temperature and pressure of hydrogen (−239.97 °C, 1.297 MPa), hydrogen will be stored in the gaseous phase, and the ideal gas law can be used to describe its low-pressure behaviour, although uncertainties arise at higher pressures, requiring more complex equations of state to accurately describe the fluid properties. Hydrogen-rich gas does not form gas hydrates as their formation requires pressures and temperatures beyond the conditions of geological storage.30 The density of hydrogen increases with increasing pressure, leading to increased hydrogen storage efficiency with depth (Fig. 4). The low density of hydrogen compared to the formation brines leads to buoyancy contributing to the formation of a hydrogen cap directly below the caprock.31 The viscosity of hydrogen is low in comparison with CH4 and CO2 and exhibits minimal variation with pressure and temperature within the range of typical subsurface storage conditions (T < 150 °C; P < 50 MPa). Commonly applied viscosity models designed for reservoir engineering software may be applicable to most non-polar gases but alternative equations are often required for hydrogen, as very early pointed out by Stiel & Thodos in 1961.32 Hydrogen also has a relatively high thermal conductivity which increases with both increasing pressure and temperature so that under deep storage conditions (e.g. at about 2 km depth and 65 °C and 20 MPa), hydrogen is almost three times more heat conductive than CH4 and CO2. In common with other gasses, hydrogen solubility in water increases with increasing pressure, and decreases with increasing temperature and increasing salinity. However, the non-polar nature of hydrogen limits its solubility in water, with a hydrogen solubility in pure water of approximately 0.14 mol l−1 (at 65 °C and 20 MPa),33 similar to that of the solubility of CH4 and one order of magnitude lower than CO2, thus causing no significant pH change. This means that negligible losses of hydrogen due to dissolution can be expected.24The uncertainties of hydrogen flow in brine-filled porous media derive from its low viscosity and high diffusivity. Its low viscosity leads to high mobility, which may enable faster filling or draining of the reservoir, but also makes it a less favourable agent for displacing other in situ fluids, especially brine. This increases the risk of viscous fingering, which could result in pockets of unrecoverable hydrogen due to uncontrolled lateral spreading.34 Despite the high diffusivity of hydrogen due to its small molecular size,35 diffusion-driven hydrogen losses from a storage site are estimated to be on the order of 0.1–1% during the lifetime of a storage site.24,36
Interphase diffusion and advection, into other fluids present in the reservoir, such as residual hydrocarbon gas or an alternative cushion gas, will result in mixing of the injected hydrogen,36 leading to contamination of the stored hydrogen. The degree of mixing of the gases will depend on the cycling rate, the injection and reproduction rates, the reservoir properties and the used cushion gas. Limited experimental data is currently available for multicomponent hydrogen-rich fluids in the published literature (e.g.ref. 33) for validation and tuning of existing thermodynamic models. The GERG-2008 EoS37 is proven to have high accuracy for hydrogen when mixed with natural gas components within its tuned range (i.e. benchmarked against experimental results). However, in the presence of a water-rich aqueous phase the model requires further improvement for accurate results.37 The presence of impurities could also lead to challenging engineering and operating issues such as toxicity, safety, and compression or dehydration requirements, as the thermo-physical properties of a hydrogen-rich stream may differ significantly from a pure hydrogen stream.
Modelling the flow of hydrogen requires an understanding of how hydrogen influences the dynamic interaction between the rock and fluid properties in the reservoir. Of particular importance are relative permeability and capillary pressure, and hence the residual hydrogen saturation in water-wet porous media, which are directly related to the phases present within the formation.38 The determination of residual hydrogen saturation is of particular importance, as it controls the irrecoverable portion of the stored gas, impacting the economic feasibility of the operation. In turn, the capillary forces controlling residual trapping also control the imbibition and drainage behaviour of the rock, and hence the relative permeability. It should be noted that the relative permeability may change over time, as a result of the multiple cycles of hydrogen injection and reproduction, as seen in CO2 flow experiments.39 There are very few relative permeability and capillary pressure measurements for the hydrogen-brine system. Experimental measurements in Triassic sandstone showed that relative permeability and capillary pressures vary little between 5.5–10 MPa and 20–45 °C, suggesting that capillary pressure is almost constant in the hydrogen–water system under these conditions.40 However, additional data taken under varying conditions and in different formations, including multi-phase flow properties of hydrogen-gas mixtures, are vital to make accurate predictions of the hydrogen plume development and hence to define optimum production strategies.
The low permeability of caprocks, and their associated high interfacial forces, may in theory prevent upward migration of hydrogen, but there is no experimental data on hydrogen breakthrough pressures in shales or other potential caprocks. The buoyancy forces of hydrogen will be approximately three times greater than those generated by CO2, for a reference depth of 1000 m. Hence, even hydrogen columns of relatively moderate height could lead to very high buoyancy pressures.
It has been demonstrated that fluids like CO2 can change rock wettability, particularly in micas, and that pressure and temperature have different effects on wettability for CO2 and CH4.41,42 However, very little is known about the influence of hydrogen on wettability. As wettability behaviour is crucial for hydrogen retention, more research is needed to identify if hydrogen influences rock wettability and what could be the potential impact of cyclic injection and extraction on wettability, as observed during CO2 storage.39
3. Hydrogen-brine-rock geochemical reactions
Hydrogen injected into a porous reservoir will change the chemical equilibrium between the formation pore water, dissolved gases and the rock matrix. Resulting geochemical reactions could lead to: (i) significant loss of hydrogen; (ii) contamination of the stored hydrogen by the production of other gases (e.g. H2S); (iii) mineral dissolution/precipitation leading to enhanced or reduced injectivity; (iv) mineral dissolution leading to opening of migration pathways through the caprock; and (v) mineral dissolution impacting the mechanical properties of the reservoir and the caprock. Any of these reactions can compromise secure and efficient UHSP, although their associated impact is still poorly constrained. Dissolved hydrogen does not directly affect the pore water pH. However, it may react with chemical components initially present in the pore water, such as dissolved sulphate, indirectly impacting fluid pH, thereby driving mineral dissolution/precipitation reactions.43 In common with standard diagenetic reactions, any geochemical reactions will occur via the aqueous phase, which is likely to be ubiquitous even very close to the wellbore. Note that abiotic geochemical reactions could be difficult to distinguish from biotic reactions (see Section 4).The types of reactions expected to occur during subsurface storage are hydrogen-driven redox reactions with iron-bearing minerals such as hematite, goethite, or with Fe3+-bearing clays and micas. Such reactions could change the mechanical strength of the rock matrix if hematite-containing cements or clay cutans at grain–grain contacts in sandstone reservoirs are reduced. The dissolution of minerals within the caprock could create new leakage pathways and hence induce the loss of containment, though research shows that such reactions are likely to be limited in their extent.44
In addition to redox reactions, reactions of hydrogen with dissolved sulphur species or sulphur-bearing minerals (e.g. pyrite) are expected to occur.45 Besides the direct impact of mineral dissolution on porosity, permeability and mechanical properties, these reactions lead to the formation of hydrogen sulphide (H2S), decreasing the quality of the stored hydrogen gas. Additionally, H2S can modify the redox potential and the pH of pore waters,46 triggering further fluid-rock reactions. H2S can also compromise the infrastructure due to its corrosive, flammable and toxic nature.47 In the case of town gas storage in Beynes (France),48 it has been argued that abiotic pyrite reduction resulted in H2S production, however, it should be noted that H2S generation may be inhibited by the presence of carbonate minerals within the reservoir.45,49 As the hydrocarbon industry has decades of experience of safely producing H2S-rich natural gas,50,51 this would be a surmountable, though costly, side-effect of hydrogen storage.
Experimental studies on reservoir sandstones under subsurface conditions (T = 40–100 °C, P = 10–20 MPa) show dissolution of carbonate and sulphate cements, leading to an increase in porosity during hydrogen exposure.52 Similar experiments on reservoir and caprock material of a natural gas storage site show an overall decrease in permeability in both rock types, due to the alteration of clay minerals.53 However, in both studies framework minerals, such as quartz and feldspar, appeared to be unaffected by hydrogen exposure. Some potential hydrogen storage reservoirs in Europe are located in Permian and Triassic sandstones,54 or Carboniferous carbonate formations.22 Therefore, the dissolution of carbonate and sulphate minerals are of importance, as it may lead to mechanical weakening of the reservoir rock or carbonate/sulphate-cemented faults in the caprock, depending on the distribution of these cements and the local fluid to rock ratio.55
Though geochemical processes may have a significant impact on the technical and economic aspects of hydrogen storage, their extents and reaction rates under subsurface conditions are associated with uncertainties. This is highlighted by the fact that there is yet no consensus on the significance26,45,56 or insignificance25,40,57 of geochemical reactions on storage operations. Understanding of both the possible extent and rate of reactions is thus crucial, and experiments determining reaction rates at conditions typical of subsurface hydrogen storage58,59 are needed.
To predict the impact of chemical reactions over the lifetime of a hydrogen storage site, geochemical modelling is needed. Note that equilibrium geochemical modelling often does not account for reaction rates and can hence overestimate the extent of reactions. Even reactive transport modelling relies upon rate constants that are derived from lab experiments, and these are known to overestimate in situ reaction rate by orders of magnitude.60 To quantify the extent of reactions in the reservoir and caprock, and to assess the probability and magnitude of the expected processes, the development of a geochemical database, analogous to those made for CO2 storage,61 containing the reactions of hydrogen with dissolved ions and mineral surfaces including their kinetics, as well as possible catalysis is crucial. In addition, complementary flow-through experiments at realistic in situ conditions, using site-specific rock from potential storage sites, as well as studies from natural hydrogen fields,62 are required to be benchmarked against reactive transport models.
4. Microbial growth in the reservoir
Microbial growth is known to be important in hydrocarbon reservoirs63 and it is also considered to be of importance for the feasibility of hydrogen storage.64 Although several studies have looked at hydrogen utilization under natural concentrations,65–67 little is known about the effects that high hydrogen pressures expected in UHSP will have on the subsurface microbial system. A limited number of studies indicate that once microorganisms are exposed to excess hydrogen, hydrogen turnover will not further increase with increasing hydrogen pressure,68,69 indicating that hydrogen turnover rates determined at excess hydrogen and standard conditions may be representative and applicable to estimate subsurface hydrogen consumption rates, providing realistic nutrient, temperature, pressure, and salinity regimes are applied. A number of classes of microorganisms, including methanogens, sulphate-reducers, homoacetogenic bacteria and iron(III)-reducers are considered as major hydrogen consumers and are frequently present in subsurface formations.70 The potential impact of the microorganisms is controlled by parameters such as temperature, salt concentration, pH-value, and substrate supply, with optimal and critical values for these parameters for each class of microorganism summarised in Table 1. However, the microbial community composition is a major uncertainty due to the non-culturability of many subsurface microorganisms71,72 and the risk of accidental introduction of allochthonous organisms from the surface, or of surface gas and/or drilling fluid during the storage operation. Other uncertainties include the bacterial nutrient demand in mixed cultures and the nutrient supply in the subsurface, and the effect of pressure on the microbial metabolism, including the toxicity of high hydrogen pressures to some microorganisms.73,74 Addressing those questions is crucial for delineating the potential hydrogen loss from storage sites by biodegradation.| Class of microorganism | Main storage impact | Hydrogen consumption (nM hour−1) | Temperature (°C) | Salinity (g L−1) | pH |
|---|---|---|---|---|---|
| a Data determined at varying hydrogen exposure concentration, substrate concentration, temperature and organic matter availability. b Data compiled and taken from ref. 91. | |||||
| Methanogens | H2 loss by CH4 production, clogging | Laboratoriala:92–95 0.008–5.8 × 105 | Optimumb: 30–40 | Optimumb: <60 | Optimumb: 6.0–7.5 |
| Oil and gas fields:96,97 0–1185 | Criticalb: 122 | Criticalb: 200 | Criticalb: 4.5–9 | ||
| Wells:97 up to 4533 | |||||
| Sulfate reducers | H2 loss by H2S production, corrosion, clogging | Laboratoriala:68,92,98,99 0.005–130 × 105 | Optimumb: 20–30 | Optimumb: <100 | Optimumb: 6.0–7.5 |
| Oil and gas fields:96,97 0.05–351 | Criticalb: 113 | Criticalb: 240 | Criticalb: 0.8–11.5 | ||
| Wells:97 up to 2544 | |||||
| Homoacetogens | H2 loss by CH3COOH production, clogging | Laboratoriala:68,98,100,101 0.2–5.0 × 105 | Optimumb: 20–30 | Optimumb: <40 | Optimumb: 6.0–7.5 |
| Criticalb: 72 | Criticalb: 300 | Criticalb: 3.6–10.7 | |||
| Iron(III) reducing bacteria | H2 loss by Fe(II) | Laboratoriala:68,102–106 0.005–2.2 × 105 | Optimum: 0–30 | Optimum: <40 | Optimum: 6–7.5 |
| Production, clogging | Critical: 90 | Critical: 200 | Critical: 1.6–>9 | ||
The main impact of microbes on hydrogen storage is the permanent loss of hydrogen due to the conversion of hydrogen into products like CH4 or H2S. Experience from storage operations of hydrogen-rich town gas, demonstrate ranges from no detected hydrogen consumption in Beynes (France),27 up to a 17% decrease in hydrogen, with a concurrent decrease of CO2 and an increase of CH4, over a seven month cycle in Lobodice, Czech Republic.75,76 The latter was likely caused by the presence of methanogens leading to microbial reactions causing CH4 generation.77 Microbial hydrogen consumption was also reported during combined storage of natural gas with additions of hydrogen and CO2 (e.g. Underground Sun.Storage and Sun.Conversion projects, Austria; HyChico project, Argentina78–80). In the Underground Sun.Storage project, a significant shift in the microbial consortium was identified and it was concluded that 3% of the injected hydrogen was converted to CH4 by methanogens.78 Although CH4 produced by the methanogens comes as an improvement to the calorific value of the stored gas, when coupled to a deterioration of the greenhouse balance, this loss of hydrogen has to be considered as a risk for hydrogen storage.81 Furthermore, the biotic generation of H2S82 has the same consequences as abiotically generated H2S.
As microbial population density increases, microbially formed biofilms or mineral precipitation could lead to pore-clogging, and therefore to a reduction of hydrogen injectivity. Loss of injectivity, or a reduction in flow rates, due to biological activity is a common problem encountered in geothermal applications83 and CO2 storage operations.84 Experiments on microbial enhanced oil recovery recorded an overall decrease of the absolute permeability by a factor of 0.56 up to 0.86 accompanied by an increasing microbial density.85 First modelling approaches of pore-clogging effects in the near well-bore area during hydrogen injection provide evidence that lateral gas flow near the wellbore improves, while vertical flow rates decrease.86 Microbial models are strongly dependent on the kinetic parameters of the specific microorganisms, which are uncertain, and simulations require detailed confirmation based on experimental research. Field data from the Sun.Conversion and the HyChico projects did not show indications of pore-clogging effects after one storage operation cycle. Overall, pore-clogging due to microbes has hardly been investigated in detail and further study is required to assess the probability and severity of the process during long-term operation of hydrogen storage.
5. Geomechanical considerations for storage integrity
Cyclical hydrogen injection and reproduction leads to (i) cyclical pressure changes on intact and fault rock behaviour, (ii) short-and long-term chemical interaction of hydrogen on intact rock and faults, and (iii) stress–strain-sorption on mechanical and transport behaviour, all of which can have crucial impact on the storage integrity.The injection of cold, pressurised hydrogen directly leads to chemical, pressure, and temperature changes in the reservoir, nearby faults and near the injection well. The near well-bore area will experience smaller temperature fluctuations,87 in comparison with CO2 storage, where Joule–Thomson cooling and the concomitant cooling of the near-wellbore area poses a serious challenge to storage integrity.88–90
The introduction of hydrogen into the subsurface will lead to pressure, and thus stress changes beyond the extent of the hydrogen and cushion gas plume, meaning that deformation may occur beyond the area of pressure change.107 Furthermore, hydrogen storage complexes will experience cyclical pore pressure changes, resulting from injection-reproduction cycles. In turn, this will lead to cyclical changes in the effective state of stress in the storage complex. Cyclic stress fluctuations in the vicinity of the wellbore, within the reservoir, and nearby faults, might cause reservoir compaction, leading to porosity reduction and reduced fluid flow,108,109 subsidence110–112 and/or fault reactivation,113,114 with or without (micro)seismicity. Furthermore, compaction of the reservoir may lead to caprock flexure,115 giving rise to the creation of fractures and hence leakage pathways within the caprock.
Since the rate of reservoir deformation is controlled by the rate of stress change, it is the rate of pore pressure cycling, i.e. the time of the hydrogen injection-reproduction cycle, which will control the deformation rate. Similarly, the cycling rate controls the normal stress on faults, and hence the rate of slip and fault behaviour. However, little is known about the response of reservoirs and/or faults to cyclic stresses,116–118 especially under relevant hydrogen storage in situ stress–pressure–temperature-chemical conditions. At the same time, interactions between hydrogen and minerals in the reservoir, caprock and pre-existing faults can affect the mechanical response of the system (Fig. 3).
Dissolution–precipitation reactions (Section 3), can lead to removal of load-bearing minerals and cements. Weakening of the load-bearing framework of a reservoir may result in increased elastic and inelastic (permanent) deformation, potentially enhanced by injection–reproduction-induced stress changes.117,119 However, the change in chemical environment will also drive other fluid-assisted, grain–scale processes that could lead to permanent deformation.118,120 Such processes include local grain–contact cement dissolution, clay mineral sorption/desorption within grain boundaries, fluid-assisted slow crack growth (stress corrosion cracking), dissolution–precipitation (disequilibrium or stress-induced) and/or intergranular frictional slip.121 These processes are not only driven by the rate of stress change, but are often also time-dependent, potentially giving rise to time-dependent (creep) deformation of the reservoir even during periods of no pore pressure change. When these processes occur within faults, it may affect their stability and frictional behaviour, thereby potentially affecting the economic and regulatory viability of the hydrogen storage complex. Although the above-mentioned grain–scale mechanisms are well studied, little is known about the influence of hydrogen on their rates. Fluid-assisted processes, impacted by fluid composition, such as mass transfer122–125 and/or slow (time-dependent) growth of subcritically stressed cracks in grains,126,127 can lead to creep deformation of the reservoir and/or any faults.
Furthermore, sorption of hydrogen to (swelling) clay minerals in clay-bearing reservoirs, caprock and faults can lead to associated swelling-induced stress changes. Though the hydrogen sorption capacity of typical swelling clays (montmorillonite, laponite)57,128–130 is two to four times less than for fluids like CO2,131 the associated stress–strain–sorption behaviour may still pose an issue for the mechanical and transport behaviour of the storage complex. It should also be noted that the swelling potential of clays is strongly influenced by the water activity of the fluid and the clays.132,133 Clay swelling is directly correlated with the water content of the clay minerals, with no swelling observed for fully dry, nor fully saturated clays.132,133 Similarly, processes like dissolution–precipitation and crack growth are assisted by the presence of water. During the lifetime of a hydrogen storage complex, repetitive injection cycles of dry hydrogen could lead to the pervasive drying out of the reservoir, particularly in the case of depleted hydrocarbon reservoirs, containing mainly residual water. Therefore, over time, the relative contribution of the various processes to the mechanical behaviour and integrity will change. On the one hand, potentially unfavourable chemical reactions may stop over time. On the other hand, drying and shrinkage of clays may reverse swelling-induced sealing of fractures and lead to the re-opening of leakage pathways.55,134
From studies into the effects of prolonged hydrocarbon production, we already know that even small amounts of compaction at the reservoir-level121 can lead to significant impacts at the surface, in terms of surface subsidence and induced seismicity.114 Therefore, it is crucial to investigate and quantify the effect hydrogen has on the rates of such grain–scale deformation mechanisms, so that the impact of prolonged seasonal hydrogen storage on the reservoir behaviour can be quantified. Furthermore, adsorption and desorption of hydrogen to swelling clays within grain contacts, and the concomitant swelling, may lead to small normal and shear strains, and hence stresses, between individual grains.133 Over the lifetime of a hydrogen storage complex, this could lead to mechanical fatigue of the reservoir and increase permanent deformation. Clay swelling can lead to fracture closure, though swelling-induced critical stressing of faults may lead to slip,135 potentially enhanced by any lubrication effect of hydrogen,136 which could result in induced seismicity and the creation of leakage pathways.116 Therefore, sorption processes not only impact retrievability, but also long-term stability and safety of the store.
6. Ensuring safe and effective storage
The unwanted loss of gas during storage operation is a concern from an economic, safety and environmental perspective of any gas storage operation. To minimise this risk during hydrogen storage, storage sites must be carefully selected and evaluated for their storage integrity, and the storage operations have to be accompanied by monitoring and verification systems.While hydrogen has been safely produced, stored, transported and utilised in industrial applications for decades, extensive experimental work in containment and failure processes and risks known from other gas storage operations137 is required to provide precise inputs for quantitative hydrogen storage risk assessments. The safety implications of hydrogen are different than those from other fuels, though not necessarily more dangerous.138 Within the gas supply network, a number of projects (e.g. H21 Spadeadam and HyHouse139) have shown that hydrogen does not carry increased inherent safety risks when compared to natural gas or liquified petroleum gas. As pure hydrogen is non-toxic, non-poisonous, non-corrosive, and environmentally benign, the environmental risks associated with leakage are limited compared to leakage of CH4 or CO2. If loss of hydrogen were to occur during storage, recoverable gas could be produced and the storage site could be abandoned.
To ensure rapid detection of the loss of containment from the storage site, it is imperative for UHSP operations to have a measurement, monitoring and verification (MMV) system in place.140 The monitoring system covers various aspects of the underground storage process (i) to guaranteed safe controllable hydrogen injection–reproduction operations, (ii) surveillance of hydrogen migration in the subsurface, (iii) control of brine displacement due to pore pressure evolution, (iv) identification of possible leakage pathways, and (v) validation of long-term safety of the storage site. Surveillance of a hydrogen porous media storage site will be built on proven multi-disciplinary monitoring concepts, as applied in other fluid storage experiences such as UGS and UCS, consisting of geophysical, geochemical and microbiological surveillance techniques.141,142 These techniques allow for the direct (i.e. at reservoir level) and indirect (i.e. from surface) detection of underground fluids at different scales. Direct methods, including downhole observation tools such as well logging, probe-sampling, and permanent sensor instrumentations in wellbores, have seen an increasing demand and technological improvements in the past years (e.g. fibre optic pressure and temperature monitoring, distributed acoustic sensing143).
Indirect methods, such as geophysical methods, provide reservoir-scale detection of the plume within the reservoir. However, most indirect monitoring tools employed in UGS (e.g. seismic, gravity or electromagnetic methods) will likely struggle to detect and quantify the hydrogen plume at sufficient resolution.144 Therefore, existing monitoring protocols will need to be tested and verified for the specific properties of hydrogen, such as high mobility and low density.
Site selection criteria are crucial to ensure safe and efficient storage, though for hydrogen storage there are no accepted selection procedures established. While these can be inspired, at least to a certain degree, by those for UCS or UGS sites,145–149 new optimal storage site criteria must be established, taking into account the underlying fundamental processes unique to hydrogen as discussed in Sections 2–5. Given the current uncertainties of UHSP operations, geological sites with different size, shape and depth to these employed in UCS or UGS might be used. This could in turn make current knowledge of the subsurface based on oil and gas reservoirs or CO2 storage atlases redundant, increasing the site selection costs accordingly.
Of particular importance is the investigation of favourable trap architectures to keep the highly mobile hydrogen in place and allow effective reproduction, such as steeply dipping anticlines.150 Reservoir heterogeneity could limit hydrogen flow, or may favour viscous fingering and hence the potential loss of hydrogen.36 Additionally, it could enhance mixing with in situ gas or alternative cushion gas. Though operational guidelines are rare, there is consensus, that lateral hydrogen spreading should be minimised, with lower injection rates leading to more stable in situ brine displacement151 but quantitative guidelines do not exist. Operational challenges, such as coning, the unwanted rise of the fluid interface, which ultimately leads to water production and a reduction of the gas pressure, can be inhibited by reducing the hydrogen production rate.152 No studies on the relationship of reservoir geology and coning during hydrogen injection–reproduction cycles, or on optimisation strategies beyond the reduction of the production rate to remediate coning have been performed.
As discussed in Section 2, hydrogen cushion gas is an initial expenditure required to provide the desired gas deliverability. The required amount of cushion gas relative to the working gas is unknown for hydrogen storage and presumably highly site- and project specific, and numbers from natural gas storage are provided as a rule of thumb, which range from 40–70%.145 Working gas and cushion gas are usually of similar composition in gas storage operations. The use of alternative cushion gas, i.e. non-hydrogen gas, to reduce storage costs (CH4, N2) or to reduce greenhouse gas emissions (CO2), has been discussed for several gas storage applications and has successfully been conducted for natural gas storage.153–156 In addition to cost-reduction, all considered alternative cushion gases can help to reduce the sharp density contrast between hydrogen and the formation water.36 Therefore, to assess the trade-off between additional cost and hydrogen contamination, future studies could focus on reducing the working to cushion gas ratio, as well as employing alternative cushion gasses.
Finally, storage security will be determined by the quality of caprock, i.e. its composition and permeability. Workflows and analytical procedures have to be developed to characterise and assess the suitability of caprocks to retain hydrogen. Databases on hydrogen reactivity and reaction kinetics of hydrogen-mineral reactions should be applied for screening subsurface lithologies, highlighting which formations contain reactive minerals that could compromise safe seasonal UHSP over the lifetime of a storage operation (i.e. the aspects discussed in Sections 2 and 3). Similarly, chemical ranges suitable for the growth of hydrogen consuming microbes could be used to eliminate potential storage candidate formations (see Section 4). As for hydrogen storage depth constraints, a minimum depth due to a distinct fluid density increase, as seen in CO2 storage, does not exist. Instead, a range of parameters including the exploitation of hydrogen density to maximise reservoir capacity and abiotic/biotic reactions leading to the loss and contamination of hydrogen will determine suitable depth.
7. Summary
This perspective paper highlights a range of scientific issues that need to be addressed in order to enable large-scale underground hydrogen storage in porous media as a driver of the energy transition. They include the fluid flow behaviour of hydrogen in subsurface reservoirs, geochemical reactions caused by the introduction of hydrogen, biotic reactions enabled by the presence of excess hydrogen, and the geomechanical response of the subsurface to hydrogen storage. The risks posed by these processes could have severe economical and safety consequences on the storage operation. The discussed processes and their coupled influences provide the fundamental basis for reservoir-scale models to accurately assess and predict the impact of seasonal hydrogen storage. These predictions can lead the way to informed decision making with regards to operational strategies to ensure safe and efficient implementation of UHSP.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was stimulated by the GEO*8 Workshop on “Hydrogen Storage in Porous Media”, November 2019 at the GFZ in Potsdam (Germany). N. H., A. H., E. T., K. E., M. W. and S. H. are funded by the Engineering and Physical Sciences Research Council (EPSRC) funded research project “HyStorPor” (grant number EP/S027815/1). J. A. is funded by the Spanish MICINN (Juan de la Cierva fellowship-IJC2018-036074-I). J. M. is co-funded by EU INTERREG V project RES-TMO (Ref: 4726/6.3). C. O. H. acknowledges funding by the Federal Ministry of Education and Research (BMBF, Germany) in the context of project H2_ReacT (03G0870C).Notes and references
- O. Y. Edelenbosch, D. L. McCollum, D. P. van Vuuren, C. Bertram, S. Carrara, H. Daly, S. Fujimori, A. Kitous, P. Kyle, E. Ó. Broin, P. Karkatsoulis and F. Sano, Transp. Res. Part D Transp. Environ., 2017, 55, 281–293 CrossRef.
- S. Lazarou, V. Vita, M. Diamantaki, D. Karanikolou-Karra, G. Fragoyiannis, S. Makridis and L. Ekonomou, Energy Sci. Eng., 2018, 6, 116–125 CrossRef.
- E. S. Hanley, J. P. Deane and B. P. Ó. Gallachóir, Renewable Sustainable Energy Rev., 2018, 82, 3027–3045 CrossRef.
- M. McPherson, N. Johnson and M. Strubegger, Appl. Energy, 2018, 216, 649–661 CrossRef CAS.
- DNV GL, Heading for Hydrogen, 2020.
- UNIDO, in On the sidelines of the 24th Session of the Conference of the Parties to the United Nations Framework Convention on Climate Change (UNFCCC) – COP24, 2018, p. 12.
- Intergovernmental Panel on Climate Change, Geneva, Switz.
- D. Heide, L. von Bremen, M. Greiner, C. Hoffmann, M. Speckmann and S. Bofinger, Renewable Energy, 2010, 35, 2483–2489 CrossRef.
- K. Engeland, M. Borga, J.-D. Creutin, B. François, M.-H. Ramos and J.-P. Vidal, Renewable Sustainable Energy Rev., 2017, 79, 600–617 CrossRef.
- P. Pinel, C. A. Cruickshank, I. Beausoleil-Morrison and A. Wills, Renewable Sustainable Energy Rev., 2011, 15, 3341–3359 CrossRef.
- P. Denholm and T. Mai, Renewable Energy, 2019, 130, 388–399 CrossRef.
- F. Dawood, M. Anda and G. M. Shafiullah, Int. J. Hydrogen Energy, 2020, 45, 3847–3869 CrossRef CAS.
- The German Federal Ministry for Economic Affairs and Energy, The National Hydrogen Strategy (in German), 2020.
- ARENA (Australian Renewable Energy Agency), Renewable Hydrogen Deployment Funding Round, https://arena.gov.au/renewable-energy/hydrogen/.
- Fuel Cells and Hydrogen Joint Undertaking, FCH2 JU call for proposals 2020, https://www.fch.europa.eu/page/call-2020.
- R. Sedlacek, W. Rott, W. Rokosz, S. Khan, G. H. Joffre, P. Ten Eyck, C. Odevall, H. Noteboom, R. Ivanov, V. Kunev, G. Radu, C. Simeoni, A. Chachine and Y. Guerrini, United nations economic commission for europe, 2013, vol. 21, p. 136 Search PubMed.
- European Commission, The role of gas storage in internal market and in ensuring security of supply, 2015.
- M. Panfilov, Compend. Hydrogen Energy, 2016, 91–115 CAS.
- U. Bünger, J. Michalski, F. Crotogino and O. Kruck, Compendium of Hydrogen Energy, Elsevier, 2016, pp. 133–163 Search PubMed.
- IEA, The Future of Hydrogen, OECD, Paris, 2019 Search PubMed.
- D. Zivar, S. Kumar and J. Foroozesh, Int. J. Hydrogen Energy, 2020 DOI:10.1016/j.ijhydene.2020.08.138.
- N. Heinemann, M. G. Booth, R. S. Haszeldine, M. Wilkinson, J. Scafidi and K. Edlmann, Int. J. Hydrogen Energy, 2018, 43, 20861–20874 CrossRef CAS.
- P. Hoffmann, The forever fuel: The story of hydrogen, 2019 Search PubMed.
- P. O. Carden and L. Paterson, Int. J. Hydrogen Energy, 1979, 4, 559–569 CrossRef.
- S. Foh, M. Novil, E. Rockar and P. Randolph, Inst. Gas Tech., DOE, Brookhaven Natl Lab, Upton, NY, 1979, p. 145 Search PubMed.
- O. Kruck and F. Crotogino, Assessment of the potential, the actors and relevant business cases for large scale and seasonal storage of renewable electricity by hydrogen underground storage in Europe – Deliverable 3.3 Benchmarking of options, 2013.
- D. Stolten and B. Emonts, Hydrogen Science and Engineering: Materials, Processes, Systems and Technology, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016 Search PubMed.
- K. Sordakis, C. Tang, L. K. Vogt, H. Junge, P. J. Dyson, M. Beller and G. Laurenczy, Chem. Rev., 2018, 118, 372–433 CrossRef CAS.
- A. Lanz, J. Heffel and C. Messer, Hydrogen Fuel Cell Engines and Related Technologies, 2001 Search PubMed.
- W. L. Vos, L. W. Finger, R. J. Hemley and H. Mao, Phys. Rev. Lett., 1993, 71, 3150–3153 CrossRef CAS.
- M. Ruith and E. Meiburg, J. Fluid Mech., 2000, 420, 225–257 CrossRef CAS.
- L. I. Stiel and G. Thodos, AIChE J., 1961, 7, 611–615 CrossRef CAS.
- A. Hassanpouryouzband, E. Joonaki, K. Edlmann, N. Heinemann and J. Yang, Sci. Data, 2020, 7, 222 CrossRef CAS.
- L. Paterson, Int. J. Hydrogen Energy, 1983, 8, 53–59 CrossRef CAS.
- J. Völkl and G. Alefeld, in Hydrogen in metals I, ed. J. Völkl and G. Alefeld, Springer Berlin Heidelberg, Berlin, 1978, vol. 28, pp. 321–348 Search PubMed.
- F. Feldmann, B. Hagemann, L. Ganzer and M. Panfilov, Environ. Earth Sci., 2016, 75, 1165 CrossRef.
- O. Kunz and W. Wagner, J. Chem. Eng. Data, 2012, 57, 3032–3091 CrossRef CAS.
- M. J. Blunt, Multiphase Flow in Permeable Media, Cambridge University Press, 2017 Search PubMed.
- K. Edlmann, S. Hinchliffe, N. Heinemann, G. Johnson, J. Ennis-King and C. I. McDermott, Int. J. Greenhouse Gas Control, 2019, 80, 1–9 CrossRef CAS.
- A. E. Yekta, M. Pichavant and P. Audigane, Appl. Geochem., 2018, 95, 182–194 CrossRef CAS.
- D. N. Espinoza and J. C. Santamarina, Water Resour. Res., 2010, 46, 1–10 CrossRef.
- B. Pan, F. Jones, Z. Huang, Y. Yang, Y. Li, S. H. Hejazi and S. Iglauer, Energy Fuels, 2019, 33, 788–795 CrossRef CAS.
- A. Lassin, M. Dymitrowska and M. Azaroual, Phys. Chem. Earth, Parts A/B/C, 2011, 36, 1721–1728 CrossRef.
- N. Kampman, A. Busch, P. Bertier, J. Snippe, S. Hangx, V. Pipich, Z. Di, G. Rother, J. F. Harrington, J. P. Evans, A. Maskell, H. J. Chapman and M. J. Bickle, Nat. Commun., 2016, 7, 12268 CrossRef CAS.
- V. Reitenbach, L. Ganzer, D. Albrecht and B. Hagemann, Environ. Earth Sci., 2015, 73, 6927–6937 CrossRef CAS.
- L. Truche, M.-C. Jodin-Caumon, C. Lerouge, G. Berger, R. Mosser-Ruck, E. Giffaut and N. Michau, Chem. Geol., 2013, 351, 217–228 CrossRef CAS.
- L. Wei, X. Pang and K. Gao, Corros. Sci., 2016, 111, 637–648 CrossRef CAS.
- J. P. Bourgeois, N. Aupaix, R. Bloise and J. L. Millet, Rev. l’Institut Français du Pétrole, 1979, 34, 371–386 CrossRef CAS.
- C. Gaucher, A. N. Sial, D. Poiré, L. Gómez Peral, V. P. Ferreira and M. M. Pimentel, in Neoproterozoic-Cambrian Tectonics, Global Change And Evolution: A Focus On South Western Gondwana, ed. C. Gaucher, N. S. Sial, H. E. Frimmel and G. P. Halverson, Elsevier, 2009, pp. 115–122 Search PubMed.
- P. Boschee, Oil Gas Facil., 2014, 3, 21–25 CrossRef.
- X. Bihua, Y. Bin and W. Yongqing, Appl. Geochem., 2018, 96, 155–163 CrossRef CAS.
- S. Flesch, D. Pudlo, D. Albrecht, A. Jacob and F. Enzmann, Int. J. Hydrogen Energy, 2018, 43, 20822–20835 CrossRef CAS.
- Z. Shi, K. Jessen and T. T. Tsotsis, Int. J. Hydrogen Energy, 2020, 45, 8757–8773 CrossRef CAS.
- J. Juez-Larré, S. van Gessel, R. Dalman, G. Remmelts and R. Groenenberg, First Break, 2019, 37, 57–66 CrossRef.
- S. J. T. Hangx, E. Bakker, P. Bertier, G. Nover and A. Busch, Earth Planet. Sci. Lett., 2015, 428, 230–242 CrossRef CAS.
- F. Crotogino, G.-S. Schneider and D. J. Evans, Proc. Inst. Mech. Eng. Part A J. Power Energy, 2018, 232, 100–114 CrossRef CAS.
- F. Bardelli, C. Mondelli, M. Didier, J. G. Vitillo, D. R. Cavicchia, J.-C. Robinet, L. Leone and L. Charlet, Appl. Geochem., 2014, 49, 168–177 CrossRef CAS.
- N. Thüns, B. M. Krooss, Q. Zhang and H. Stanjek, Int. J. Hydrogen Energy, 2019, 44, 27615–27625 CrossRef.
- L. Truche, G. Berger, C. Destrigneville, A. Pages, D. Guillaume, E. Giffaut and E. Jacquot, Geochim. Cosmochim. Acta, 2009, 73, 4824–4835 CrossRef CAS.
- M. Wilkinson, Clay Miner., 2015, 50, 275–281 CrossRef CAS.
- L. Nghiem, P. Sammon, J. Grabenstetter and H. Ohkuma, in SPE/DOE Symposium on Improved Oil Recovery, Society of Petroleum Engineers, 2004.
- A. Prinzhofer, C. S. Tahara Cissé and A. B. Diallo, Int. J. Hydrogen Energy, 2018, 43, 19315–19326 CrossRef CAS.
- I. M. Head, D. M. Jones and S. R. Larter, Nature, 2003, 426, 344–352 CrossRef CAS.
- S. Gregory, M. Barnett, L. Field and A. Milodowski, Microorganisms, 2019, 7, 53 CrossRef CAS.
- R. Conrad, T. J. Phelps and J. G. Zeikus, Appl. Environ. Microbiol., 1985, 50, 595 LP–601 LP CrossRef.
- K.-U. Hinrichs, J. M. Hayes, W. Bach, A. J. Spivack, L. R. Hmelo, N. G. Holm, C. G. Johnson and S. P. Sylva, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 14684–14689 CrossRef CAS.
- Q. Jin, Geobiology, 2007, 5, 35–48 CAS.
- M. Berta, F. Dethlefsen, M. Ebert, D. Schäfer and A. Dahmke, Environ. Sci. Technol., 2018, 52, 4937–4949 CrossRef CAS.
- D. Schieche, M. V. S. Murty, R. I. Kermode and D. Bhattacharyya, J. Chem. Technol. Biotechnol., 1997, 70, 316–322 CrossRef CAS.
- B. Hagemann, PhD thesis, Clausthal University of Technology, 2017.
- S. J. Payler, J. F. Biddle, B. Sherwood Lollar, M. G. Fox-Powell, T. Edwards, B. T. Ngwenya, S. M. Paling and C. S. Cockell, Front. Microbiol., 2019, 10, 426 CrossRef.
- R. J. Parkes and H. Sass, in Encyclopedia of Microbiology, ed. M. Schaechter, Elsevier, 3rd edn, 2009, pp. 64–79 Search PubMed.
- J. F. Miller, N. N. Shah, C. M. Nelson, J. M. Ludlow and D. S. Clarck, Appl. Environ. Microbiol., 1988, 54, 3039–3342 CrossRef CAS.
- L. C. Stewart, C. K. Algar, C. S. Fortunato, B. I. Larson, J. J. Vallino, J. A. Huber, D. A. Butterfield and J. F. Holden, ISME J., 2019, 13, 1711–1721 CrossRef CAS.
- P. Šmigáň, M. Greksák, J. Kozánková, F. Buzek, V. Onderka and I. Wolf, FEMS Microbiol. Lett., 1990, 73, 221–224 CrossRef.
- C. Gniese, P. Bombach, J. Rakoczy, N. Hoth, M. Schlömann, H.-H. Richnow and M. Krüger, in Geobiotechnology II: Energy Resources, Subsurface Technologies, Organic Pollutants and Mining Legal Principles, ed. A. Schippers, F. Glombitza and W. Sand, Springer Berlin Heidelberg, Berlin, Heidelberg, 2014, pp. 95–121 Search PubMed.
- A. Liebscher, J. Wackerl and M. Streibel, in Hydrogen Science and Engineering: Materials, Processes, Systems and Technology, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016, pp. 629–658 Search PubMed.
- S. Bauer, Underground Sun.Storage: Publizierbarer Endbericht – Projektnummer 840705, 2017 Search PubMed.
- S. Bauer, Underground Sun.Conversion: Erneuerbares Erdgas zur Speicherung von Sonne und Wind, 2018 Search PubMed.
- A. Pérez, S. Dupraz and J. Bolcich, 21st World Hydrogen Energy Conference 2016, Zaragoza, Spain, 2016 Search PubMed.
- G. Strobel, B. Hagemann, T. M. Huppertz and L. Ganzer, Renewable Sustainable Energy Rev., 2020, 123, 109747 CrossRef CAS.
- C. H. Chou and World Health Organization, Hydrogen sulfide: human health aspects, 2003 Search PubMed.
- H. Würdemann, A. Westphal, A. Kleyböcker, R. Miethling-Graff, S. Teitz, M. Kasina, A. Seibt, M. Wolfgramm, F. Eichinger and S. Lerm, Grundwasser, 2016, 21, 93–106 CrossRef.
- M. Zettlitzer, F. Moeller, D. Morozova, P. Lokay and H. Würdemann, Int. J. Greenhouse Gas Control, 2010, 4, 952–959 CrossRef CAS.
- C. Gaol, J. Wegner, L. Ganzer, N. Dopffel, F. Koegler, A. Borovina and H. Alkan, in SPE Europec featured at 81st EAGE Conference and Exhibition, Society of Petroleum Engineers, 2019 Search PubMed.
- M. Panfilov and N. Eddaoui, InterPore 10th Annual Meeting and Jubilee, New Orleans, USA, 2018 Search PubMed.
- M. Klell, Handbook of Hydrogen Storage, 2010, pp. 1–37 Search PubMed.
- S. A. Mathias, J. G. Gluyas, C. M. Oldenburg and C.-F. Tsang, Int. J. Greenhouse Gas Control, 2010, 4, 806–810 CrossRef CAS.
- C. M. Oldenburg, Energy Convers. Manage., 2007, 48, 1808–1815 CrossRef CAS.
- M. Preisig and J. H. Prévost, Int. J. Greenhouse Gas Control, 2011, 5, 1055–1064 CrossRef CAS.
- E. M. Thaysen, S. McMahon, G. Strobel, I. Butler, B. T. Ngwenya, N. Heinemann, M. Wilkinson, A. Hassanpouryouzband, C. I. McDermott and K. Edlmann, EarthArXiv, 2020 DOI:10.31223/X5HC7H.
- J. A. Robinson and J. M. Tiedje, Arch. Microbiol., 1984, 137, 26–32 CrossRef CAS.
- F. Karadagli and B. E. Rittmann, Environ. Sci. Technol., 2005, 39, 4900–4905 CrossRef CAS.
- RAG AUSTRIA AG, Eur. Pat., EP3280807A1, 2016 Search PubMed.
- C. R. Smatlak, J. M. Gossett and S. H. Zinder, Environ. Sci. Technol., 1996, 30, 2850–2858 CrossRef CAS.
- E. A. Bonch-Osmolovskaya, M. L. Miroshnichenko, A. V. Lebedinsky, N. A. Chernyh, T. N. Nazina, V. S. Ivoilov, S. S. Belyaev, E. S. Boulygina, Y. P. Lysov, A. N. Perov, A. D. Mirzabekov, H. Hippe, E. Stackebrandt, S. L’Haridon and C. Jeanthon, Appl. Environ. Microbiol., 2003, 69, 6143–6151 CrossRef CAS.
- A. E. Ivanova, I. A. Borzenkov, A. L. Tarasov, E. I. Milekhina and S. S. Belyaev, Microbiology, 2007, 76, 461–468 CAS.
- L. R. Krumholz, S. H. Harris, S. T. Tay and J. M. Suflita, Appl. Environ. Microbiol., 1999, 65, 2300–2306 CrossRef CAS.
- R. T. van Houten, S. Y. Yun and G. Lettinga, Biotechnol. Bioeng., 1997, 55, 807–814 CrossRef CAS.
- L. H. Lynd and J. G. Zeikus, J. Bacteriol., 1983, 153, 1415–1423 CrossRef CAS.
- J. A. Breznak, J. M. Switzer and H.-J. Seitz, Arch. Microbiol., 1988, 150, 282–288 CrossRef CAS.
- A. I. Slobodkin and J. Wiegel, Extremophiles, 1997, 1, 106–109 CrossRef CAS.
- K. Kashefi and D. R. Lovley, Appl. Environ. Microbiol., 2000, 66, 1050–1056 CrossRef CAS.
- S. Hedrich and A. Schippers, Curr. Issues Mol. Biol., 2021, 25–48 CrossRef.
- H. Huber and K. O. Stetter, Arch. Microbiol., 1989, 151, 479–485 CrossRef CAS.
- D. G. G. McMillan, I. Velasquez, B. L. Nunn, D. R. Goodlett, K. A. Hunter, I. Lamont, S. G. Sander and G. M. Cook, Appl. Environ. Microbiol., 2010, 76, 6955–6961 CrossRef CAS.
- J. Rutqvist, Geotech. Geol. Eng., 2012, 30, 525–551 CrossRef.
- R. M. Ostermeier, SPE Form. Eval., 1995, 10, 79–85 CrossRef.
- J. Dautriat, N. Gland, J. Guelard, A. Dimanov and J. L. Raphanel, Pure Appl. Geophys., 2009, 166, 1037–1061 CrossRef.
- D. Doornhof, T. G. Kristiansen, N. B. Nagel, P. D. Pattillo and C. Sayers, Oilf. Rev., 2006, 18, 50–68 Search PubMed.
- M. Hettema, E. Papamichos and P. Schutjens, Oil Gas Sci. Technol., 2002, 57, 443–458 CrossRef.
- N. B. Nagel, Phys. Chem. Earth, Part A Solid Earth Geod., 2001, 26, 3–14 CrossRef.
- P. Segall, Geology, 1989, 17, 942 CrossRef.
- J. Suckale, Leading Edge, 2010, 29, 310–319 CrossRef.
- S. J. T. Hangx, C. J. Spiers and C. J. Peach, J. Geophys. Res., 2010, 115, B07402 Search PubMed.
- J. E. Elkhoury, A. Niemeijer, E. E. Brodsky and C. Marone, J. Geophys. Res.: Solid Earth, 2011, 116 DOI:10.1029/2010jb007759.
- K. Peng, J. Zhou, Q. Zou and F. Yan, Int. J. Fatigue, 2019, 127, 82–100 CrossRef.
- M. T. W. Schimmel, S. J. T. Hangx and C. J. Spiers, Rock Mech. Rock Eng., 2020, 169 DOI:10.1007/s00603-020-02267-0.
- K. Peng, J. Zhou, Q. Zou and X. Song, Int. J. Fatigue, 2020, 131, 105349 CrossRef.
- S. J. T. Hangx, C. J. Spiers and C. J. Peach, J. Geophys. Res., 2010, 115, B09205 Search PubMed.
- C. J. Spiers, S. J. T. Hangx and A. R. Niemeijer, Netherlands J. Geosci., 2017, 96, s55–s69 CrossRef.
- F. K. Lehner, in Deformation processes in minerals ceramics and rocks, ed. D. J. Barber and P. G. Meredith, Unwin Hyman, 1990, pp. 296–333 Search PubMed.
- F. K. Lehner, Tectonophysics, 1995, 245, 153–170 CrossRef.
- E. H. Rutter, Philos. Trans. R. Soc., A, 1976, 283, 203–219 CrossRef.
- C. J. Spiers, S. De Meer, A. R. Niemeijer and X. Zhang, in Physicochemistry of water in geological and biological systems, ed. S. Nakashima, C. J. Spiers, L. Mercury, P. A. Fenter and J. M. F. Hochella, Universal Academy Press, Inc., Tokyo, Japan, 44th edn, 2004, pp. 129–158 Search PubMed.
- B. K. Atkinson and P. G. Meredith, Tectonophysics, 1981, 77, T1–T11 CrossRef CAS.
- N. Brantut, M. J. Heap and P. G. Meredith, J. Struct. Geol., 2013, 52, 17–43 CrossRef.
- M. Didier, L. Leone, J.-M. Greneche, E. Giffaut and L. Charlet, Environ. Sci. Technol., 2012, 46, 3574–3579 CrossRef CAS.
- J. Edge, PhD thesis, University College London, 2015.
- C. Mondelli, F. Bardelli, J. G. Vitillo, M. Didier, J. Brendle, D. R. Cavicchia, J.-C. Robinet and L. Charlet, Int. J. Hydrogen Energy, 2015, 40, 2698–2709 CrossRef CAS.
- A. Busch, S. Alles, Y. Gensterblum, D. Prinz, D. N. Dewhurst, M. D. Raven, H. Stanjek and B. M. Krooss, Int. J. Greenhouse Gas Control, 2008, 2, 297–308 CrossRef CAS.
- A. Busch, P. Bertier, Y. Gensterblum, G. Rother, C. J. Spiers, M. Zhang and H. M. Wentinck, Geomech. Geophys. Geo-Energy Geo-Resources, 2016, 2, 111–130 CrossRef.
- M. Zhang, PhD thesis, Utrecht University, 2019.
- J. Song and D. Zhang, Environ. Sci. Technol., 2013, 47, 9–22 CrossRef CAS.
- H. M. Wentinck and A. Busch, Spec. Publ. - Geol. Soc. London, 2017, 454, 155–173 CrossRef.
- C. Cornelio, E. Spagnuolo, G. Di Toro, S. Nielsen and M. Violay, Nat. Commun., 2019, 10, 1274 CrossRef CAS.
- J. Alcalde, S. Flude, M. Wilkinson, G. Johnson, K. Edlmann, C. E. Bond, V. Scott, S. M. V. Gilfillan, X. Ogaya and R. Stuart Haszeldine, Nat. Commun., 2018, 9, 25–28 CrossRef.
- M. A. Rosen and S. Koohi-Fayegh, Energy, Ecol. Environ., 2016, 1, 10–29 CrossRef.
- Kiwa Gastec, Energy Storage Component Research & Feasibility Study Scheme – HyHouse – Safety Issues Surrounding Hydrogen as an Energy Storage Vector, 2015.
- S. Themann, H. M. Schmidt and D. Esser, SPE International Conference on CO2 Capture, Storage, and Utilization, Society of Petroleum Engineers, 2009 Search PubMed.
- S. Martens, R. Conze, M. De Lucia, J. Henninges, T. Kempka, A. Liebscher, S. Lüth, F. Möller, B. Norden, B. Prevedel, C. Schmidt-Hattenberger, A. Szizybalski, A. Vieth-Hillebrand, H. Würdemann, K. Zemke and M. Zimmer, in Geological Storage of CO2 -- Long Term Security Aspects: Geotechnologien Science Report No. 22, ed. A. Liebscher and U. Münch, Springer International Publishing, Cham, 2015, pp. 1–32 Search PubMed.
- S. Hannis, A. Chadwick, D. Connelly, J. Blackford, T. Leighton, D. Jones, J. White, P. White, I. Wright, S. Widdicomb, J. Craig and T. Dixon, Energy Procedia, 2017, 114, 5967–5980 CrossRef CAS.
- B. Freifeld, T. Daley, P. Cook, R. Trautz and K. Dodds, Energy Procedia, 2014, 63, 3500–3515 CrossRef CAS.
- W. T. Pfeiffer, S. A. Al Hagrey, D. Köhn, W. Rabbel and S. Bauer, Environ. Earth Sci., 2016, 75, 1177 CrossRef.
- B. R. Misra, S. E. Foh, Y. A. Shikari, R. M. Berry and F. Labaune, Proceedings of SPE Gas Technology Symposium, Society of Petroleum Engineers, 1988 Search PubMed.
- D. B. Bennion, F. B. Thomas, T. Ma and D. Imer, SPE/CERI Gas Technology Symposium, Society of Petroleum Engineers, 2000 Search PubMed.
- D. J. Evans and R. A. Chadwick, Spec. Publ. - Geol. Soc. London, 2009, 313, 1–11 CrossRef.
- A. Chadwick, R. Arts, C. Bernstone, F. May, S. Thibeau and P. Zweigel, Best practice for the storage of CO2 in saline aquifers – observations and guidelines from the SACS and CO2STORE projects, 2008, vol. 14 Search PubMed.
- J. M. Miocic, S. M. V. Gilfillan, J. J. Roberts, K. Edlmann, C. I. McDermott and R. S. Haszeldine, Int. J. Greenhouse Gas Control, 2016, 51, 118–125 CrossRef CAS.
- A. Sainz-Garcia, E. Abarca, V. Rubi and F. Grandia, Int. J. Hydrogen Energy, 2017, 42, 16657–16666 CrossRef CAS.
- B. Hagemann, M. Rasoulzadeh, M. Panfilov, L. Ganzer and V. Reitenbach, Environ. Earth Sci., 2015, 73, 6891–6898 CrossRef CAS.
- K. Luboń and R. Tarkowski, Int. J. Hydrogen Energy, 2020, 45, 2068–2083 CrossRef.
- J. P. Laille, J. E. Molinard and A. Wents, SPE Gas Technology Symposium, Society of Petroleum Engineers, 1988 Search PubMed.
- M. Dussaud, Underground Storage of Natural Gas, Springer Netherlands, Dordrecht, 1989, pp. 371–383 Search PubMed.
- C. M. Oldenburg, Energy Fuels, 2003, 17, 240–246 CrossRef CAS.
- W. T. Pfeiffer and S. Bauer, Energy Procedia, 2015, 76, 565–572 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |