Open Access Article
Open Access ArticleRecyclable, sustainable, and stronger than portland cement: a composite from unseparated biomass and fossil fuel waste†
Moira K.
Lauer
a,
Menisha S.
Karunarathna
a,
Andrew G.
Tennyson
 ab and
Rhett C.
Smith
ab and
Rhett C.
Smith
 *a
*a
aDepartment of Chemistry, Clemson University, Clemson, South Carolina 29634, USA. E-mail: rhett@clemson.edu
bDepartment of Materials Science and Engineering, Clemson University, Clemson, South Carolina 29634, USA
First published on 24th June 2020
Abstract
A composite was prepared from biomass and waste sulfur from fossil fuel refining. The composite has higher compressive and flexural strength than portland cement. Avoiding expensive biomass separation and achieving metrics exceeding those of commercial products is a notable step towards a green economy.
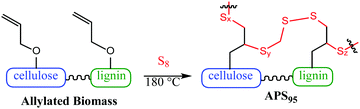
Effective biomass utilization is the centrepiece of a sustainable future. Fuels, petrochemical plastics and portland cement building materials must ultimately be replaced with greener surrogates such as biofuels, plant plastics and carbon-negative cement products. A primary barrier to the affordability of biomass products is the high cost of separating lignin, cellulose and other components.1 Current leading technologies for valorising biomass are energy or solvent intensive – pyrolysis, steam explosion, extraction and filtration or pressing, etc.2–10 Herein is reported a method to prepare a composite using unseparated biomass as a starting material. The other comonomer is sulfur, a waste product of fossil fuel refining. The biomass derivative and sulfur are combined to form composite APS95 (Fig. 1), a material having markedly improved flexural and compressional strength versus portland cement.
 | ||
| Fig. 1 A carbon-negative cement product (APS95) is prepared by harnessing waste products of energy production and agricultural industries. | ||
Efforts to unveil sustainable fuels and plastics have attracted significantly more attention than finding sustainable portland cement replacements, yet portland cement manufacturing is responsible for 7–10% of anthropogenic greenhouse gas emissions as well as accounting for 30% of global material utilization.11–14 Couple this with the fact that global portland cement production has soared by more than 150% since 2000, and the need for sustainable cement surrogates to counter climate change is evident. Furthermore, only a small percentage of portland cement and traditional plastic produced are recycled,15 so a more readily recyclable alternative should be sought.14
We previously demonstrated that purified samples of either cellulose16 or lignin17–19 could be modified with olefin moieties that allowed them to undergo inverse vulcanization, a 100% atom-economical process whereby the olefin units are crosslinked with sulfur.20–40 We previously observed that it was necessary to modify lignin17 or cellulose16 with olefin moieties for effective mixing with molten sulfur under the typical inverse vulcanization conditions. For the current work, we thus hypothesized that a raw biomass sample treated to incorporate olefinic units could undergo inverse vulcanization to give a composite in an overall two-step process that does not require separation of the biomass. Finely ground peanut shells were used as the biomass source to test this hypothesis. Of the ∼44 megatons of peanuts produced each year, ∼25–30% of the mass – over 11 megaton/year – is peanut shells.41 The dry mass of peanut shells is comprised by 87% lignocellulose (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 lignin
3 lignin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
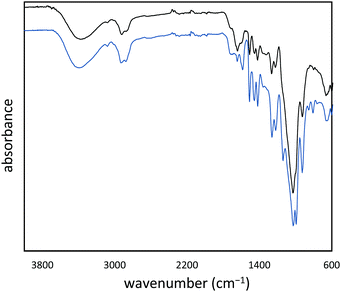
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cellulose ratio), with ∼1% peanut oil, 7% total protein, and the balance inorganic salts (ash content). In order to append olefin units to the lignin and cellulose components of the shells, they were treated with allyl bromide in alkaline aqueous solution and filtered. The allylated biomass was characterized by infrared (IR) spectroscopy by comparison to spectra for independently prepared samples of allyl cellulose42 and allyl lignin.17 The IR spectrum for allylated biomass is nearly identical to the spectrum that results from adding the scaled spectra for ally cellulose and allyl lignin (Fig. 2). The total amount of olefins in allylated biomass was further quantified to be 1.90 ± 0.04 mmol g−1 by iodometric titration and elemental composition consistent with the structure was confirmed by elemental analysis. The allylated biomass was subjected to inverse vulcanization (Scheme 1) with 95 wt% elemental sulfur to give composite APS95 as a visually homogeneous black solid (Fig. 1). The homogeneity was further confirmed by scanning electron microscopy (SEM) imaging with element mapping by energy dispersive X-ray analysis (EDX), which show uniform distribution of sulfur and carbon in the material (Fig. 3 and ESI† Fig. S3). These data confirm the absence of fibres or particles that might be responsible for mechanical reinforcement the material. IR spectroscopy confirms consumption of the alkene units within detection limits (ESI† Fig. S7). APS95 is remeltable and can be fabricated into various shapes by simple melt processing and shaping in stainless steel or silicone moulds. Many high sulfur- content materials prepared by the inverse vulcanization route contain extractable sulfur in addition to the sulfur that is covalently bound as crosslinking chains. The presence of free sulfur has proven important for the mechanical strength, processability and stability of polymeric sulfur domains in previous lignin–sulfur or cellulose sulfur materials prepared by inverse vulcanization.16,17 Sulfur that is not covalently bound is readily extractable into CS2. The CS2 -extractable sulfur accounted for 89% of the mass of APS95. This data, and the amount of covalently bound sulfur known, allowed a sulfur rank of 20 to be calculated for APS95. This sulfur rank compares well to the sulfur rank for other cellulose–sulfur materials but is somewhat lower than the rank observed in lignin–sulfur composites comprising 95 wt% sulfur (sulfur rank of 31–48).
cellulose ratio), with ∼1% peanut oil, 7% total protein, and the balance inorganic salts (ash content). In order to append olefin units to the lignin and cellulose components of the shells, they were treated with allyl bromide in alkaline aqueous solution and filtered. The allylated biomass was characterized by infrared (IR) spectroscopy by comparison to spectra for independently prepared samples of allyl cellulose42 and allyl lignin.17 The IR spectrum for allylated biomass is nearly identical to the spectrum that results from adding the scaled spectra for ally cellulose and allyl lignin (Fig. 2). The total amount of olefins in allylated biomass was further quantified to be 1.90 ± 0.04 mmol g−1 by iodometric titration and elemental composition consistent with the structure was confirmed by elemental analysis. The allylated biomass was subjected to inverse vulcanization (Scheme 1) with 95 wt% elemental sulfur to give composite APS95 as a visually homogeneous black solid (Fig. 1). The homogeneity was further confirmed by scanning electron microscopy (SEM) imaging with element mapping by energy dispersive X-ray analysis (EDX), which show uniform distribution of sulfur and carbon in the material (Fig. 3 and ESI† Fig. S3). These data confirm the absence of fibres or particles that might be responsible for mechanical reinforcement the material. IR spectroscopy confirms consumption of the alkene units within detection limits (ESI† Fig. S7). APS95 is remeltable and can be fabricated into various shapes by simple melt processing and shaping in stainless steel or silicone moulds. Many high sulfur- content materials prepared by the inverse vulcanization route contain extractable sulfur in addition to the sulfur that is covalently bound as crosslinking chains. The presence of free sulfur has proven important for the mechanical strength, processability and stability of polymeric sulfur domains in previous lignin–sulfur or cellulose sulfur materials prepared by inverse vulcanization.16,17 Sulfur that is not covalently bound is readily extractable into CS2. The CS2 -extractable sulfur accounted for 89% of the mass of APS95. This data, and the amount of covalently bound sulfur known, allowed a sulfur rank of 20 to be calculated for APS95. This sulfur rank compares well to the sulfur rank for other cellulose–sulfur materials but is somewhat lower than the rank observed in lignin–sulfur composites comprising 95 wt% sulfur (sulfur rank of 31–48).
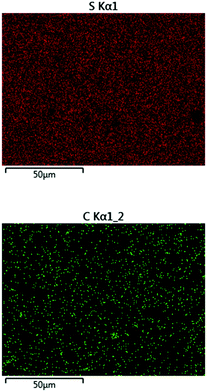 | ||
| Fig. 3 Surface analysis of APS95 by EDX revealed the homogeneous distribution of sulfur (upper) and carbon (lower) on the composite surface. | ||
Thermogravimetric analysis (TGA) revealed a major mass loss step characteristic of elemental sulfur with an onset of 252 °C whereas the influence of allyl peanut shells could be seen by a second mass loss step with an inflection point at 316 °C (ESI† Fig. S9). Differential scanning calorimetry (DSC) demonstrated characteristic peaks for sulfur melting (115–120 °C) and crystallization (10–30 °C) as well as a cold crystallization at ∼30 °C (ESI† Fig. S11). Similar cold crystallization peaks are reported in the DSC analysis of other cellulose–sulfur composites (PCSx where x = wt% sulfur) and lignin–sulfur composites (SALx and CLSx, where x = wt% sulfur).16–18
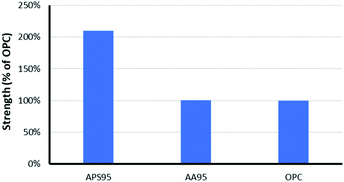
The mechanical properties of APS95 are the most striking finding from this study. Many efforts have been undertaken to understand the mechanical and other properties of plant-derived composites having high sulfur content.43–50 Despite the presence of just 5 wt% organic crosslinker in the composite, significant physical strength enhancement over elemental sulfur was observed. An elemental sulfur sample breaks upon mounting in the instrument at the minimum clamping force. Previous work likewise revealed significant mechanical property enhancement, with an 8-fold increase in storage modulus when sulfur was crosslinked with just 1 wt% of oleic acid.35 Previous biopolymer–sulfur composites PCSx, SALx and CLSx, for example generally have flexural strengths lower or similar to that of portland cement (Table 1).16–18 The flexural strength of APS95 is 30% higher than that of portland cement, making it a good candidate for certain applications where flexural stress is applied. In contrast to flexural strength, the compressional strength of a material reflects its ability to support a load applied perpendicular to its surface. The high compressional strength of portland cement is the primary feature that has made it the most-produced synthetic product of civilization. When previously-reported biopolymer–sulfur composites are handled, it is clear that they lack substantial compressional strength. In contrast, when APS95 was handled it exhibited substantial resistance to crushing, leading to further study of its compressional strength. When quantified, the compressional strength of APS95 was found to be 35.7 ± 1.8 MPa, more than twice that required by building regulation ACI 332 for residential building (17 MPa, Fig. 4).51 The compressional strength of APS95 also exceeds that of light bricks used in wall construction (7 MPa). It should be noted that the compressional strength of portland cement varies with aging time and humidity. While much higher values are often quoted for materials referred to as “portland cement”, those are often materials having added fines (sand) and/or aggregate (gravel), which can improve compressional strength by over an order of magnitude. Neither the portland cement nor APS95 had any added fines or aggregate for the current study. The remarkable improvement in compressional strength of APS95versus previously-reported cellulose– or lignin–sulfur composites must derive from the synergistic combination of lignin with cellulose. Specifically, the allylated biomass could contain naturally-occurring lignin–cellulose crosslinks or potentially base-catalysed esterification of lignin carboxylates during the biomass etherification reaction. It is this same combination primarily of crosslinked lignin and cellulose that provides plants and timber-built structures with their impressive mechanical strength despite the fact that either lignin or cellulose alone has very poor mechanical strength. Prior work on high sulfur content material prepared by inverse vulcanization showed that he modulus can be increased by nearly an order of magnitude upon increasing the crosslink density by only 1%.35
| Sulfur rank | Compressive strength (MPa) | Flexural strength/modulus (MPa) | Modulus of resilience (kPa) | |
|---|---|---|---|---|
a Compressive strength is for the minimum requirement of ACI 332. Flexural data refers to portland cement without added fines or aggregate, mixed with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 water to cement ratio, and cured very gradually over an eight-day time period and after drying at 200 °C to screen out humidity effects.
b Data refers to portland cement without added fines or aggregate, mixed with a 1 2 water to cement ratio, and cured very gradually over an eight-day time period and after drying at 200 °C to screen out humidity effects.
b Data refers to portland cement without added fines or aggregate, mixed with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 water to cement ratio, and cured very gradually over a twelve day time period and after drying at 200 °C to screen out humidity effects.
c Sample could not be measured beyond this level due to instrumental limitations. 2 water to cement ratio, and cured very gradually over a twelve day time period and after drying at 200 °C to screen out humidity effects.
c Sample could not be measured beyond this level due to instrumental limitations.
|
||||
| APS95 | 20 | 35.7 ± 1.8 | 4.8/690 | 0.2 |
| Portland cementa | NA | 17 | 3.7/580b | 0.6b |
| AA95 | X | 17.1 | ND | ND |
| PCS90 | 24 | ND | Too brittle | Too brittle |
| PCS85 | 31 | ND | 3.2c/320 | 2.7 |
| PCS80 | 22 | ND | 3.8c/520 | 1.8 |
| CLS95 | 31 | ND | 2.1/156 | ND |
| SAL95 | 48 | ND | 2.1/90 | ND |
In an effort to confirm the hypothesis that the high compressional strength is due to lignin–cellulose crosslinking, separately-prepared allyl cellulose and allyl lignin were blended in the same cellulose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin ratio and having the same unsaturation per unit mass as in the allylated peanut shells used to prepare APS95. This synthetic blend was polymerized with 95 wt% sulfur under the same conditions used to prepare APS95, yielding AA95. The compressional strength of AA95 was only about 50% that of APS95 (Table 1 and Fig. 4). Using unseparated biomass thus improves material properties over materials having the same putative composition.
lignin ratio and having the same unsaturation per unit mass as in the allylated peanut shells used to prepare APS95. This synthetic blend was polymerized with 95 wt% sulfur under the same conditions used to prepare APS95, yielding AA95. The compressional strength of AA95 was only about 50% that of APS95 (Table 1 and Fig. 4). Using unseparated biomass thus improves material properties over materials having the same putative composition.
Although the high compressive strength of APS95 is promising for its use as a structural cement replacement, APS95 requires hot casting. This means that blocks of APS95 would need to be made by melting it and pouring it into moulds, while paving with APS95 would require hot-casting in a manner similar to asphalt paving. Shell oil has developed a sulfur asphalt, Thiopave® that can be cast in this way, and several stretches of Thiopave® highways showed durability on par or better than traditional asphalt installations.52–54
Conclusions
In conclusion, we report a protocol to prepare a composite from waste products of energy and agriculture industries. The biomass can be used as a comonomer without separation after one simple modification step in aqueous solution, followed by a 100% atom economical polymerization step. The resulting composite (APS95) has mechanical properties that significantly exceed that of portland cement. APS95 also outperforms portland cement in its recyclability by simple melt processing and is a carbon-negative/carbon sequestering product whereas portland cement production is one of the leading sources of anthropogenic CO2. Given the similar cellulose and lignin content for other agricultural biproducts, we anticipate that the approach herein should be applicable to leveraging a wide range of lignocellulosic biomass waste products, thus allowing for their straightforward conversion to durable structural materials. Studies involving application of the current procedure to other biomass resources are underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank Jake Harrell of Golden Peanut and Tree Nuts (Alpharetta, Georgia, USA) for supplying peanut shell product ES used in this study. Funding for this project from the National Science Foundation (CHE-1708844) is gratefully acknowledged.Notes and references
- R. Ma, Y. Xu and X. Zhang, ChemSusChem, 2015, 8, 24–51 CrossRef CAS PubMed.
- A. K. Varshney and D. P. Patel, J. Sci. Ind. Res., 1989, 47, 315–319 CAS.
- D. Chiaramonti, M. Prussi, S. Ferrero, L. Oriani, P. Ottonello, P. Torre and F. Cherchi, Biomass Bioenergy, 2012, 46, 25–35 CrossRef CAS.
- P. Dominguez de Maria, J. Chem. Technol. Biotechnol., 2014, 89, 11–18 CrossRef CAS.
- Y. Zhao, W. Lu, J. Chen, X. Zhang and H. Wang, Front. Environ. Sci. Eng., 2014, 8, 151–161 CrossRef CAS.
- H. Sarip, M. S. Hossain, A. M. N. Mohamad and K. Allaf, BioResources, 2016, 11, 1–29 CrossRef CAS.
- M. N. Borand and F. Karaosmanoglu, J. Renewable Sustainable Energy, 2018, 10, 033104 CrossRef.
- P. Halder, S. Kundu, S. Patel, A. Setiawan, R. Atkin, R. Parthasarthy, J. Paz-Ferreiro, A. Surapaneni and K. Shah, Renewable Sustainable Energy Rev., 2019, 105, 268–292 CrossRef CAS.
- V. Oriez, J. Peydecastaing and P.-Y. Pontalier, Molecules, 2019, 24, 4373 CrossRef PubMed.
- A. Ferreira Jorge and J. Taherzadeh Mohammad, Bioresour. Technol., 2020, 299, 122695 CrossRef CAS PubMed.
- K. L. Scrivener, V. M. John and E. M. Gartner, Cem. Concr. Res., 2018, 114, 2–26 CrossRef CAS.
- F. Krausmann, A. Schaffartzik, A. Mayer, S. Gingrich and N. Eisenmenger, MRS Online Proc. Libr., 2013, 1545 Search PubMed , opl 2013 1075, 2011 pp.
- R. M. Andrew, Earth Syst. Sci. Data, 2019, 11, 1675–1710 CrossRef.
- J. M. Allwood, J. M. Cullen and R. L. Milford, Environ. Sci. Technol., 2010, 44, 1888–1894 CrossRef CAS PubMed.
- T. Thiounn and R. C. Smith, J. Polym. Sci., 2020, 58, 1347–1364 CrossRef CAS.
- M. K. Lauer, T. A. Estrada-Mendoza, C. D. McMillen, G. Chumanov, A. G. Tennyson and R. C. Smith, Adv. Sustainable Syst., 2019, 3, 1900062 CrossRef CAS.
- M. S. Karunarathna, M. K. Lauer, T. Thiounn, R. C. Smith and A. G. Tennyson, J. Mater. Chem. A, 2019, 7, 15683–15690 RSC.
- M. S. Karunarathna, A. G. Tennyson and R. C. Smith, J. Mater. Chem. A, 2020, 8, 548–553 RSC.
- M. S. Karunarathna and R. C. Smith, Sustainability, 2020, 12, 734–748 CrossRef CAS.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- Y. Zhang, R. S. Glass, K. Char and J. Pyun, Polym. Chem., 2019, 10, 4078–4105 RSC.
- J. M. Chalker, M. J. H. Worthington, N. A. Lundquist and L. J. Esdaile, Top. Curr. Chem., 2019, 377, 1–27 CrossRef CAS PubMed.
- M. J. H. Worthington, R. L. Kucera and J. M. Chalker, Green Chem., 2017, 19, 2748–2761 RSC.
- X. Wu, A. Smith Jessica, S. Petcher, B. Zhang, J. Parker Douglas, T. Hasell and M. Griffin John, Nat. Commun., 2019, 10, 647 CrossRef PubMed.
- B. Zhang, S. Petcher and T. Hasell, Chem. Commun., 2019, 55, 10681–10684 RSC.
- B. Zhang, H. Gao, P. Yan, S. Petcher and T. Hasell, Mater. Chem. Front., 2020, 4, 669–675 RSC.
- J. A. Smith, S. J. Green, S. Petcher, D. J. Parker, B. Zhang, M. J. H. Worthington, X. Wu, C. A. Kelly, T. Baker, C. T. Gibson, J. A. Campbell, D. A. Lewis, M. J. Jenkins, H. Willcock, J. M. Chalker and T. Hasell, Chem. – Eur. J., 2019, 25, 10433–10440 CrossRef CAS PubMed.
- M. J. H. Worthington, C. J. Shearer, L. J. Esdaile, J. A. Campbell, C. T. Gibson, S. K. Legg, Y. Yin, N. A. Lundquist, J. R. Gascooke, I. S. Albuquerque, J. G. Shapter, G. G. Andersson, D. A. Lewis, G. J. L. Bernardes and J. M. Chalker, Adv. Sustainable Syst., 2018, 2, 1800024 CrossRef.
- L. J. Esdaile and J. M. Chalker, Chem. – Eur. J., 2018, 24, 6905–6916 CrossRef CAS PubMed.
- C. Herrera, J. Ysinga Kristen and L. Jenkins Courtney, ACS Appl. Mater. Interfaces, 2019, 11, 35312–35318 CrossRef CAS PubMed.
- C. R. Westerman and C. L. Jenkins, Macromolecules, 2018, 51, 7233–7238 CrossRef CAS.
- A. D. Smith, C. D. McMillin, R. C. Smith and A. G. Tennyson, J. Polym. Sci., 2020, 58, 438–445 CrossRef CAS.
- M. S. Karunarathna, M. K. Lauer, A. G. Tennyson and R. C. Smith, Polym. Chem., 2020, 11, 1621–1628 RSC.
- T. Thiounn, A. G. Tennyson and R. C. Smith, RSC Adv., 2019, 9, 31460–31465 RSC.
- A. D. Smith, T. Thiounn, E. W. Lyles, E. K. Kibler, R. C. Smith and A. G. Tennyson, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 1704–1710 CrossRef CAS.
- T. Thiounn, M. K. Lauer, M. S. Bedford, R. C. Smith and A. G. Tennyson, RSC Adv., 2018, 8, 39074–39082 RSC.
- A. D. Smith, R. C. Smith and A. G. Tennyson, Sustainable Chem. Pharm., 2020, 16, 100249 CrossRef.
- T. Hasell, P. Yan, W. Zhao, B. Zhang, S. Petcher, A. Smith Jessica, J. Parker Douglas, I. Cooper Andrew, L. Jiang and J. Lei, Angew. Chem., Int. Ed., 2020, 59, 2–10 CrossRef.
- S. J. Tonkin, C. T. Gibson, J. A. Campbell, D. A. Lewis, A. Karton, T. Hasell and J. M. Chalker, Chem. Sci., 2020, 11, 5537–5546 RSC.
- N. Lundquist, A. Tikoalu, M. Worthington, R. Shapter, S. Tonkin, F. Stojcevski, M. Mann, C. Gibson, J. Gascooke, A. Karton, L. Henderson, L. Esdaile and J. Chalker, Chem. – Eur. J., 2020, 26, 1–11 CrossRef.
- M.-A. Perea-Moreno, F. Manzano-Agugliaro, Q. Hernandez-Escobedo and A.-J. Perea-Moreno, Sustainability, 2018, 10, 3254 CrossRef CAS.
- H. Hu, J. You, W. Gan, J. Zhou and L. Zhang, Polym. Chem., 2015, 6, 3543–3548 RSC.
- D. J. Parker, H. A. Jones, S. Petcher, L. Cervini, J. M. Griffin, R. Akhtar and T. Hasell, J. Mater. Chem. A, 2017, 5, 11682–11692 RSC.
- D. J. Parker, S. T. Chong and T. Hasell, RSC Adv., 2018, 8, 27892–27899 RSC.
- A. D. Tikoalu, N. A. Lundquist and J. M. Chalker, Adv. Sustainable Syst., 2020, 4, 1900111 CrossRef CAS.
- M. J. H. Worthington, R. L. Kucera, I. S. Albuquerque, C. T. Gibson, A. Sibley, A. D. Slattery, J. A. Campbell, S. F. K. Alboaiji, K. A. Muller, J. Young, N. Adamson, J. R. Gascooke, D. Jampaiah, Y. M. Sabri, S. K. Bhargava, S. J. Ippolito, D. A. Lewis, J. S. Quinton, A. V. Ellis, A. Johs, G. J. L. Bernardes and J. M. Chalker, Chem. – Eur. J., 2017, 23, 16106 CrossRef CAS.
- M. P. Crockett, A. M. Evans, M. J. H. Worthington, I. S. Albuquerque, A. D. Slattery, C. T. Gibson, J. A. Campbell, D. A. Lewis, G. J. L. Bernardes and J. M. Chalker, Angew. Chem., Int. Ed., 2016, 55, 1714–1718 CrossRef CAS PubMed.
- I. Gomez, O. Leonet, J. A. Blazquez and D. Mecerreyes, ChemSusChem, 2016, 9, 3419–3425 CrossRef CAS PubMed.
- A. Hoefling, Y. J. Lee and P. Theato, Macromol. Chem. Phys., 2017, 218, 1600303 CrossRef.
- A. Hoefling, D. T. Nguyen, Y. J. Lee, S.-W. Song and P. Theato, Mater. Chem. Front., 2017, 1, 1818–1822 RSC.
- A. R.-. Guide to Residential Concrete Construction, American Concrete Institute, Farmington Hills, MI., Journal.
- X.-D. Hu, Y.-M. Gao, L.-R. Lin, S. Zhong and X.-W. Dai, J. Wuhan Inst. Technol., 2013, 35, 10–13 CAS.
- Z.-G. Zuo, G.-S. Cao and H.-L. Song, J. Qingdao Technol. Univ., 2011, 32, 26–29 CAS.
- M. Al-Ansary, E. Masad and D. Strickland, Adv. Gas Process., 2010, 2, 121–130 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectra, thermal analysis, mechanical analysis and SEM/EDX imaging. See DOI: 10.1039/d0ma00270d |
| This journal is © The Royal Society of Chemistry 2020 |