Optimizing graphene content in a NiSe/graphene nanohybrid counter electrode to enhance the photovoltaic performance of dye-sensitized solar cells†
Vignesh
Murugadoss
 ab,
Jing
Lin
ab,
Jing
Lin
 *c,
Hu
Liu
*c,
Hu
Liu
 d,
Xianmin
Mai
*e,
Tao
Ding
*f,
Zhanhu
Guo
d,
Xianmin
Mai
*e,
Tao
Ding
*f,
Zhanhu
Guo
 *b and
Subramania
Angaiah
*b and
Subramania
Angaiah
 *a
*a
aElectro-Materials Research Laboratory, Centre for Nanoscience and Technology, Pondicherry University, Puducherry – 605 014, India. E-mail: a.subramania@gmail.com
bIntegrated Composites Laboratory (ICL), Department of Chemical and Biomolecular Engineering, University of Tennessee, Knoxville, TN 37996, USA. E-mail: zguo10@utk.edu; nanomaterilas2000@gmail.com
cSchool of Chemistry and Chemical Engineering, Guangzhou University, Guangzhou 510006, China. E-mail: linjing@gzhu.edu.cn
dKey Laboratory of Materials Processing and Mold (Zhengzhou University), Ministry of Education, National Engineering Research Center for Advanced Polymer Processing Technology, Zhengzhou University, Zhengzhou, China
eSchool of Urban Planning and Architecture, Southwest Minzu University, Chengdu 610041, China. E-mail: maixianmin@foxmail.com
fCollege of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, China
First published on 9th September 2019
Abstract
Nickel selenide (NiSe) nanoparticles were grown on graphene nanosheets (GN) with different mass ratios to obtain their corresponding NiSe/GNx (x = 0.25 to 1.00) nanohybrids by a facile in situ hydrothermal process to integrate the advantages of the high specific surface area of graphene and the homogeneously immobilized catalytic sites of NiSe. The nanohybrid with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 (i.e., NiSe/GN0.50) exhibited higher electrocatalytic activity and electrolyte diffusion. Thus, NiSe/GN0.50 exhibited an improved photo-conversion efficiency (PCE) of 12% (η = 8.62%) compared to a standard Pt (η = 7.68%)-based dye-sensitized solar cell (DSSC). This improved PCE mainly originated from the catalytic ability of NiSe and the multiple interfacial electron transfer pathways of graphene, resulting in enhanced charge transfer and fast tri-iodide reduction kinetics at the counter electrode/electrolyte interface. The results obtained from the cyclic voltammetry (CV), electrochemical AC-impedance (EIS) and Tafel polarization studies validated the synergistic effects of NiSe and GN and the high potential of this nanohybrid as an efficient counter electrode (CE) for DSSCs.
0.50 (i.e., NiSe/GN0.50) exhibited higher electrocatalytic activity and electrolyte diffusion. Thus, NiSe/GN0.50 exhibited an improved photo-conversion efficiency (PCE) of 12% (η = 8.62%) compared to a standard Pt (η = 7.68%)-based dye-sensitized solar cell (DSSC). This improved PCE mainly originated from the catalytic ability of NiSe and the multiple interfacial electron transfer pathways of graphene, resulting in enhanced charge transfer and fast tri-iodide reduction kinetics at the counter electrode/electrolyte interface. The results obtained from the cyclic voltammetry (CV), electrochemical AC-impedance (EIS) and Tafel polarization studies validated the synergistic effects of NiSe and GN and the high potential of this nanohybrid as an efficient counter electrode (CE) for DSSCs.
1. Introduction
Dye-sensitized solar cells (DSSCs) are promising alternatives to silicon solar cells owing to their inherent advantages, such as high conversion efficiency under diffuse light and low illumination along with decreased angular dependence of efficiency, easy fabrication, and low cost.1–5 A typical DSSC consists of a redox couple electrolyte assembled between a dye-sensitized semiconductor photoanode and an electrocatalytic platinum (Pt) counter electrode (CE).6–8 However, the low abundance, high cost and corrosion of Pt by the I3−/I− electrolyte hinder the large-scale commercialization of DSSCs.9 In order to eliminate the dependence on the noble Pt metal, a low-cost CE with high electrocatalytic activity is required. Various interesting earth-abundant materials, including carbon nanostructures, conducting polymers, metal nitrides, metal carbides, metal sulfides and their hybrids/nanocomposites, have been reported as replacements for Pt.10–14The transition metal selenide differs from the above-mentioned alternative materials due to its high electrical conductivity, special geometry, unique heat and chemical stabilities and excellent electrocatalytic activity.15–17 Recent studies have shown that NiSe provides good electrocatalytic properties for tri-iodide reduction due to its unique electronic structure, higher catalytic activity and earth abundance; it can serve as a potential CE material.18–20
However, the facile agglomeration of NiSe can lead to poor electronic conductivity, low material utilization and poor electrocatalytic performance for the tri-iodide reduction reaction.21–30 To avoid these problems, Yue et al. introduced different amounts of graphene into NiSe2 nanoparticles by physical mixing and achieved a photoconversion efficiency higher than that of platinum (7.28%) for NiSe2/6 wt% graphene (7.47%).31 However, physical mixing may result in insufficient charge transfer capacity and electrocatalytic activity due to the weak interactions between the two components.
Many reports in the literature state that transition metal compounds chemically modified with graphene (including Pt-decorated graphene) as effective alternatives to platinum CEs can satisfy the inevitable requirements of high abundance, low cost and long-term stability as well as excellent catalytic functionality and high electrical conductivity for a wide range of applications, such as dye-sensitized solar cells, the oxygen reduction reaction, water splitting, and fuel cells.32–38 Most of these studies focus on the regulation of the morphology and composition of the nanohybrids.39–41 Our recent studies on CoSe nanoparticles/graphene nanohybrids exhibited the desired electrocatalytic activity for the reduction of tri-iodide (I3−).42 From the above literature, it is rational to anticipate the development of in situ grown NiSe on graphene to use as CE for DSSCs. Furthermore, the mass ratio between NiSe and graphene may greatly influence the electrochemical activity of the counter electrode, which has not been studied yet.
Herein, Ni0.85Se nanoparticles were grown onto different mass ratios of graphene nanosheets (GN) by a facile in situ hydrothermal method and characterized by field emission scanning electron microscopy (FE-SEM), high-resolution transmission electron microscopy (HR-TEM), X-ray diffraction (XRD), Raman spectroscopy and X-ray photoelectron spectroscopy (XPS) studies. Further, the electrocatalytic abilities and tri-iodide reduction kinetics of the prepared nanohybrids were analyzed using cyclic voltammetry (CV), electrochemical AC-impedance (EIS) and Tafel polarization studies. The optimum mass ratio of GN in the NiSe/GN nanohybrid was investigated for use as a good counter electrode for DSSCs. Finally, the DSSC was assembled using the optimized NiSe/GN nanohybrid, and its higher photoconversion efficiency was measured and compared with that of a Pt-based DSSC.
2. Experimental
2.1 Materials
Sodium nitrate (NaNO3, 99% pure), nickel nitrate (Ni(NO)3·6H2O), hydrazine hydrate (N2H4·H2O), N-methyl-2-pyrrolidinone (NMP), iodine (I2), and sulfuric acid (H2SO4) were obtained from Merck, India. Lithium perchlorate (LiClO4) and graphite flakes were procured from HIMEDIA. Lithium iodide (LiI) and potassium permanganate (KMnO4) were purchased from SRL (Sisco Research Laboratories) Pvt. Ltd. Selenium powder (Se), polyvinylidene fluoride (PVdF), 1-butyl-3-methylimidazolium iodide (BMImI), acetonitrile (ACN), 4-tert-butylpyridine (TBP), ITO glass plates and N719 dye were obtained from Sigma Aldrich. All the chemicals were of analytical grade and were used without any further purification.2.2 Preparation of NiSe/GN nanohybrids
Graphene oxide nanosheets (GO) were prepared by a modified Hummer's method.43,44 The typical synthesis of the NiSe/GN nanohybrids followed the procedures reported in our previous study.45 First, GO (5, 10, 15, or 20 mg) was dispersed ultrasonically in 50 mL deionized water. To this, nickel nitrate (Ni(NO)3·6H2O, 04225 g) and selenium (Se) powder (0.01145 g) were added to obtain NiSe/GN nanohybrids with mass ratios between NiSe and GN of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, 1
0.25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50, 1
0.50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 and 1
0.75 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00, respectively. Hydrazine was added to this mixture under constant stirring after 30 minutes. Hydrazine acted as a reductant to enhance the graphitization properties of graphene and the formation of nickel selenide nanoparticles. Subsequently, the mixture was maintained at 120 °C for 12 h in an autoclave for the hydrothermal reaction. After cooling, the precipitates were washed thoroughly with deionized water and then dried at 50 °C under vacuum. The prepared NiSe/GN nanohybrids with different mass ratios between NiSe and graphene of 1
1.00, respectively. Hydrazine was added to this mixture under constant stirring after 30 minutes. Hydrazine acted as a reductant to enhance the graphitization properties of graphene and the formation of nickel selenide nanoparticles. Subsequently, the mixture was maintained at 120 °C for 12 h in an autoclave for the hydrothermal reaction. After cooling, the precipitates were washed thoroughly with deionized water and then dried at 50 °C under vacuum. The prepared NiSe/GN nanohybrids with different mass ratios between NiSe and graphene of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, 1
0.25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50, 1
0.50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 and 1
0.75 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 were represented as NiSe/GN0.25, NiSe/GN0.50, NiSe/GN0.75 and NiSe/GN1.00, respectively. Pristine NiSe nanoparticles were also synthesized for comparison by following the same procedures without the addition of GO.
1.00 were represented as NiSe/GN0.25, NiSe/GN0.50, NiSe/GN0.75 and NiSe/GN1.00, respectively. Pristine NiSe nanoparticles were also synthesized for comparison by following the same procedures without the addition of GO.
2.3 Physical characterization
The morphologies of the prepared NiSe nanoparticles, GO and NiSe/GN nanohybrids were observed using FE-SEM (JSM, JEOL 7600F). Their microstructures were investigated in detail using HR-TEM (Philips, Model: CM 200). The chemical natures and compositions of the NiSe/GN nanohybrids were evaluated using XPS (Kratos Analytical Ltd, Model: Kratos AXIS Ultra DLD). XPS data were obtained with mono Al-Kα radiation at an operating power of 75 W. The data were acquired at an inclination of 90° between the detector and the substrate in steps of 1 eV and for a residence time of 100 ms. The C 1s peak (284.6 eV) was set as the reference.The crystallinities and the phases of the prepared samples were characterized by a powder X-ray diffractometer (Rigaku, Ultima IV) with nickel-filtered Cu-Kα radiation between 20° and 80° at an augmentation of 0.05°. The Raman spectroscopy analysis was carried out using a confocal micro-Raman spectrometer (Renishaw RM 2000) with an excitation wavelength of 514 nm.
2.4 Fabrication of CE
The ITO glass substrates were cleaned with deionized water, acetone and ethanol by ultrasonication and then dried in air. Because of their higher transmittance and low surface roughness, ITO glass plates were chosen instead of FTO glass plates.46 Scotch tape was used as a spacer to control the film thickness. The CEs with the prepared samples were fabricated by following reported slurry coating procedures.42 Briefly, a mixture of 95 wt% active CE material and 5 wt% PVdF (binder) in NMP was ground well in a mortar to afford a slurry paste, coated on the cleaned ITO glass substrate by the doctor blade technique at a thickness of 15 μm and then dried at 80 °C in a vacuum oven for 12 h.7For comparison purposes, the counter electrode was fabricated by coating a 15 μm thick layer of Pt paste (Dyesol Ltd) on the ITO glass substrate by the doctor blade technique and then sintering at 450 °C in air for 30 min.47
2.5 Electrochemical studies
Cyclic voltammetry (CV), electrochemical impedance and Tafel polarization measurements were carried out to quantify the electrocatalytic ability of the CE materials prior to the DSSC fabrication using an electrochemical workstation (Biologic: VSP). The CV measurements were carried out in a three-electrode cell with an anhydrous acetonitrile (ACN) solution containing 0.01 M LiI, 0.001 M I2 and 0.1 M LiClO4 as the I−/I3− redox electrolyte at a scan rate of 50 mV s−1, using Pt as the CE, Ag/AgCl as the reference electrode and the fabricated CE (NiSe and NiSe/GN nanohybrid) as the working electrodes.48Electrochemical impedance measurements were analyzed using a symmetric cell assembled with two ITO glass substrates coated with an identical counter electrode in the redox electrolyte. The samples were tested between 100 kHz and 1 mHz at 10 mV of AC amplitude.9 The Tafel polarization measurements were carried out using the symmetric cell assembly at a scan rate of 50 mV s−1.27
2.6 Fabrication and photovoltaic performance of the DSSCs
The DSSCs were assembled with N719 dye-sensitized TiO2 photoanodes and the fabricated CE as per the procedure reported in our previous work.49,50 An ACN solution containing 0.5 M LiI, 0.05 M I2, 0.5 M BMImI (ionic liquid) and 0.5 M TBP was used as the I−/I3− redox electrolyte.The photovoltaic performance of the fabricated DSSC was investigated after an aging time of 24 h using a calibrated solar simulator (Newport, Oriel instruments U.S.A. 150 W, Model: 67005) equipped with a computer controlled digital source meter (Keithley, Model: 2420) under illumination of AM 1.5 with a light intensity of 100 mW cm−2.51,52 The measurement for each DSSC was repeated thrice under the same experimental conditions in an ambient environment.
3. Results and discussion
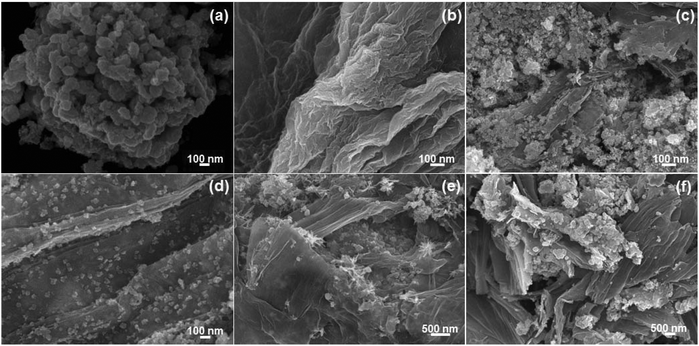
The FE-SEM images of the NiSe nanoparticles, GO and the NiSe/GNx nanohybrids are shown in Fig. 1(a–f). Fig. 1a shows the severe agglomeration of the NiSe nanoparticles. Fig. 1b shows that pure GO has a 2-D wrinkled sheet-like structure. It can be observed from Fig. 1(c–f) that the dispersity of the NiSe nanoparticles varies with the inclusion of graphene nanosheets according to the mass ratio of NiSe to graphene. This indicates that the GO regulates the nucleation, growth and dispersion of the NiSe nanoparticles. At a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50, NiSe/GN0.50 exhibits good dispersion of NiSe nanoparticles on the graphene (Fig. 1d). The oxygen-containing functional groups, such as hydroxy, epoxide and carboxylic groups, present on the basal plane and edges of graphene oxide acted as preferred sites for nickel ion adsorption, which directed the uniform distribution of NiSe on the GN surface without aggregation of the NiSe nanoparticles. At the mass ratio of 1
0.50, NiSe/GN0.50 exhibits good dispersion of NiSe nanoparticles on the graphene (Fig. 1d). The oxygen-containing functional groups, such as hydroxy, epoxide and carboxylic groups, present on the basal plane and edges of graphene oxide acted as preferred sites for nickel ion adsorption, which directed the uniform distribution of NiSe on the GN surface without aggregation of the NiSe nanoparticles. At the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, the amount of graphene oxide that served as nucleation sites for NiSe nanoparticles was comparatively lower than at the mass ratio of 1
0.25, the amount of graphene oxide that served as nucleation sites for NiSe nanoparticles was comparatively lower than at the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50. This led to the formation of NiSe aggregates on the graphene nanosheets (Fig. 1c). With mass ratios of 1
0.50. This led to the formation of NiSe aggregates on the graphene nanosheets (Fig. 1c). With mass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 and 1
0.75 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 (Fig. 1e and f), most of the graphene nanosheets remained uncovered due to the presence of small amounts of NiSe nanoparticles and restacked due to the π–π interactions and the van der Waals forces between the planar surfaces. In addition, it was observed that the graphene nanosheets were covered with NiSe nanoparticles. Therefore, NiSe/GN0.50 nanohybrid was selected as the optimal counter electrode material; it was expected to provide better electrocatalytic activity for the reduction of tri-iodides.
1.00 (Fig. 1e and f), most of the graphene nanosheets remained uncovered due to the presence of small amounts of NiSe nanoparticles and restacked due to the π–π interactions and the van der Waals forces between the planar surfaces. In addition, it was observed that the graphene nanosheets were covered with NiSe nanoparticles. Therefore, NiSe/GN0.50 nanohybrid was selected as the optimal counter electrode material; it was expected to provide better electrocatalytic activity for the reduction of tri-iodides.
Fig. 2 shows the HR-TEM images of pristine NiSe nanoparticles and the NiSe/GN0.50 nanohybrid. Fig. 2a shows that the NiSe nanoparticles are spherical in shape, with an average particle size of 16 nm. It can be observed that the layered reduced graphene nanosheets serve as a support that is uniformly decorated with NiSe nanoparticles (Fig. 2b). The presence of wrinkles in graphene nanosheets provides better contact with the NiSe nanoparticles. Fig. 2c depicts the lattice fringes with an interplanar spacing of 2.04 and 3.47 Å, which are ascribed to the (102) plane of hexagonal NiSe and the graphitic layers (3 to 9 layers) of GN, respectively. The visible set of fringes confirms the high crystallinity of NiSe. The selected area electron diffraction (SAED) rings (Fig. 2d) of NiSe/GN0.50 can be matched well with the (101), (110), (201) and (212) planes of hexagonal NiSe (JCPDS no. 18-0888, space group: P63/mmc), demonstrating the polycrystallinity of NiSe.
 | ||
| Fig. 1 FE-SEM images of (a) NiSe nanoparticles, (b) graphene oxide, and the (c–f) NiSe/GN0.25, NiSe/GN0.50, NiSe/GN0.75 and NiSe/GN1.00 nanohybrids. | ||
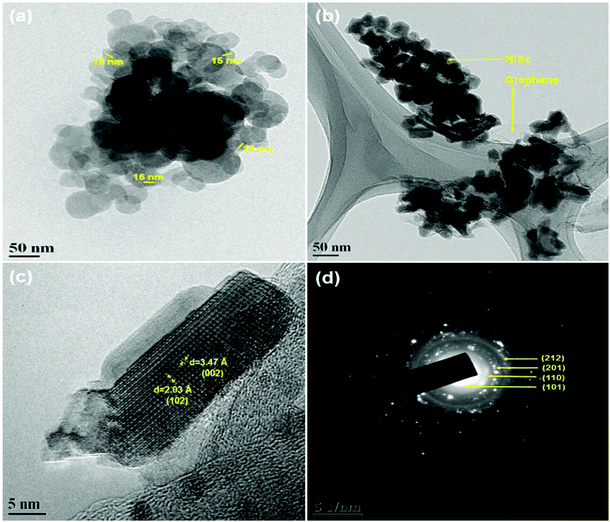 | ||
| Fig. 2 HR-TEM images of (a) NiSe nanoparticles, (b) NiSe/GN0.50 nanohybrid, and (c) NiSe/GN0.50 nanohybrid; (d) SAED image of the NiSe/GN0.50 nanohybrid. | ||
As shown in Fig. 3a, the peaks of O 1s, C 1s, Ni 2p, and Se 3d were observed for the NiSe/GN0.50 nanohybrid. The appearance of the O 1s peak is due to the inevitable oxygen adsorption on the nanohybrid surface.53 The high-resolution XPS spectrum of C 1s shows that the graphene oxide nanosheets are reduced to graphene nanosheets (Fig. 3b). The peaks at 284.8 and 284.1 eV correspond to the C–C and sp2 hybridized (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) carbon atoms of graphene nanosheets, respectively. The sp2 hybridized C
C) carbon atoms of graphene nanosheets, respectively. The sp2 hybridized C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C carbon atoms in the graphene nanosheets contribute to their high electrical conductivity.42 The low intensity peaks at 286.9 and 290.01 eV correspond to the C–O and O–C
C carbon atoms in the graphene nanosheets contribute to their high electrical conductivity.42 The low intensity peaks at 286.9 and 290.01 eV correspond to the C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively, of the reduced graphene oxide. The high-resolution Ni 2p spectrum displays the peaks corresponding to Ni 2p3/2 and Ni 2p1/2 at 855.6 and 873.1 eV, respectively, representing the Ni2+ characteristics of NiSe (Fig. 3c).54 The Se 3d5/2 and Se 3d3/2 peaks at 54.9 and 58.8 eV, respectively (Fig. 3d), refer to the Ni–Se bond of NiSe.55 These results reveal the existence of C, Ni2+ and Se2− species in the NiSe/GN0.50 nanohybrids, confirming the in situ growth of NiSe nanoparticles on the GN.
O groups, respectively, of the reduced graphene oxide. The high-resolution Ni 2p spectrum displays the peaks corresponding to Ni 2p3/2 and Ni 2p1/2 at 855.6 and 873.1 eV, respectively, representing the Ni2+ characteristics of NiSe (Fig. 3c).54 The Se 3d5/2 and Se 3d3/2 peaks at 54.9 and 58.8 eV, respectively (Fig. 3d), refer to the Ni–Se bond of NiSe.55 These results reveal the existence of C, Ni2+ and Se2− species in the NiSe/GN0.50 nanohybrids, confirming the in situ growth of NiSe nanoparticles on the GN.
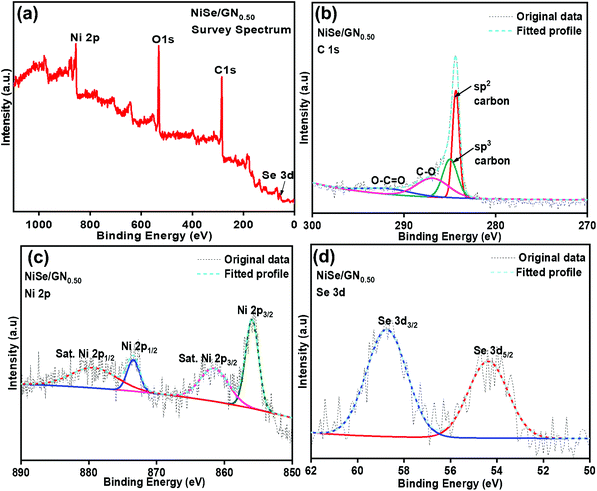 | ||
| Fig. 3 (a) XPS spectrum of the NiSe/GN0.50 nanohybrid; (b) XPS spectrum of C 1s; (c) XPS spectrum of Ni 2p; (d) XPS spectrum of Se 3d in the NiSe/GN0.50 nanohybrid. | ||
Fig. 4 shows the Raman spectra of GO, the pristine NiSe nanoparticles and the NiSe/GN nanohybrids. For the NiSe nanoparticles, their characteristic longitudinal optical (LO) phonon mode is represented by the peak at 506 cm−1. Another peak at 204 cm−1 corresponds to either the stretching (Ag) or libration (Tg) mode of the Se–Se pairs or a combination of both.54 No characteristic peaks were observed for impurities, such as elemental nickel, elemental selenium or NiO.56 The peaks representing the typical D-band (k-point phonons of A1g symmetry) and the G-band (E2g phonon of sp2 bonded carbon atoms) of graphene oxide appear at around 1340 and 1582 cm−1, respectively, with an ID/IG ratio of 0.71. The ID/IG ratio is used to obtain insight into the structural distortion in graphene oxide during the reduction process, as reported elsewhere.57,58 The slight change in the ID/IG ratio is attributed to imperfections (defects) due to the removal of oxygen functional groups on the basal and edge planes of graphene oxide. In the NiSe/GN nanohybrids, the NiSe Raman modes are suppressed due to the higher intensities of the D and G bands of graphene. Enlarged spectra of the NiSe/GN nanohybrids in the range between 150 and 800 cm−1 are included in Fig. S1.† The ID/IG ratios are 1.38, 1.43, 1.32 and 1.29 for NiSe/GN0.25, NiSe/GN0.50, NiSe/GN0.75 and NiSe/GN1.00, respectively. This demonstrates both the reduction of graphene oxide and the formation of NiSe/GN nanohybrids.59 The D band of the NiSe/GN nanohybrids is slightly blue shifted compared with that of graphene oxide. This is due to the high structural disorder arising from the decreased volume of the GN upon oxygen removal in the NiSe/GN nanohybrids.60,61 For NiSe/GN0.75 and NiSe/GN1.00, the decrease in the ID/IG ratio is due to the attenuation of the Raman peak caused by the increased defect density.62 No significant change was observed for the vibration modes of the NiSe nanoparticles, which indicates that they do not deviate from their phase after the incorporation of GN.
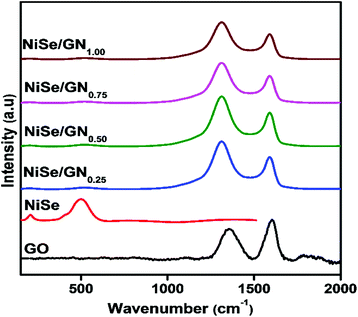 | ||
| Fig. 4 Raman spectra of graphene oxide, the NiSe nanoparticles and the NiSe/GNx (x = 0.25 to 1.00) nanohybrids. | ||
Fig. 5 shows the XRD patterns of the pristine NiSe nanoparticles and NiSe/GN nanohybrids. The diffraction peaks of the pristine NiSe nanoparticles and NiSe/GN nanohybrids match well with JCPDS card No. 18-0888 of hexagonal Ni0.85Se.63 The peaks at 21° and 42.6° representing the (002) and (101) planes of graphene further confirm the in situ growth of the NiSe nanoparticles on the GN. No impurity peaks or peak shifts were observed in the diffraction patterns, which indicates that the pristine NiSe nanoparticles and NiSe/GN nanohybrids prepared by the hydrothermal method have high phase purity and crystallinity. The grain size calculated using Scherrer's formula revealed a slight change in the grain size of the pristine NiSe nanoparticles (∼21 nm) after the addition of graphene (∼19 nm for the NiSe nanoparticles in the NiSe/GN0.50 nanohybrid). However, in the NiSe/GN0.50 nanohybrid, NiSe nanoparticles are homogenously distributed on the graphene nanosheets (Fig. 1d), which offer a more accessible surface for the electrolyte than the pristine NiSe nanoparticles (Fig. 1a). This is beneficial for enhancing the electrocatalytic activity in DSSCs.64
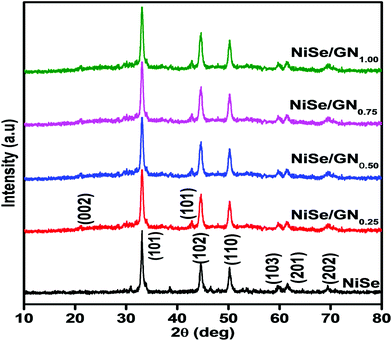 | ||
| Fig. 5 X-ray diffraction patterns of the pristine NiSe nanoparticles and NiSe/GNx (x = 0.25 to 1.00) nanohybrids. | ||
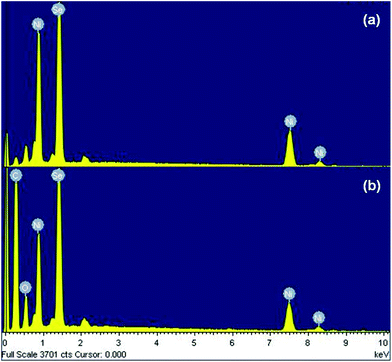
The existence of nickel and selenium species in the pristine NiSe nanoparticles as well as the carbon, nickel and selenium species in the NiSe/GN0.50 nanohybrid was confirmed from their energy dispersive X-ray (EDX) spectra (Fig. 6(a and b)). In both the pristine NiSe nanoparticles and the NiSe/GN0.50 nanohybrid, it was found that the atomic percentages of Ni and Se correspond to the stoichiometric ratio of Ni0.85Se.
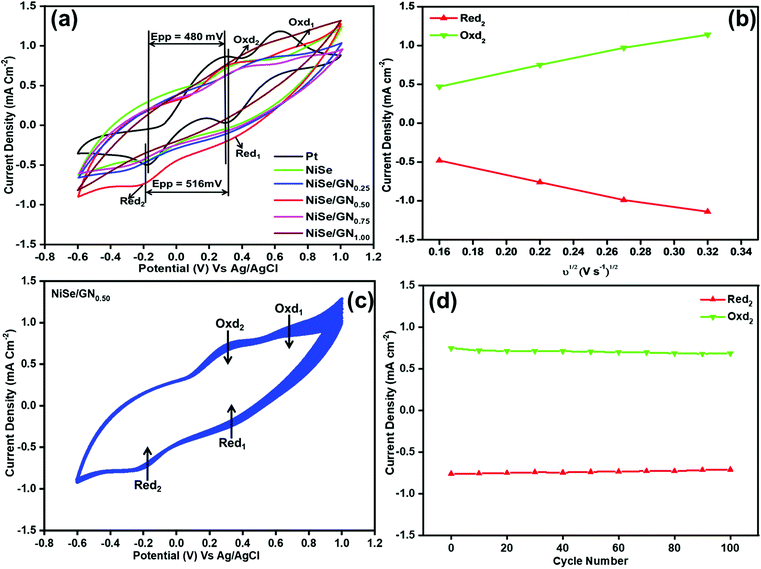
The electrocatalytic activities of Pt, NiSe and the NiSe/GN nanohybrids towards tri-iodide reduction were characterized by their typical cyclic voltammogram (CV) curves, as shown in Fig. 7a. All the CV curves demonstrate two pairs of redox responses; the left pair at the negative potential (Oxd2/Red2) corresponds to the redox reaction of I3−/I−, as represented in eqn (1), and the other pair at the right (Oxd1/Red1) corresponds to the redox reaction of I2−/I3−, as represented in eqn (2).65
3I2− + 2e− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) 2I3− 2I3− | (1) |
I3− + 2e− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) 3I− 3I− | (2) |
The current density of the catholic peak (Jpc) of the Red2 reaction and the potential difference between the anodic peak and cathodic peak (Epp) of the Oxd2/Red2 redox reaction are crucial parameters for assessing the electrocatalytic activity of CEs. The CV curves of Pt, the NiSe nanoparticles, NiSe/GN0.25, NiSe/GN0.50, NiSe/GN0.75 and NiSe/GN1.00 are shown in Fig. 7a. Their Jpc values for Red2 are −0.52, −0.49, −0.51, −0.76, −0.47 and −0.42 mA cm−2, respectively. The comparable cathodic peak of the NiSe CE to that of platinum demonstrates the catalytic properties of NiSe. However, the low peak current is due to its aggregated structure and lower number of active sites (Fig. 1a). NiSe/GN0.50 has the highest Jpc because of the well-dispersed NiSe nanoparticles on the graphene nanosheets, which increase the active surface area.
Fig. 7b shows the Jpc values of the NiSe/GN0.50 CE as a function of the square root of the scan rate. The linear correlation between them indicates that the redox reaction rate of I3− at the NiSe/GN0.50 CE is determined by the diffusion of I3− in the electrolyte, and there is no specific interaction between the NiSe/GN0.50 CE and the I3−/I−redox electrolyte.66 The diffusion coefficient (Dn) of the iodide species can be obtained from the Jpc using the Randles–Sevcik equation:67
| Jpc = K ·n3/2·A·C·Dn1/2·ν1/2 | (3) |
The Dn values of I3− for the fabricated CEs are given in Table 1, and the values are in the following order: NiSe/GN0.50 (3.20 × 10−6 cm2 S−1) > Pt (1.49 × 10−6 cm2 S−1) > NiSe/GN0.25 (1.44 × 10−6 cm2 S−1) > NiSe (1.33 × 10−6 cm2 S−1) > NiSe/GN0.75 (1.22 × 10−6 cm2 S−1) > NiSe/GN1.00 (0.97 × 10−6 cm2 S−1). The higher Dn of NiSe/GN0.50 (3.20 × 10−6 cm2 S−1) is attributed to its higher active surface area. This reveals that the NiSe/GN0.50 CE has efficient electrocatalytic activity towards I3− reduction. The aggregation of NiSe and its poor connection with GN (Fig. 1c) limit the conductivity and electrolyte diffusion at a mass ratio of NiSe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GN = 1
GN = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25. Beyond the mass ratio of 1
0.25. Beyond the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, the low density of NiSe nanoparticles and the covering of the NiSe nanoparticles with aggregated graphene reduced the available active sites. Hence, at an optimal mass ratio of 1
50, the low density of NiSe nanoparticles and the covering of the NiSe nanoparticles with aggregated graphene reduced the available active sites. Hence, at an optimal mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50, the NiSe nanoparticles act as spacers to prevent the aggregation of graphene nanosheets, thereby providing high conductivity, an effective diffusion path for the electrolyte and high electrocatalytic ability.
0.50, the NiSe nanoparticles act as spacers to prevent the aggregation of graphene nanosheets, thereby providing high conductivity, an effective diffusion path for the electrolyte and high electrocatalytic ability.
| Counter electrode | R s (Ω cm2) | R ct (Ω cm2) | D (cm2 s−1) | J 0 (mA cm−2) |
|---|---|---|---|---|
| Pt | 0.52 | 2.06 | 1.49 × 10−6 | 6.23 |
| NiSe | 2.45 | 2.46 | 1.33 × 10−6 | 5.22 |
| NiSe/GN0.25 | 0.76 | 2.37 | 1.44 × 10−6 | 5.42 |
| NiSe/GN0.50 | 0.51 | 1.92 | 3.20 × 10−6 | 6.69 |
| NiSe/GN0.75 | 1.47 | 2.59 | 1.22 × 10−6 | 4.96 |
| NiSe/GN1.00 | 1.97 | 3.48 | 0.97 × 10−6 | 3.69 |
E pp is inversely correlated to the tri-iodide reduction reaction rate and its reversibility. A smaller Epp indicates better catalytic reversibility with a higher reduction reaction rate.68 The Epp of NiSe/GN0.50 (516 mV) is close to that of Pt (480 mV), demonstrating that NiSe/GN0.50 has rapid redox kinetics and higher electrocatalytic activity than the other prepared NiSe/GN nanohybrids. The CV curves for 100 consecutive cycles at a scan rate of 50 mV S−1 for the NiSe/GN0.50 nanohybrid CE are shown in Fig. 7c. It can be observed that the peak current did not obviously change after 100 cycles (Fig. 7d), whereas it decreased significantly for the platinum CE within 10 cycles (Fig. S1†). For Pt, the Jpc (−0.55 mA cm−2) decreased to nearly 37% (−0.34 mA cm−2) of its initial value, accompanied by a slight shift in the reduction peak towards the overpotential within 10 cycles. Meanwhile, the NiSe/GN0.50 nanohybrid retained nearly ∼93% of its initial value after 100 cycles. This suggests that the NiSe/GN0.50 CE has high electrochemical stability in the I3−/I− redox electrolyte solution.
EIS of symmetric cells with two identical electrodes (CE/electrolyte/CE) of Pt, NiSe and the NiSe/GN nanohybrids was performed under dark conditions to study their charge transfer at the CE/electrolyte interface. Fig. 8 shows the Nyquist plots of the corresponding symmetrical cells with the equivalent circuit in the inset, where Rs represents the series resistance, which includes the ohmic resistance between the substrate and the electrocatalytic film and electrolyte, Rct denotes the resistance to charge transfer at the CE/electrolyte interface and Cdl is its corresponding capacitance, and Zw represents the Nernstian diffusion element. The two key parameters, Rs and Rct, obtained using EC lab software are summarized in Table 1. The NiSe/GN0.50 CE has lower Rs and Rct values than the other CEs, indicating its superior electrocatalytic activity for I3− reduction. The Rs value of the NiSe CE is larger than that of the platinum CE, which is attributed to the agglomeration and lower conductivity of the NiSe nanoparticles. After in situ growth onto graphene, the Rs values of the NiSe/GN nanohybrids decrease obviously because the graphene improves the conductivity as well as the bonding of the NiSe nanoparticles as counter electrodes on the conducting glass substrate (ITO). The relatively small Rct exhibited by NiSe/GN0.50 indicates its lower intrinsic interfacial resistance than those of the other NiSe/GN nanohybrids, which leads to a higher efficiency value. The lower values of Rs and Rct for the NiSe/GN0.50 CE than of the other CEs indicate its superior electrocatalytic activity for I3− reduction. The increase in the values of RS and Rct beyond the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 is due to excessive graphene loading, which causes the disordered aggregation that obstructs the electron transport and increases the charge transfer resistance.
0.50 is due to excessive graphene loading, which causes the disordered aggregation that obstructs the electron transport and increases the charge transfer resistance.
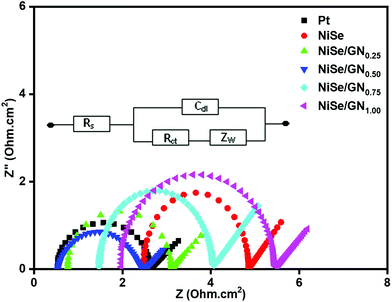 | ||
| Fig. 8 Nyquist plots of symmetrical cells fabricated with platinum, NiSe and NiSe/GNx (x = 0.25 to 1.00) nanohybrid CEs. | ||
The values of the exchange current density (Jo) corresponding to the EIS plots were calculated using the following expression69 and are listed in Table 1.
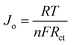 | (4) |
The Tafel polarization of the symmetrical cells of Pt, NiSe and the NiSe/GN nanohybrids was studied to further validate their electrochemical catalytic activity. Fig. 9 shows the Tafel polarization curves, displaying the logarithmic current density as a function of voltage. Normally, a Tafel polarization curve is separated into three zones as follows: (i) polarization zone at the lower potential region (|V| < 120 mV), (ii) Tafel zone at the middle potential region (120 mV < |V| < 400 mV) and (iii) a diffusion zone at the higher frequency region (|V| > 400 mV). The slope for the anodic or cathodic branch in the Tafel zone represents the exchange current density (Jo). In general, a larger Jo indicates a larger number of electrons transmitting through the CE/electrolyte interface, which indicates better electrocatalytic performance of the corresponding CE.70 The cathodic or anodic branches with steeper slopes indicate higher exchange current density (Jo). Accordingly, Jo of these CEs shows the tendency of NiSe/GN0.50 > Pt > NiSe/GN0.25 > NiSe > NiSe/GN0.75 > NiSe/GN1.00. This is highly consistent with the tendency of Jo obtained from the EIS (Table 1). The Jo value obtained from eqn (4) for the NiSe/GN0.50 CE (6.69 mA cm−2) is slightly higher than that obtained for the platinum CE (6.23 mA cm−2). This can be attributed to the NiSe nanoparticles filled within the graphene wrinkles (Fig. 1d), which create a smooth surface on the entire NiSe/GN0.50 nanohybrid. This structure can improve the binding of NiSe/GN0.50 to the conductive substrate, which further results in improved electron transport at the CE/electrolyte and CE/conductive substrate interfaces; this facilitates improved electrocatalytic performance.
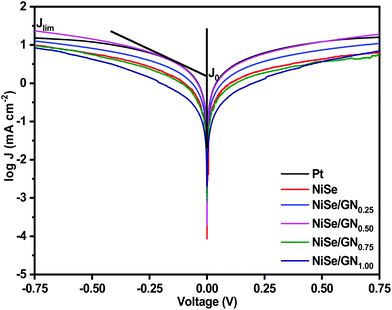 | ||
| Fig. 9 Tafel polarization curves of the symmetrical cells fabricated with platinum, NiSe and NiSe/GNx (x = 0.25 to 1.00) nanohybrid CEs. | ||
Moreover, the intersection of the cathodic branch with the y-axis is a limiting diffusion current (Jlim), and it depends directly on the Dn of tri-iodide, according to eqn (5):71
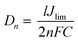 | (5) |
The photocurrent density–voltage (J–V) curves of the DSSCs based on Pt, NiSe and NiSe/GN0.50 are shown in Fig. 10. Their corresponding short-circuit current density (Jsc), open circuit voltage (Voc), fill factor (FF) and efficiency (η) are summarized in Table 2. The FF and photoconversion efficiency (η) were obtained using the following equations:51,52
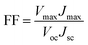 | (6) |
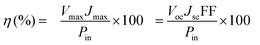 | (7) |
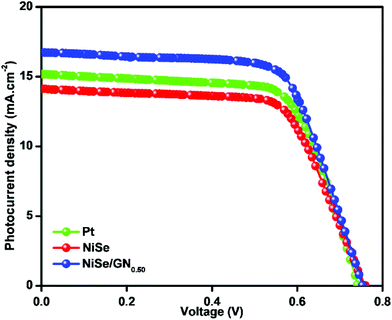 | ||
| Fig. 10 Photocurrent density–voltage (J–V) curves of the DSSCs based on (a) Pt, (b) NiSe and (c) NiSe/GN0.50 nanohybrid CEs. | ||
| Counter electrode | V oc (V) | J sc (mA cm−2) | FF | η (%) |
|---|---|---|---|---|
| Pt (Std.) | 0.74 | 15.18 | 0.684 | 7.68 |
| NiSe | 0.76 | 14.12 | 0.669 | 7.18 |
| NiSe/GN0.50 | 0.75 | 16.73 | 0.687 | 8.62 |
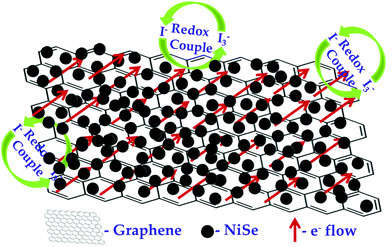
The NiSe/GN0.50 CE exhibited superior photovoltaic performance compared to the Pt and pristine NiSe CEs. The relatively poor performance of the NiSe electrode can be attributed to severe aggregation of the NiSe nanoparticles (as observed in Fig. 1a) and poor connections between the nanoparticles, which decrease the electrocatalytic active sites and electrical conductivity. This results in lower FF and η values. The DSSC fabricated with the NiSe/GN0.50 nanohybrid as a CE has a higher efficiency (8.62%) than those fabricated with platinum (7.68%) and NiSe (7.18%) as CEs. This is because of the high electrical conductivity offered by graphene and the exceptional catalytic activity of NiSe towards the tri-iodide reduction, as illustrated in Fig. 11. NiSe/GN0.50 synergistically offered an increased number of NiSe electrocatalytic active sites and faster electron transfer pathways of graphene, which enhanced the I3− reduction and photo-generated electron transfer at the CE/electrolyte interface.72 NiSe/GN0.50 was observed to exhibit a 12% improvement in the photo-conversion efficiency over the standard Pt (Table 3), which is much higher than those of other reported nanohybrids.
| Counter electrode | V oc (V) | J sc (mA cm−2) | FF | Cell efficiency, η (%) | % of improvement in PCE | Ref. | |
|---|---|---|---|---|---|---|---|
| Std. Pt | Prepared CE | ||||||
| NiSe2/rGO | 0.73 | 15.82 | 0.610 | 6.82 | 6.94 | 2 | 21 |
| Ni0.85Se nano-spheres/rGO | 0.76 | 13.20 | 0.660 | 7.54 | 7.82 | 4 | 23 |
| NiSe2/6 wt% GN | 0.75 | 16.32 | 0.61 | 7.28 | 7.47 | 3 | 31 |
| NiSe/GN 0.50 | 0.75 | 16.73 | 0.687 | 7.68 | 8.62 | 12 | This work |
4. Conclusion
NiSe nanoparticles were in situ grown on graphene nanosheets by a facile hydrothermal process. The formation mechanisms and corresponding morphologies of the NiSe/GNx nanohybrids relative to the different mass ratios of graphene were studied. The mass ratio of NiSe to graphene (x = 0.25 to 1.00) was controlled to achieve effective electrolyte diffusion channels and high electrocatalytic activity. Extensive electrochemical analysis showed that the NiSe/GN0.50 nanohybrid exhibited enhanced photo-generated electron transfer at the CE/electrolyte interface, with excellent electrocatalytic ability for tri-iodide reduction. These excellent abilities are mainly due to structural configurations, namely the many active sites, large specific surface area and faster electron transport of the NiSe/GN0.50 nanohybrid. This, in turn, endowed the NiSe/GN0.50 nanohybrid with higher photoconversion efficiency than Pt. The excellent electrochemical stability and photovoltaic performance of the NiSe/GN0.50 nanohybrid counter electrode have great potential for the substitution of Pt counter electrodes in DSSCs.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Dr AS gratefully acknowledges the CSIR, New Delhi (Ref. No. 01/2810/14/EMR-II, dt. 26/04/2017) and UGC, New Delhi (BSR Mid-Career Award No. F.19-214/2018) for their financial support. Mr MV is grateful to the DST, New Delhi for providing a DST-Inspire Award (IF160290) fellowship and the Indo-US Science and Technology Forum (IUSSTF), DST, New Delhi for a Bhaskara Advanced Solar Energy (BASE) Internship award (IUSSTF BASE Internships 2018/12/Vignesh M, dt. 09/04/2018).References
- S. Casaluci, M. Gemmi, V. Pellegrini, A. Di Carlo and F. Bonaccorso, Nanoscale, 2016, 8, 5368–5378 RSC.
- M. Rameez, K. Saranya, A. Subramania, N. Sivasankar and S. Mallick, Appl. Phys. A, 2016, 122, 71 CrossRef.
- H. He, C. Zhang, T. Liu, Y. Cao, N. Wang and Z. Guo, J. Mater. Chem. A, 2016, 4, 9362–9369 RSC.
- L. Yang, X. Wang, X. Mai, T. Wang, C. Wang, X. Li, V. Murugadoss, Q. Shao, S. Angaiah and Z. Guo, J. Colloid Interface Sci., 2019, 534, 459–468 CrossRef CAS PubMed.
- S. Angaiah, V. Murugadoss, S. Arunachalam, P. Panneerselvam and S. Krishnan, Eng. Sci., 2018, 4, 44–51 CrossRef.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef.
- K. Saranya, N. Sivasankar and A. Subramania, RSC Adv., 2014, 4, 36226–36233 RSC.
- T. Liu, J. Hou, B. Wang, F. Bai, H. Chen, L. Gao, Y. Cao, H. He, J. Wang, N. Wang, G. Cao and Z. Guo, J. Mater. Chem. A, 2016, 4, 10794–10800 RSC.
- K. Saranya, A. Subramania and N. Sivasankar, RSC Adv., 2015, 5, 43611–43619 RSC.
- Y. Wang, J. Li, J. Li, C. Duan, H. Zhang, H. Jian, L. He, G. Tao, C. Yan and T. Jiu, Sol. Energy, 2018, 160, 353–359 CrossRef CAS.
- N. Singh, V. Murugadoss, S. Nemala, S. Mallick and S. Angaiah, Sol. Energy, 2018, 171, 571–579 CrossRef CAS.
- K. Saranya, M. Rameez and A. Subramania, Eur. Polym. J., 2015, 66, 207–227 CrossRef CAS.
- K. Saranya, A. Subramania, N. Sivasankar and S. Mallick, Mater. Res. Bull., 2016, 75, 83–90 CrossRef CAS.
- R. Ramachandran, S. Felix, M. Saranya, C. Santhosh, V. Velmurugan, B. P. C. Ragupathy, S. K. Jeong and A. N. Grace, IEEE Trans. Nanotechnol., 2013, 12, 985–990 CAS.
- F. Gong, H. Wang, X. Xu, G. Zhou and Z.-S. Wang, J. Am. Chem. Soc., 2012, 134, 10953–10958 CrossRef CAS PubMed.
- Y.-Y. Yao, H.-J. Chao, T.-H. Chou, S. H. Chang, C.-G. Wu, Y.-C. Ling and J.-Y. Chang, Sol. Energy, 2016, 137, 401–408 CrossRef CAS.
- P. Yang and Q. Tang, Electrochim. Acta, 2016, 188, 560–565 CrossRef CAS.
- J. Jia, J. Wu, Y. Tu, J. Huo, M. Zheng and J. Lin, J. Alloys Compd., 2015, 640, 29–33 CrossRef CAS.
- J. Dong, J. Wu, J. Jia, J. Ge, Q. Bao, C. Wang and L. Fan, Appl. Surf. Sci., 2017, 401, 1–6 CrossRef CAS.
- Y. Duan, Q. Tang, B. He, R. Li and L. Yu, Nanoscale, 2014, 6, 12601–12608 RSC.
- X. Zhang, T.-z. Jing, S.-q. Guo, G.-d. Gao and L. Liu, RSC Adv., 2014, 4, 50312–50317 RSC.
- J. Dong, J. Wu, J. Jia, L. Fan and J. Lin, J. Colloid Interface Sci., 2017, 498, 217–222 CrossRef CAS PubMed.
- X. Zhang, Y. Yang, S. Guo, F. Hu and L. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 8457–8464 CrossRef CAS PubMed.
- C. Wang, V. Murugadoss, J. Kong, Z. He, X. Mai, Q. Shao, Y. Chen, L. Guo, C. Liu, S. Angaiah and Z. Guo, Carbon, 2018, 140, 696–733 CrossRef CAS.
- X. Pan, Q. Du, Y. Zhou, L. Liu, G. Xu and C. Yan, J. Nanosci. Nanotechnol., 2018, 18, 7231–7240 CrossRef CAS.
- X. Li, W. Zhao, R. Yin, X. Huang and L. Qian, Eng. Sci., 2018, 3, 89–95 CrossRef.
- B. Yang, X. Zuo, P. Chen, L. Zhou, X. Yang, H. Zhang, G. Li, M. Wu, Y. Ma, S. Jin and X. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 137–143 CrossRef CAS PubMed.
- B. Kirubasankar, V. Murugadoss and S. Angaiah, RSC Adv., 2017, 7, 5853–5862 RSC.
- L. Wei, K. Lozano and Y. Mao, Eng. Sci., 2018, 3, 62–66 CrossRef.
- C. Hou, Z. Tai, L. Zhao, Y. Zhai, Y. Hou, Y. Fan, F. Dang, J. Wang and H. Liu, J. Mater. Chem. A, 2018, 6, 9723–9736 RSC.
- Z. Yue, G. Wu, X. Chen, Y. Han, L. Liu and Q. Zhou, Mater. Lett., 2017, 192, 84–87 CrossRef CAS.
- S. Guo, S. Zhang, L. Wu and S. Sun, Angew. Chem., Int. Ed., 2012, 51, 11770–11773 CrossRef CAS.
- V.-D. Dao, N. T. Q. Hoa, L. L. Larina, J.-K. Lee and H.-S. Choi, Nanoscale, 2013, 5, 12237–12244 RSC.
- C. Zhang, J. Sha, H. Fei, M. Liu, S. Yazdi, J. Zhang, Q. Zhong, X. Zou, N. Zhao, H. Yu, Z. Jiang, E. Ringe, B. I. Yakobson, J. Dong, D. Chen and J. M. Tour, ACS Nano, 2017, 11, 6930–6941 CrossRef CAS PubMed.
- H. Wang, K. Sun, F. Tao, D. J. Stacchiola and Y. H. Hu, Angew. Chem., 2013, 125, 9380–9384 CrossRef.
- S. Guo and S. Sun, J. Am. Chem. Soc., 2012, 134, 2492–2495 CrossRef CAS PubMed.
- X. Li, L. Zhang, M. Huang, S. Wang, X. Li and H. Zhu, J. Mater. Chem. A, 2016, 4, 14789–14795 RSC.
- G. Wang, J. Zhang, S. Kuang, S. Liu and S. Zhuo, J. Power Sources, 2014, 269, 473–478 CrossRef CAS.
- H. Wu, Y. Wang, L. Zhang, Z. Chen, C. Wang and S. Fan, J. Alloys Compd., 2018, 745, 222–227 CrossRef CAS.
- Q. Jiang, R. Chen, H. Chen, J. Jiang, X. Yang, Y. Ju, R. Ji and Y. Zhang, J. Mater. Sci., 2018, 53, 7672–7682 CrossRef CAS.
- X. Zhang, J. Bai, M. Zhen and L. Liu, RSC Adv., 2016, 6, 89614–89620 RSC.
- V. Murugadoss, N. Wang, S. Tadakamalla, B. Wang, Z. Guo and S. Angaiah, J. Mater. Chem. A, 2017, 5, 14583–14594 RSC.
- M. H. Yeh, L. Y. Lin, L. Y. Chang, Y. A. Leu, W. Y. Cheng, J. J. Lin and K. C. Ho, ChemPhysChem, 2014, 15, 1175–1181 CrossRef CAS.
- Y. Xu, H. Bai, G. Lu, C. Li and G. Shi, J. Am. Chem. Soc., 2008, 130, 5856–5857 CrossRef CAS.
- B. Kirubasankar, V. Murugadoss, J. Lin, T. Ding, M. Dong, H. Liu, J. Zhang, T. Li, N. Wang, Z. Guo and S. Angaiah, Nanoscale, 2018, 10, 20414–20425 RSC.
- H. Bisht, H. T. Eun, A. Mehrtens and M. A. Aegerter, Thin Solid Films, 1999, 351, 109–114 CrossRef CAS.
- Z. Salam, E. Vijayakumar, A. Subramania, N. Sivasankar and S. Mallick, Sol. Energy Mater. Sol. Cells, 2015, 143, 250–259 CrossRef CAS.
- X. Duan, Z. Gao, J. Chang, D. Wu, P. Ma, J. He, F. Xu, S. Gao and K. Jiang, Electrochim. Acta, 2013, 114, 173–179 CrossRef CAS.
- T. Liu, K. Yu, L. Gao, H. Chen, N. Wang, L. Hao, T. Li, H. He and Z. Guo, J. Mater. Chem. A, 2017, 5, 17848–17855 RSC.
- P. Panneerselvam, V. Murugadoss, V. Elayappan, N. Lu, Z. Guo and S. Angaiah, ES Energy Environ., 2018, 1, 99–105 Search PubMed.
- A. Subramania, E. Vijayakumar, N. Sivasankar, S. A. R. Priya and K.-J. Kim, Ionics, 2013, 19, 1649 CrossRef CAS.
- V. Murugadoss, S. Arunachalam, V. Elayappan and S. Angaiah, Ionics, 2018, 24, 4071–4080 CrossRef CAS.
- C. Mattevi, G. Eda, S. Agnoli, S. Miller, A. K. Mkhoyan, O. Celik, D. Mastrogiovanni, G. Granozzi, E. Garfunkel and M. Chhowalla, Adv. Funct. Mater., 2009, 19, 2577–2583 CrossRef CAS.
- W. Shi, X. Zhang and G. Che, Int. J. Hydrogen Energy, 2013, 38, 7037–7045 CrossRef CAS.
- H. Chen, S. Chen, M. Fan, C. Li, D. Chen, G. Tian and K. Shu, J. Mater. Chem. A, 2015, 3, 23653–23659 RSC.
- A. T. Swesi, J. Masud and M. Nath, Energy Environ. Sci., 2016, 9, 1771–1782 RSC.
- S. Muhammad Hafiz, R. Ritikos, T. J. Whitcher, N. Md. Razib, D. C. S. Bien, N. Chanlek, H. Nakajima, T. Saisopa, P. Songsiriritthigul, N. M. Huang and S. A. Rahman, Sens. Actuators, B, 2014, 193, 692–700 CrossRef.
- H.-H. Huang, K. K. H. De Silva, G. R. A. Kumara and M. Yoshimura, Sci. Rep., 2018, 8, 6849 CrossRef.
- J. Shen, Y. Hu, M. Shi, N. Li, H. Ma and M. Ye, J. Phys. Chem. C, 2010, 114, 1498–1503 CrossRef CAS.
- R. P. Vidano, D. B. Fischbach, L. J. Willis and T. M. Loehr, Solid State Commun., 1981, 39, 341–344 CrossRef CAS.
- M. Zhu, X. Li, W. Liu and Y. Cui, J. Power Sources, 2014, 262, 349–355 CrossRef CAS.
- J.-F. Zhao, J.-M. Song, C.-C. Liu, B.-H. Liu, H.-L. Niu, C.-J. Mao, S.-Y. Zhang, Y.-H. Shen and Z.-P. Zhang, CrystEngComm, 2011, 13, 5681–5684 RSC.
- X. Zhang, S. Guo, M. Zhen, G. Gao and L. Liu, J. Electrochem. Soc., 2015, 162, H774–H779 CrossRef CAS.
- S. Das, P. Sudhagar, S. Nagarajan, E. Ito, S. Lee, Y. Kang and W. Choi, Carbon, 2012, 50, 4815–4821 CrossRef CAS.
- M.-S. Fan, C.-P. Lee, C.-T. Li, Y.-J. Huang, R. Vittal and K.-C. Ho, Electrochim. Acta, 2016, 211, 164–172 CrossRef CAS.
- G. Yue, J.-Y. Lin, S.-Y. Tai, Y. Xiao and J. Wu, Electrochim. Acta, 2012, 85, 162–168 CrossRef CAS.
- C.-J. Liu, S.-Y. Tai, S.-W. Chou, Y.-C. Yu, K.-D. Chang, S. Wang, F. Chien, J.-Y. Lin and T.-W. Lin, J. Mater. Chem., 2012, 22, 21057–21064 RSC.
- V.-D. Dao, I.-K. Jin and H.-S. Choi, Electrochim. Acta, 2016, 201, 1–7 CrossRef CAS.
- C.-K. Cheng, C.-H. Lin, H.-C. Wu, C.-C. M. Ma, T.-K. Yeh, H.-Y. Chou, C.-H. Tsai and C.-K. Hsieh, Nanoscale Res. Lett., 2016, 11, 1–9 CrossRef CAS PubMed.
- Y.-J. Huang, M.-S. Fan, C.-T. Li, C.-P. Lee, T.-Y. Chen, R. Vittal and K.-C. Ho, Electrochim. Acta, 2016, 211, 794–803 CrossRef CAS.
- V. Elayappan, V. Murugadoss, S. Angaiah, Z. Fei and P. Dyson, J. Appl. Polym. Sci., 2015, 132, 42777 CrossRef.
- X. Miao, K. Pan, G. Wang, Y. Liao, L. Wang, W. Zhou, B. Jiang, Q. Pan and G. Tian, Chem. – Eur. J., 2014, 20, 474–482 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nr07060e |
| This journal is © The Royal Society of Chemistry 2019 |