Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Leachate emissions of short- and long-chain per- and polyfluoralkyl substances (PFASs) from various Norwegian landfills†
Heidi
Knutsen
 *a,
Trond
Mæhlum
*a,
Trond
Mæhlum
 b,
Ketil
Haarstad
b,
Gøril Aasen
Slinde
a and
Hans Peter H.
Arp
b,
Ketil
Haarstad
b,
Gøril Aasen
Slinde
a and
Hans Peter H.
Arp
 *ac
*ac
aNorwegian Geotechnical Institute (NGI), P.O. Box 3930 Ullevål Stadion, N-0806 Oslo, Norway. E-mail: hkn@ngi.no; hpa@ngi.no; Tel: +47 478 51 733, +47 950 02 667
bNorwegian Institute of Bioeconomy Research (NIBIO), P.O. Box 115, N-1431 Ås, Norway
cNorwegian University of Science and Technology (NTNU), NO-7491 Trondheim, Norway
First published on 9th August 2019
Abstract
Restrictions on the use of long-chain per- and polyfluoralkyl substances (PFASs) has led to substitutions with short-chain PFASs. This study investigated the presence of four short-chain PFASs and twenty-four long-chain PFASs in leachate and sediment from ten Norwegian landfills, including one site in Svalbard, to assess whether short-chain PFASs are more dominant in leachate. PFASs were detected in all sites. Short-chain PFASs were major contributors to the total PFAS leachate concentrations in six of ten landfills, though not in Svalbard. In sediment, long-chain PFASs such as perfluorooctanesulfonate (PFOS) and PFOS-precursors were dominant. Short-chain PFAS leachate concentrations ranged from 68 to 6800 ng L−1 (mean: 980 ± 1800; median: 360 ng L−1), whereas long-chain concentrations ranged from 140 to 2900 ng L−1 (mean: 530 ± 730; median: 290 ng L−1). Sediment concentrations, which contained mainly long-chain PFASs, ranged from 8.5 to 120 μg kg−1 (mean: 47 ± 36; median: 41 μg kg−1). National 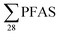 release from Norwegian landfills to the environment was estimated to be 17 ± 29 kg per year (median: 6.3 kg per year), which is in the same range as national emissions from the US, China and Germany after normalizing the data to a per capita emission factor (3.2 ± 5.5 mg per person per year). Results from this study are compared with previous and current studies in other countries, indicating a general trend that short-chain PFASs are dominating over long-chain PFASs in landfill leachate emissions.
release from Norwegian landfills to the environment was estimated to be 17 ± 29 kg per year (median: 6.3 kg per year), which is in the same range as national emissions from the US, China and Germany after normalizing the data to a per capita emission factor (3.2 ± 5.5 mg per person per year). Results from this study are compared with previous and current studies in other countries, indicating a general trend that short-chain PFASs are dominating over long-chain PFASs in landfill leachate emissions.
1. Introduction
Per- and polyfluoroalkyl substances (PFASs) in the environment are of concern because of their general persistence in combination with either potential aquatic mobility, long range transport, bioaccumulation, toxicity, or some combination thereof.1 PFASs have been used in a variety of industrial processes and in commercial products over the past 60 years,2,3 due to their unique chemical and physical properties, such as their thermal and chemical stability as well as their hydrophobic/lipophobic behavior.2,4–6Regulations of PFAS in recent years3,7,8 have led to restrictions of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA) and other so-called “long-chain” PFASs.9,10 This has resulted in these being replaced on the market with alternatives including so-called “short-chain” PFASs, such as perfluorobutanesulfonate (PFBS), which are considered to be less bioaccumulative.9–11 Short-chain PFASs are defined by Buck et al. (2011)10 and the Organisation for Economic Co-operation and Development (OECD) as perfluoroalkyl carboxylic acids (PFCAs) with a chain length of <C7, and <C6 for perfluoroalkane sulfonates (PFSAs).9,10 Though short-chain PFASs are less bioaccumulative, they are of concern because of their environmental persistence and aquatic mobility,1 and because currently little is known about their (eco)toxicity.12 As such, it is important to investigate to what extent these short-chain PFASs are being emitted to and occurring in the environment.
Landfill leachate from municipal solid waste are potential emission hotspots for PFASs,13–16 though concentrations vary widely.13–15,17,18 There are few available data on PFAS occurrence in Norwegian landfill leachates;19,20 though the first available data, comprising data from ten landfills from 2003–2007 based on non-target screening, indicated short-chain PFASs were infrequently detected (or analysed for (ref. 21)), and long-chain PFASs clearly dominated.20 Studies from North American landfills from 2006 to 2009 reported that specific short-chain PFASs were amongst the most dominant in landfills,13,15 and this has also been reported in more recent studies from elsewhere such as China,22 Germany,17 Sweden,23 and Spain.24 It is therefore anticipated based on this shift of usage and data from other countries, that short-chain PFASs are emitted to a greater extent than long-chain PFASs from Norwegian landfill leachate.
The aims of this study are three-fold. The first is to characterize the aqueous (leachate) concentration and composition of PFASs being emitted from a diverse array of Norwegian landfills, alongside PFAS composition in leachate sediment. The second aim is, based on this data, to test the hypothesis that short-chain PFASs dominate landfill emissions over long-chain PFASs. The third aim is to compare these results with other regions, through the derived national per capita emission factors of short and long-chain PFASs. For this study, leachate and sediment was collected from ten diverse Norwegian landfills, including one in Svalbard, and analysed for PFASs. The collected data is further discussed in terms of the distribution of PFASs between collected sediment and water leachate, the influence of leachate characteristics, including pH, electrical conductivity (EC), leachate flow rates, dissolved organic carbon (DOC) and meteorological data (24 h and 2 week precipitation).
2. Methods
2.1. Site descriptions
Ten diverse Norwegian landfills, including one site in Svalbard (Table S1, (ESI†)), receiving primarily municipal solid waste (MSW) and in some cases industrial waste and contaminated soil and sewage sludge, were included. Yearly leachate volumes for each landfill are given in Table S2.† Due to confidentiality reasons, the identities of the landfills are anonymized. Some of the landfills were established in a period where there was few or no requirements for liners, leachate drainage nor control of the influence of storm water and groundwater dilution of the raw leachate. However, it is our opinion that the selected landfills represent typical Norwegian, and that unpolluted storm water and groundwater have relatively limited influence on the sampled raw leachate. A description of landfills, the sampling points, their hydrology and whether dilution by storm water/groundwater can be a consideration is presented in the ESI.†2.2. Sampling
As an aim of this study was to characterize PFAS landfill emissions and sediment concentrations from all of Norway, available resources were used to sample from many locations rather than obtaining replicates or time series in individual locations. Sampling at each landfill was conducted mainly between April to June 2018 by landfill operators following sampling protocols and equipment provided by the authors. Sampling dates are given in Table S2.† Sampling of leachate (0.5 L, HDPE bottles, Eurofins Environment, Norway) was conducted as close as possible to where leachate leaves the landfill (either a retention pond, borehole, pumping station, stream, or underground culvert/leachate pipe accessed by a manhole; see Table S2, ESI†), in order to be representative of landfill emissions. Not all samples are considered raw leachate, but raw leachate diluted by storm water and groundwater (see the ESI†).Sediment samples (500 g, Rilsan bags, Eurofins Environment, Norway) were collected from sedimentation ponds, if present, or by sandtraps in the underground culverts. The samples were placed in coolers with cooling elements and bubble wrap and shipped generally overnight and stored cold (ca. 4 °C) until analysis. To compliment this data, existing, recent PFAS data was also included when possible, provided by the site owners. These were obtained for four of the landfills from the same sampling points using similar protocols and analysis laboratories as in this study.
2.3. Analyses
The pH and EC were measured following method NS-EN ISO 10523:2012 and NS-ISO 7888:1985 = EN 27888:1993, respectively (Table S2†).28 PFASs were quantified in leachate: short-chain PFCAs (PFBA, PFPeA and PFHxA), a short-chain PFSA (PFBS), long-chain PFCAs (PFHpA, HPFHpA, PFOA, PFNA, PFDA, PF-3,7-DMOA, PFUnDA, PFDoA, PFTrA, PFTA and PFHxDA), long-chain PFSAs (PFHxS, PFHpS, PFOS and PFDS), as well as fluorotelomer sulfonates (FTSAs: 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA, 6
2 FTSA, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA and 8
2 FTSA and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA), a fluorotelomer alcohol (FTOH: 8
2 FTSA), a fluorotelomer alcohol (FTOH: 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTOH) and PFOS-precursors (perfluorosulfonamides (FOSAs): FOSA, EtFOSA, MeFOSA and perfluoroalkylsulfonamide alcohols (FOSEs): EtFOSE and MeFOSE). In Table S3† the full names, PFAS class, and molecular formula for these are provided. In sediment, perfluorooctane sulfonamido acetic acids (FOSAAs) were also analysed (EtFOSAA, MeFOSAA and FOSAA). All sediment analyses and most leachate analyses (all except for leachate from landfill V and VIII), including the previous data provided by site owners, were carried out at the accredited laboratory Eurofins Environment Testing AS (sediment: centrifugation in methanol, followed ENVI-carb clean-up and UPLC/MS/MS analysis; leachate: analyzed by SPE-methanol elutriation followed by UPLC/MS/MS analysis, instrument: Agilent 6495 MS/MS. Column: Waters BEH 50 × 2.1 mm. Mobile phase: ammonium acetate (aq) and methanol). Due to logistical reasons, including contractual obligations, leachate from Landfill V and VIII were analyzed by the accredited ALS Laboratory Group Norway AS (method EPA537: SPE-methanol elutriation followed by LC/MS/MS analysis), which did not analyse PFBS, PFHpS, PFDS, PFBA, PFPeA, PFHpA, PFHxDA, 4
2 FTOH) and PFOS-precursors (perfluorosulfonamides (FOSAs): FOSA, EtFOSA, MeFOSA and perfluoroalkylsulfonamide alcohols (FOSEs): EtFOSE and MeFOSE). In Table S3† the full names, PFAS class, and molecular formula for these are provided. In sediment, perfluorooctane sulfonamido acetic acids (FOSAAs) were also analysed (EtFOSAA, MeFOSAA and FOSAA). All sediment analyses and most leachate analyses (all except for leachate from landfill V and VIII), including the previous data provided by site owners, were carried out at the accredited laboratory Eurofins Environment Testing AS (sediment: centrifugation in methanol, followed ENVI-carb clean-up and UPLC/MS/MS analysis; leachate: analyzed by SPE-methanol elutriation followed by UPLC/MS/MS analysis, instrument: Agilent 6495 MS/MS. Column: Waters BEH 50 × 2.1 mm. Mobile phase: ammonium acetate (aq) and methanol). Due to logistical reasons, including contractual obligations, leachate from Landfill V and VIII were analyzed by the accredited ALS Laboratory Group Norway AS (method EPA537: SPE-methanol elutriation followed by LC/MS/MS analysis), which did not analyse PFBS, PFHpS, PFDS, PFBA, PFPeA, PFHpA, PFHxDA, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA, HPFHpA and PF-3,7-DMOA.
2 FTSA, HPFHpA and PF-3,7-DMOA.
2.4. Statistical analysis
Statistical analysis was performed with Statistica v. 13.1 (©1984–2016 by Statsoft, Tulsa, USA). All concentrations were Box–Cox transformed prior to statistical analysis. Physical–chemical properties (pH, DOC, EC and precipitation) were not transformed. Pearson product moment was used for testing for significant correlations. The significance level was set at p = 0.05. Principal component analysis was performed exploratively to check for correlations.3. Results and discussion
3.1 Leachate concentrations
PFASs were detected in all landfill leachate samples. The total sum of 28 PFASs per landfill ranged from 320 to 11
per landfill ranged from 320 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 (mean ± standard deviation: 1700 ± 2900; median: 630 ng L−1), see Table 1 (data for individual PFAS is presented in Tables S4 and S5†). For the four short-chain PFASs these were from 68 to 6800 ng L−1 (mean: 980 ± 1800; median: 360 ng L−1), whereas the 15 long-chain PFASs ranged from 140 to 2900 (mean: 530 ± 730; median: 290 ng L−1). Hence, a substantial variation in leachate concentrations, more than two orders of magnitude, can be found at these diverse Norwegian landfills.
000 ng L−1 (mean ± standard deviation: 1700 ± 2900; median: 630 ng L−1), see Table 1 (data for individual PFAS is presented in Tables S4 and S5†). For the four short-chain PFASs these were from 68 to 6800 ng L−1 (mean: 980 ± 1800; median: 360 ng L−1), whereas the 15 long-chain PFASs ranged from 140 to 2900 (mean: 530 ± 730; median: 290 ng L−1). Hence, a substantial variation in leachate concentrations, more than two orders of magnitude, can be found at these diverse Norwegian landfills.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA and 8
2 FTSA and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTOH were not detected > LOQ in any of the samples. n.a. = not analysed. I-S to III-S and VI-S = supplementary PFAS results obtained from the landfill operators (site-owner measurements) at the same respective sampling points as in this study
2 FTOH were not detected > LOQ in any of the samples. n.a. = not analysed. I-S to III-S and VI-S = supplementary PFAS results obtained from the landfill operators (site-owner measurements) at the same respective sampling points as in this study
| ID | Year, month (n) | ∑Short-chain PFSAsa (<C6) | ∑Long-chain PFSAsb (≥C6) | ∑Short-chain PFCAsc (<C7) | ∑Long-chain PFCAsd (≥C7) | ∑Short-chain PFAS | ∑Long-chain PFAS | Short![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) long-chain ratio long-chain ratio |
PFOS | PFOA | ∑PFAS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a PFBS. b PFHxS, PFHpS and PFOS. c PFBA, PFPeA and PFHxA. d PFHpA, HPFHpA, PFOA, PFNA, PFDA, PFUnDA and PFDoA. e Sample analysis by ALS (different from others in this table); PFBS was not analysed in these samples, which could explain their relatively lower ∑short-chain concentrations. | |||||||||||
| I | 2018, Jan. (1) | 30 | 51 | 140 | 85 | 170 | 140 | 1.3 | 29 | 66 | 320 |
| I-S | 2017, Apr., June, Aug., Nov., 2018, May (5) | 24 ± 10 | 150 ± 210 | 160 ± 24 | 110 ± 17 | 180 ± 26 | 260 ± 210 | 0.70 ± 0.40 | 100 ± 140 | 69 ± 10 | 470 ± 240 |
| II | 2018, April (1) | 260 | 90 | 240 | 140 | 500 | 230 | 2.1 | 70 | 94 | 780 |
| II-S | 2016, April (1) | 320 | 110 | 260 | 170 | 580 | 280 | 2.1 | 74 | 100 | 900 |
| III | 2018, April (1) | 100 | 110 | 250 | 180 | 350 | 290 | 1.2 | 70 | 120 | 670 |
| III-S | 2018, April (1) | 110 | 110 | 260 | 200 | 370 | 310 | 1.2 | 65 | 130 | 740 |
| IV | 2018, April (1) | 47 | 26 | 380 | 110 | 430 | 140 | 3.1 | 15 | 72 | 590 |
| Ve | 2018, May (1) | n.a. | 87 | 68e | 98 | 68e | 190 | 0.37 | 51 | 98 | 432 |
| VI | 2018, April (1) | 4200 | 200 | 2600 | 2700 | 6800 | 2900 | 2.3 | 120 | 1800 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| VI-S | 2017, Sept. (1) | 660 | 73 | 490 | 420 | 1200 | 490 | 2.3 | 36 | 270 | 2500 |
| VII | 2018, May (1) | 850 | 220 | 1900 | 1000 | 2800 | 1200 | 2.3 | 65 | 660 | 4200 |
| VIIIe | 2018, May (1) | n.a. | 98 | 95e | 240 | 95e | 340 | 0.28 | 59 | 200 | 590 |
| IX | 2018, May (1) | 7.3 | 71 | 130 | 210 | 130 | 280 | 0.47 | 36 | 170 | 420 |
| X | 2018, June (1) | 7.2 | 200 | 130 | 200 | 130 | 400 | 0.33 | 160 | 120 | 540 |
| Median | 2016–2018 (18) | 105 | 100 | 250 | 190 | 360 | 290 | 1.3 | 65 | 120 | 630 |
| Mean ± SD | 550 ± 1200 | 110 ± 58 | 510 ± 760 | 420 ± 690 | 980 ± 1800 | 530 ± 730 | 1.8 ± 0.94 | 68 ± 38 | 280 ± 460 | 1700 ± 2900 | |
| Min-max | 7.1–4200 | 26–220 | 68–2600 | 85–2700 | 68–6800 | 140–2900 | 0.28–3.1 | 15–160 | 66–1800 | 320–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Most abundant | (PFBS) | PFOS | PFHxA | PFOA | PFBS | PFOA | — | — | — | PFBS | |
For four of the landfills (I, II, III and VI), there was data available from previous, recent sampling campaigns for comparison. The concentrations for landfills I, II and III were quite similar from the current and previous campaign (generally within a factor 1.5 of each other, or not statistically different), though landfill VI exhibited substantially higher concentrations in the current April 2018 data compared to the previous September 2017 data. The only landfill with a time series available was Landfill I, having 6 time points from 2017 to 2018. This time series found more variation in long-chain PFAS concentrations (relative standard deviation, rsd, of 81%) compared to short-chain PFASs (rsd of 14%). Time trends in PFAS leachate concentrations were studied for a Canadian landfill by Benskin et al.,13 this study observed a peak in leachate emissions around mid-March to April for long-chain PFASs, but not for short-chain PFASs, which tended to be consistent throughout the year. Though the mechanisms of this are complex, and related to meteorological conditions (e.g. snow melt and precipitation), sorption and water properties (e.g. pH and ionic strength), less variability for short-chain PFASs than long-chain PFASs in landfill I is consistent with the Canadian landfill studied by Benskin et al.13 However, what is inconsistent is the apparent difference in both short-chain and long-chain PFASs observed in landfill VI between September 2017 to April 2018, where the September 2017 concentrations seems more of a diluted version compared to the April 2018 sample; and may simply be due to a dilution event e.g. storm water. Later in this manuscript a correlation analysis is presented to other leachate parameters (pH, DOC, EC and precipitation).
Regarding the second-aim of this study to see if short-chain PFAS dominate in leachate emissions, in six out of ten landfills, there were higher concentrations of short-chain PFASs than long-chain PFASs, with short-chain to long-chain ratios ranging from 0.28 to 3.1 (Table 1). Overall, the short-chain PFBS contributed most to the 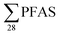 -concentration (30% based on mean concentrations). In leachate from Svalbard (landfill X), the major contributor was the long-chain PFOS (30%). At other landfills, PFOS contributed 1 to 20% of the
-concentration (30% based on mean concentrations). In leachate from Svalbard (landfill X), the major contributor was the long-chain PFOS (30%). At other landfills, PFOS contributed 1 to 20% of the 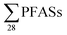 . Relatively lower overall PFOS-abundance and higher abundance of short-chain PFASs (especially PFBS) could indicate that short-chain PFASs are now the major contributors in landfill leachate, supporting the study's hypothesis. The role of time trends could also be considered here, as mentioned above, Benskin et al.13 noticed a drop in long-chain PFASs after April, but not short-chain PFASs. Since sampling was mainly done in April, short-chain PFAS may have dominated further if sampling occurred later in the year. Sorption may also play a role. PFOS and other long-chain perfluoroalkyl acids sorb stronger to organic solids than some of their short-chain analogues;25–27 for instance, average observed organic carbon partition coefficient, Koc of PFOS and PFBS is 3.0 and 2.2, respectively.28,29 Thus one would expect long-chain PFASs to leach slower than short-chain PFASs. The relatively high abundance of short chain PFAS, particularly PFBS, in leachate demonstrates that considerable amounts of PFBS (and PFBS precursor) containing waste have been deposed of in Norwegian landfills. The presence of PFOS in the leachates shows that the phase-out in new commercial products has not reduced their concentrations in leachate entirely; due to both the presence in waste being currently deposited at the landfills, and the lag-time of leaching from waste previously landfilled. There were significant correlations between
. Relatively lower overall PFOS-abundance and higher abundance of short-chain PFASs (especially PFBS) could indicate that short-chain PFASs are now the major contributors in landfill leachate, supporting the study's hypothesis. The role of time trends could also be considered here, as mentioned above, Benskin et al.13 noticed a drop in long-chain PFASs after April, but not short-chain PFASs. Since sampling was mainly done in April, short-chain PFAS may have dominated further if sampling occurred later in the year. Sorption may also play a role. PFOS and other long-chain perfluoroalkyl acids sorb stronger to organic solids than some of their short-chain analogues;25–27 for instance, average observed organic carbon partition coefficient, Koc of PFOS and PFBS is 3.0 and 2.2, respectively.28,29 Thus one would expect long-chain PFASs to leach slower than short-chain PFASs. The relatively high abundance of short chain PFAS, particularly PFBS, in leachate demonstrates that considerable amounts of PFBS (and PFBS precursor) containing waste have been deposed of in Norwegian landfills. The presence of PFOS in the leachates shows that the phase-out in new commercial products has not reduced their concentrations in leachate entirely; due to both the presence in waste being currently deposited at the landfills, and the lag-time of leaching from waste previously landfilled. There were significant correlations between 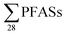 and several PFCAs (PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA), PFBS and 6
and several PFCAs (PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA), PFBS and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA (Table S6†), indicating that these substances have correlating concentration and emission pathways across landfills.
2 FTSA (Table S6†), indicating that these substances have correlating concentration and emission pathways across landfills.
In Table 2, leachate concentrations from the literature are compiled, in which it was possible for us to calculate the short-chain and long-chain concentrations and ratios in a manner similar to this study, because similar PFASs were analysed and the raw data was available. General caveats however with such comparisons are that the analysis methods can differ, PFASs analysed can differ, as can the type of leachate sampled (raw, diluted, treated), as indicated in Table 2, thus preventing exact comparisons, Eggen et al.19 using non-target analysis (Table 2) reported relatively high 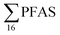 concentrations of 6123 ng L−1 for raw leachate in Norwegian landfills sampled in 2006 (which in comparison is higher than all landfills in this study except landfill VI), and a short- to long-chain ratio of 0.16; thus, indicating that short-chain PFASs have become more dominant in Norway recent years (though this data is influenced by a non-target analysis method being used).
concentrations of 6123 ng L−1 for raw leachate in Norwegian landfills sampled in 2006 (which in comparison is higher than all landfills in this study except landfill VI), and a short- to long-chain ratio of 0.16; thus, indicating that short-chain PFASs have become more dominant in Norway recent years (though this data is influenced by a non-target analysis method being used).
| Area, year (number of landfills/raw, diluted, mixed) | ∑Short-chain PFASs mean ± SD (min–max) | ∑Long-chain PFASs mean ± SD (min–max) | Short![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) long-chain ratio long-chain ratio |
n PFASs | Reference | |
|---|---|---|---|---|---|---|
| a With the exclusion of PFHpS, HPFHpA, PF-3,7-DMOA, PFTA and PFHxDA; note this was measured using non-target analysis. b With the exclusion of PFHpS, HPFHpA, PFDA, PF-3,7-DMOA, PFTrA and PFHxDA. c With the exclusion of PFHpS, HPFHpA, PFOA, PFDA and PF-3,7-DMOA. d With the exclusion of HPFHpA, PF-3,7-DMOA and PFHxDA. | ||||||
| Norway, 2017–2018 (10, mixed) | 980 ± 1800 (68–6800) | 530 ± 730 (140–2900) | 1.8 ± 0.94 (0.28–3.1) | 28 | 1700 ± 2900 (320–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
This study |
| Norway, 2006 (2, raw) | 757 | 4784a | 0.16 | 16 | 6123 | Eggen et al., 201019 |
| Canada, 2009 (1, raw) | 2812 ± 1109 (1424–5150) | 2719 ± 2160 (1021–7738)b | 1.0 | 24 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 10 000 ± 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (3800–3600) 000 (3800–3600) |
Benskin et al., 201213 |
| Sweden, 2015 (10, unknown) | 171 ± 137 (<LOQ–508) | 123 ± 78 (<LOQ–269)c | 1.4 | 26 | 487 (0.30–1300) | Gobelius et al., 201823 |
| Spain, 2015 (4, raw) | 576 ± 317 (125–852) | 506 ± 113 (413–663)d | 1.1 | 16 | 1082 (639–1379) | Fuertes et al., 201724 |
Benskin et al.13 reported higher 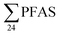 concentrations in flow-through (raw) leachate from a landfill in Canada (11
concentrations in flow-through (raw) leachate from a landfill in Canada (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 10
000 ± 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1) in 2010 than the Norwegian concentrations presented in this study. The mean short- to long-chain ratio was 1.0 in Benskin et al.,13 indicating short-chain PFASs were not dominant as in six of the landfills in this study. It is noteworthy that the short-chain PFBS was found in lower concentrations in this 2010 Canadian landfill (mean: 94 ± 41 ng L−1) than the mean of the 2018 Norwegian landfills (550 ± 1200 ng L−1); potentially indicating increased landfilling of PFBS.
000 ng L−1) in 2010 than the Norwegian concentrations presented in this study. The mean short- to long-chain ratio was 1.0 in Benskin et al.,13 indicating short-chain PFASs were not dominant as in six of the landfills in this study. It is noteworthy that the short-chain PFBS was found in lower concentrations in this 2010 Canadian landfill (mean: 94 ± 41 ng L−1) than the mean of the 2018 Norwegian landfills (550 ± 1200 ng L−1); potentially indicating increased landfilling of PFBS.
A study of Swedish landfills analysed in 2015 (ref. 23) reported 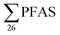 concentrations in the same range, although somewhat lower than this study (Table 2). The short- to long-chain ratio we calculate from their data was 1.4 (Table 2), which agrees with the general dominance of short-chain PFASs in landfills from this study. Four landfills from northern Spain were also sampled in 2015,24 and the concentration of s in raw leachate was in the same range as
concentrations in the same range, although somewhat lower than this study (Table 2). The short- to long-chain ratio we calculate from their data was 1.4 (Table 2), which agrees with the general dominance of short-chain PFASs in landfills from this study. Four landfills from northern Spain were also sampled in 2015,24 and the concentration of s in raw leachate was in the same range as 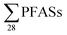 in this study (Table 2). The short- to long-chain ratio from the data in Fuertes et al.24 was 1.1 (Table 2).
in this study (Table 2). The short- to long-chain ratio from the data in Fuertes et al.24 was 1.1 (Table 2).
There are other leachate studies in the literature; however, it is not as clear to calculate the short-chain![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) long-chain PFAS ratio as done here. Thus, instead the sum PFAS concentrations and the presence of short-chain PFAS are discussed. According to Kallenborn et al.,21
long-chain PFAS ratio as done here. Thus, instead the sum PFAS concentrations and the presence of short-chain PFAS are discussed. According to Kallenborn et al.,21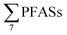 in landfill leachate from five Norwegian landfills in 2004 ranged from 199 to 1538 ng L−1 (mean: 673 ± 552, median: 468), which is lower than this study, but also with fewer PFASs, with many short-chain PFCAs not analysed for or found in low levels. A 2010 study of 22 landfills in Germany by Busch et al.17 reported
in landfill leachate from five Norwegian landfills in 2004 ranged from 199 to 1538 ng L−1 (mean: 673 ± 552, median: 468), which is lower than this study, but also with fewer PFASs, with many short-chain PFCAs not analysed for or found in low levels. A 2010 study of 22 landfills in Germany by Busch et al.17 reported 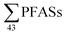 in untreated leachate from 31 to 12
in untreated leachate from 31 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 922 ng L−1 (mean: 6086 ng L−1), which is relatively higher than the
922 ng L−1 (mean: 6086 ng L−1), which is relatively higher than the 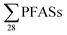 in this study, and perhaps influenced by including more congeners. This study reported short-chain PFASs were dominating, with the two most abundant congeners PFBA (mean contribution 27%) and PFBS (mean contribution 24%).17 In 2012, Li et al. published
in this study, and perhaps influenced by including more congeners. This study reported short-chain PFASs were dominating, with the two most abundant congeners PFBA (mean contribution 27%) and PFBS (mean contribution 24%).17 In 2012, Li et al. published 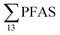 concentrations from 30 to 21
concentrations from 30 to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 in leachate from 28 landfills and dumpsites in Canada,18 these appeared dominated by long-chain PFCAs. In a study of four U.S. landfills sampled in 2006 by Huset et al.,15
000 ng L−1 in leachate from 28 landfills and dumpsites in Canada,18 these appeared dominated by long-chain PFCAs. In a study of four U.S. landfills sampled in 2006 by Huset et al.,15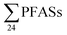 ranged from 2688 to 7415 ng L−1 in raw leachates, though interestingly already with high concentration of PFBA (up to 1700 ± 63 ng L−1) and PFBS (up to 890 ± 100 ng L−1). In raw leachate from five municipal landfill sites in China, sampled in 2013, the
ranged from 2688 to 7415 ng L−1 in raw leachates, though interestingly already with high concentration of PFBA (up to 1700 ± 63 ng L−1) and PFBS (up to 890 ± 100 ng L−1). In raw leachate from five municipal landfill sites in China, sampled in 2013, the 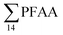 concentrations in 2015 ranged from 7280 to 292000 ng L−1 (mean: 82
concentrations in 2015 ranged from 7280 to 292000 ng L−1 (mean: 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ng L−1),22 which is much higher than
100 ng L−1),22 which is much higher than 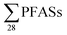 this study. PFOA and PFBS were the most abundant.22 It appears from this review that short-chain PFBS and PFBA have been a dominant component of leachate in some areas since 2006, though in the more recent studies this seems to be more typically the case, as in Norway.
this study. PFOA and PFBS were the most abundant.22 It appears from this review that short-chain PFBS and PFBA have been a dominant component of leachate in some areas since 2006, though in the more recent studies this seems to be more typically the case, as in Norway.
3.2 Emissions
The yearly amount of leachate generated at each landfill were provided by the landfill operators. This was used for estimation of the yearly PFAS release from each site, assuming the concentrations in Table 1 were consistent all year round (Table S7,† illustrated in Fig. 1); though as indicated above, sampling was done at time when concentrations of long-chain PFAS, in particular, are highest. The annual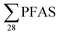 release at each landfill was estimated to range from 9.2 to 510 g per year (mean: 160 ± 160 g per year; median: 100 g per year). For short-chain PFASs these were from 2.2 to 310 g per year (mean: 87 ± 98; median: 49 g per year), and long-chain PFASs ranged from 5.9 to 130 g per year (mean: 47 ± 39; median: 46 g per year). One of the sites with the lowest PFAS emissions per year was in the high Arctic Site in Svalbard (landfill X), which is located in the Arctic, where the climate is relatively cold, causing relatively lower leachate production volumes of approximately 25
release at each landfill was estimated to range from 9.2 to 510 g per year (mean: 160 ± 160 g per year; median: 100 g per year). For short-chain PFASs these were from 2.2 to 310 g per year (mean: 87 ± 98; median: 49 g per year), and long-chain PFASs ranged from 5.9 to 130 g per year (mean: 47 ± 39; median: 46 g per year). One of the sites with the lowest PFAS emissions per year was in the high Arctic Site in Svalbard (landfill X), which is located in the Arctic, where the climate is relatively cold, causing relatively lower leachate production volumes of approximately 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per year compared to other landfills in the present study (mean: 113000 ± 135000 m3 per year, Table S2†); and therefore leaching from waste is also slowed.
000 m3 per year compared to other landfills in the present study (mean: 113000 ± 135000 m3 per year, Table S2†); and therefore leaching from waste is also slowed.
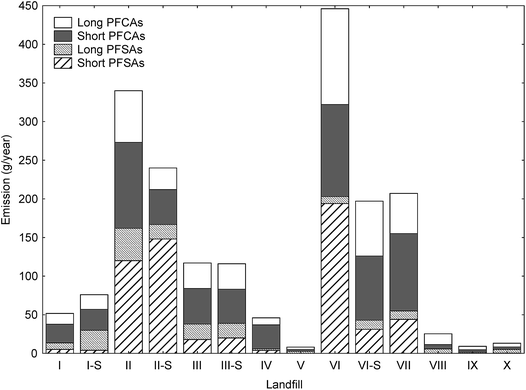 | ||
| Fig. 1 Stacked bar chart of estimated yearly PFAS emissions (g per year, based on the amount of leachate generated at each landfill (Table S2†) and the concentrations in Table 1) of long-chain and short-chain perfluorinated sulfonates (PFSAs) and perfluoroalkyl carboxylic acids (PFCAs) for the Norwegian landfills in this study (Table S7†). | ||
It is important to bear in mind that there are several limitations to the emission estimates provided in the present study. These are (1) most sites had one sampling day, so seasonal, climate and hydrological factors could not be evaluated; (2) sampling occurred around April, which as discussed above is potentially when peak emissions for long-chain PFAS occur;13 (3) discharge volume is provided by the operators and their accuracy may vary; and (4) the leachate samples could to some degree be influenced by storm water and groundwater.
Comparisons with emission estimates in the literature need to take into account different PFASs being considered, as well as annual leachate volumes, which can vary tremendously. Therefore comparisons with emissions in the literature presented below must be considered with these limitations in mind. The study on Norwegian landfills in 2006 (ref. 19) reported emissions of 2.1 kg per year, which is higher than any of the landfills in the present study, due to relatively higher PFAS concentrations (Table 2), as well as a relatively large annual loading of 345![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 leachate per year.19 The study of the Canadian landfill in 201013 reported annual
000 m3 leachate per year.19 The study of the Canadian landfill in 201013 reported annual 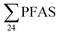 emissions from 8.5 to 25 kg per year (mean: 16 kg per year) which is considerably higher than the landfills in this study. This is mainly due to the high annual volume of leachate produced at the Canadian site, of 2.2 × 106 m3 per year,13 compared to volumes from 2.2 × 104 to 4.6 × 105 m3 per year in this study (Table S2†).
emissions from 8.5 to 25 kg per year (mean: 16 kg per year) which is considerably higher than the landfills in this study. This is mainly due to the high annual volume of leachate produced at the Canadian site, of 2.2 × 106 m3 per year,13 compared to volumes from 2.2 × 104 to 4.6 × 105 m3 per year in this study (Table S2†).
To extrapolate these results to the national level, which introduces new uncertainties, a previous report on leachate emissions in Norway concluded that a total volume of up to 1.0 × 107 m3 is emitted from Norwegian landfills, with 55% of emissions being sent to wastewater treatment plants (WTP) and 45% to the environment.30 Based on the mean concentrations provided in Table 1 multiplied by national leachate volume, this implies national 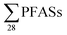 release from Norwegian landfills from 3.2 to 110 kg per year (mean: 17 ± 29; median: 6.3 kg per year) (Table S8†). Considering the current Norwegian population is approximately 5.3 million, this would correspond to a mean per capita emission factor of 3.2 ± 5.5 mg per year per person. For short-chain PFASs these were from 0.68 to 68 kg per year (mean: 9.8 ± 18; median: 3.6 kg per year), and long-chain from 1.4 to 29 kg per year (mean: 5.3 ± 7.3; median: 2.9 kg per year) (Table S8†).
release from Norwegian landfills from 3.2 to 110 kg per year (mean: 17 ± 29; median: 6.3 kg per year) (Table S8†). Considering the current Norwegian population is approximately 5.3 million, this would correspond to a mean per capita emission factor of 3.2 ± 5.5 mg per year per person. For short-chain PFASs these were from 0.68 to 68 kg per year (mean: 9.8 ± 18; median: 3.6 kg per year), and long-chain from 1.4 to 29 kg per year (mean: 5.3 ± 7.3; median: 2.9 kg per year) (Table S8†).
National estimates have also been presented for other countries, such as China,22 Germany,17 and the U.S.16 These were derived by multiplying average emission rates for landfills included in their study by the number of landfills in the country, rather than based on leachate volumes as here. For the purpose of comparison, the national emission levels are divided by population to give per capita emission factors. Yan et al.22 estimated in 2015 the Chinese national emission of 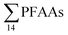 to groundwater from landfill leachate to be 3110 kg per year, based on the mean concentration of 82
to groundwater from landfill leachate to be 3110 kg per year, based on the mean concentration of 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ng L−1 from five municipal landfill sites in China, and the average amount of leachate generated per year (4.74 × 107 m3 per year), assuming that 80% of the landfills were not lined. This would correspond to 2.2 mg per person per year (assuming a Chinese population of 1.39 billion), which is similar to this study (though this study considered all aqueous emissions from landfills, not just groundwater emissions). The German national landfill emission in 2009 of
100 ng L−1 from five municipal landfill sites in China, and the average amount of leachate generated per year (4.74 × 107 m3 per year), assuming that 80% of the landfills were not lined. This would correspond to 2.2 mg per person per year (assuming a Chinese population of 1.39 billion), which is similar to this study (though this study considered all aqueous emissions from landfills, not just groundwater emissions). The German national landfill emission in 2009 of 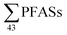 was estimated at 88 kg per year,17 corresponding to 1.1 mg per person per year (assuming population 81.8 million in 2009). A 2013 survey of U.S. landfills estimated
was estimated at 88 kg per year,17 corresponding to 1.1 mg per person per year (assuming population 81.8 million in 2009). A 2013 survey of U.S. landfills estimated 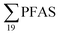 emissions from 563 to 638 kg per year,16 or 1.8 to 2.0 mg per person per year. A study from Spain24 estimated the annual discharge of
emissions from 563 to 638 kg per year,16 or 1.8 to 2.0 mg per person per year. A study from Spain24 estimated the annual discharge of 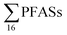 from four landfill sites serving 1.8 million people was 1.2 kg per year, which implies an emission rate of 0.7 mg per person per year.
from four landfill sites serving 1.8 million people was 1.2 kg per year, which implies an emission rate of 0.7 mg per person per year.
Despite limitations, this comparison resulted in per capita emission factors that were very similar of those that can be derived in other regions, from 0.7 (Northern Spain), 1.1 (German), 1.8–2.0 (U.S.), 2.2 to groundwater (China), to 3.2 (Norway) mg per day per person.
Compared to total per capita emission factors from all sources of PFAS, not just landfills, based on river and water treatment plant data in the literature, the contributions of landfills are relatively low. For instance, considering just PFOS, total per capita emissions from all sources for the EU in 2009 were estimated at 9.9 mg per year per person.31 In our 2018 study, Norwegian landfill emissions contribute roughly 0.35 mg per day per person emissions of PFOS, which would be 3.5% of the European per capita emissions in 2009. Total flux of sewage-derived PFOS from Japan was in 2008 estimated to be 3.6 tonnes per year, corresponding to 28 mg per year per person,32 which is almost two orders of magnitude higher than the per capita emission factors derived here. Part of this may be attributable to PFOS emissions having declined in recent years, since these other per capita emissions were derived.
3.3 Sediment concentrations
PFASs were detected in all sediment samples (n = 8). The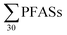 concentration (dry weight) ranged from 8.5 to 120 μg kg−1 (mean: 47 ± 36; median: 41 μg kg−1) (Table 3, with data for individual substances in Table S9†). The major contributors were long-chain PFASs, such as PFUnDA and PFOS. In addition, long-chain PFOS-precursors (especially EtFOSAA and MeFOSAA) contributed to a substantial amount of the
concentration (dry weight) ranged from 8.5 to 120 μg kg−1 (mean: 47 ± 36; median: 41 μg kg−1) (Table 3, with data for individual substances in Table S9†). The major contributors were long-chain PFASs, such as PFUnDA and PFOS. In addition, long-chain PFOS-precursors (especially EtFOSAA and MeFOSAA) contributed to a substantial amount of the 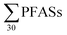 (46%). Long-chain PFASs are more likely to adsorb onto surfaces and partition into soils and sediments.25,26
(46%). Long-chain PFASs are more likely to adsorb onto surfaces and partition into soils and sediments.25,26
| ID | ∑Short-chain PFSAsa (<C6) | ∑Long-chain PFSAsb (≥C6) | ∑Short-chain PFCAsc (<C7) | ∑Long-chain PFCAsd (≥C7) | ∑Short-chain PFAS | ∑Long-chain PFAS | PFOS | PFOA | ∑PFOS precursorse | ∑PFAS |
|---|---|---|---|---|---|---|---|---|---|---|
| a PFBS. b PFHxS, PFOS and PFDS. c PFBA, PFPeA and PFHxA. d PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoA and PFTrA. e The PFOS precursors, EtFOSAA, EtFOSE, MeFOSAA, MeFOSE and FOSAA, were only analyzed in sediments and not in leachate, and hence are not included in the ∑Short-chain and ∑Long-chain data for consistency. f X-I was sampled from the outlet of a leachate pond, at the same place as the leachate sample from landfill X. X–II was sampled from a stream downstream the leachate pond. | ||||||||||
| I | <LOQ | 26 | <LOQ | 6.6 | <LOQ | 32 | 25 | 1.6 | 33 | 72 |
| IV | <LOQ | 11 | 0.25 | 3.4 | 0.25 | 14 | 11 | 1.2 | 24 | 46 |
| V | <LOQ | 14 | 0.49 | 1.9 | 0.49 | 16 | 14 | 1.3 | 12 | 37 |
| VI | <LOQ | 1.3 | <LOQ | 0.69 | <LOQ | 2.0 | 1.3 | 0.21 | 0.74 | 8.8 |
| VII | 3.3 | 9.4 | 4.9 | 7.5 | 8.2 | 17 | 9.0 | 3.6 | 84 | 120 |
| IX | <LOQ | 15 | <LOQ | 1.8 | <LOQ | 17 | 15 | 1.8 | 7.9 | 40 |
| X–If | <LOQ | 24 | <LOQ | 20 | <LOQ | 44 | 24 | 0.54 | 8.26 | 42 |
| X–IIf | <LOQ | 3.9 | <LOQ | 1.4 | <LOQ | 5.3 | 3.9 | 0.23 | 0.59 | 8.5 |
| Median | 3.3 | 13 | 0.49 | 2.6 | 0.49 | 16 | 13 | 1.3 | 10 | 41 |
| Mean ± SD | 3.3 | 13 ± 8.7 | 1.9 ± 2.6 | 5.4 ± 6.4 | 3.0 ± 4.5 | 18 ± 14 | 13 ± 9 | 1.3 ± 1.1 | 21 ± 28 | 47 ± 36 |
| Min–max | 3.3–3.3 | 1.3–26 | 0.25–4.9 | 0.69–20 | 0.25–8.2 | 2.0–44 | 1.3–25 | 0.21–3.6 | 0.59–84 | 8.5–120 |
| Most abundant | (PFBS) | PFOS | PFHxA | PFUnDA | PFBS | PFOS | — | — | EtFOSAA | EtFOSAA |
Eggen et al.19 reported that long-chain PFCAs, FOSA and EtFOSA were detected at relatively high concentrations in sediment samples from a Norwegian landfill sampled in 2006, with total PFAS concentrations (dry weight) ranging from 1.3 to 382 μg kg−1 (mean: 61 μg kg−1), which is in the same range as this study (Table 3), indicating little change. According to a literature survey by the Norwegian Environment Agency from 2008,20 PFHxS and PFOS were detected in all sediment samples from 8 Norwegian landfills, wherein the 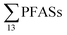 ranged from approximately 0.0013 to 0.024 μg per kg d.w.,20 which is lower than the present study. However, it is noted that PFAS substances such as EtFOSAA and MeFOSAA were not measured in that study, whereas these were measured at relatively high concentrations in this study (Table 3).
ranged from approximately 0.0013 to 0.024 μg per kg d.w.,20 which is lower than the present study. However, it is noted that PFAS substances such as EtFOSAA and MeFOSAA were not measured in that study, whereas these were measured at relatively high concentrations in this study (Table 3).
3.4 Sediment-leachate distribution
The ratio of Csediment to Cleachate, which will be referred to as Qsed/leachate (units L kg−1), measured at the specific landfills is presented in Table S10.† It is noted that the Qsed/leachate value cannot be considered an equilibrium distribution coefficient, KD, as it is uncertain if at each site Csediment is in equilibrium with Cleachate, particularly for sites where sediments and leachates were sampled in different locations. Among the analysed PFASs, only PFOA and PFOS were detected > LOQ for both leachate and sediment samples at all landfills. The calculated, average log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qsed/leachate − values for PFOA and PFOS were 0.74 ± 0.78 and 2.3 ± 0.64, respectively, ranging from −0.93 to 1.38 and from 1.0 to 2.9 (Table S10†). These happen to be similar to log
Qsed/leachate − values for PFOA and PFOS were 0.74 ± 0.78 and 2.3 ± 0.64, respectively, ranging from −0.93 to 1.38 and from 1.0 to 2.9 (Table S10†). These happen to be similar to log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD (L kg−1) reported in the literature for different soils and sediments, which cover broad ranges; e.g.: Schedin:33 2013: 0.3 ± 0.4 and 1.2 ± 0.6 for PFOA and PFOS; Kwadijk et al.:28 1.83 ± 0.40 and 2.35 ± 0.35 for PFOA and PFOS. According a literature review by Zareitalabad et al.,29 log
KD (L kg−1) reported in the literature for different soils and sediments, which cover broad ranges; e.g.: Schedin:33 2013: 0.3 ± 0.4 and 1.2 ± 0.6 for PFOA and PFOS; Kwadijk et al.:28 1.83 ± 0.40 and 2.35 ± 0.35 for PFOA and PFOS. According a literature review by Zareitalabad et al.,29 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd-values for PFOA and PFOS ranged from −1.2 to 0.96 and from −0.7 to 1.9, respectively.
Kd-values for PFOA and PFOS ranged from −1.2 to 0.96 and from −0.7 to 1.9, respectively.
3.5 Correlation analysis
PFAS concentrations in landfill leachates are to some extent dependent on the leachate's water properties.13,34 Principal component analysis on the variables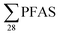 , DOC, pH and EC were conducted for all obtained samples (Fig. S1†). There was a clear correlation between
, DOC, pH and EC were conducted for all obtained samples (Fig. S1†). There was a clear correlation between 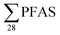 with increasing EC. Significant correlations between
with increasing EC. Significant correlations between 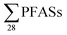 and some individual PFASs (PFHxS, PFBA, PFPeA, PFHxA, PFHpA and PFOA) with EC were found (Table S11†), which is in agreement with a study by Benskin et al.,13 as they observed significant correlations between PFBA, PFPeA and PFHxA with increasing EC. Possible explanations given in Benskin et al.13 were the observation of decreased sorption to some clays with increasing EC due to competition effects with other anions, e.g. chloride, for the anionic sorption sites (this is discussed further below). However, because in this study a correlation with
and some individual PFASs (PFHxS, PFBA, PFPeA, PFHxA, PFHpA and PFOA) with EC were found (Table S11†), which is in agreement with a study by Benskin et al.,13 as they observed significant correlations between PFBA, PFPeA and PFHxA with increasing EC. Possible explanations given in Benskin et al.13 were the observation of decreased sorption to some clays with increasing EC due to competition effects with other anions, e.g. chloride, for the anionic sorption sites (this is discussed further below). However, because in this study a correlation with 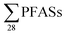 and EC was evident, there may be some influence of the dilution of raw leachate with storm water or groundwater that helps account for this observation. There were no clear associations between PFAS-concentrations and pH or DOC in leachate. On the contrary, Gallen et al.34 reported increasing concentrations of several PFAS compounds with increasing pH and DOC in leachate. In our case, this may be in part due to a limited range of pH values (from 6.4 to 7.8) and DOC values being relatively clustered between 28–125 mg L−1, except for one outlier at 1322 mg L−1 (ESI, Table S2†).
and EC was evident, there may be some influence of the dilution of raw leachate with storm water or groundwater that helps account for this observation. There were no clear associations between PFAS-concentrations and pH or DOC in leachate. On the contrary, Gallen et al.34 reported increasing concentrations of several PFAS compounds with increasing pH and DOC in leachate. In our case, this may be in part due to a limited range of pH values (from 6.4 to 7.8) and DOC values being relatively clustered between 28–125 mg L−1, except for one outlier at 1322 mg L−1 (ESI, Table S2†).
In principle, factors such as pH, EC and DOC would influence the sorption of ionic PFASs. Thus, PCA biplots were made for the Qsed/leachate − values of PFOS and PFOA, where Qsed/leachate is considered a proxy for sorption. As their Qsed/leachate − values were strongly correlated (r = 0.9, p = 0.001), only the PCA-biplot with Qsed/leachate − values for PFOS is shown in Fig. S2.†Qsed/leachate and yearly leachate production volume were positively correlated, implying higher sorption to the sediment phase with increasing leachate volume. This could be accounted for by considering a Freundlich like sorption behaviour (i.e. sorption increases with increasing water to sediment ratios, leaving behind the more strongly sorbed residues). There were no significant correlations between Qsed/leachate and pH or DOC. However, there was a negative correlation between PFOS Qsed/leachate and EC, which indicates relatively less partitioning to the sediment phase with increasing EC. This could be accounted for by increasing competition for anionic sorption sites. It is not uncommon for leachate sediments to be rich in metal oxides,35 which can be positively-charged and therefore contain anion exchange sites, depending on the pH and salt composition. Studies by Wang et al.36,37 indicated that the sorption of PFOS and PFOA on the aluminium oxides boehmite and alumina decreased with increasing ionic strength. In contrast, negatively-charged clays like the phyllosilicate bentonite show negligible sorption of PFAS,38 and therefore would be less influenced by EC than positively-charged metal oxides. Thus, the stronger sorption with higher flow volumes and lower EC seems to match well with mechanistic expectations for a Freundlich like behaviour: dilution would lower EC and PFAS concentrations, and therefore increase sorption via less competition for anion-exchange sites to e.g. metal oxide surfaces.
4. Environmental implications
This study estimated that the release of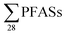 from Norwegian landfills was in the range 3.2 to 110 kg per year (mean: 17 ± 29; median: 6.3 kg per year); though, it should be kept in mind there were several assumptions used to make this data, ranging from the (limited) sampling campaign to the assumption that the obtained data were representative of yearly emissions. Future sampling campaigns should address this. As hypothesized, due to the shift towards short-chain PFAS chemistry, emissions of short-chain PFASs appear to dominate over emissions of long-chain PFASs. Short-chain PFASs are generally more mobile, as made evident by the difficulty in this study in obtaining sediment concentrations of PFBS, despite it being the most dominating PFAS in leachate. Future studies could confirm this trend, and relate emission levels with current production levels, uses and disposal of specific PFASs. Landfills are one of the many sources of PFAS emissions. Here we presented a very rough estimation based on a literature comparison that landfills contribute approximately 3.5% of all European per capita emissions of PFOS. A similar estimation for other PFASs could not be made. Future studies could seek to quantify these values better through comparison of different emission sources, e.g. households, fire training facilities, airports, and industries, to their respective contributions to wastewater treatment plants, groundwater, rivers, and other recipients. Even though the composition of PFASs in commerce and in landfills change over time, landfills can continue to release contaminants like PFASs for years to come. If the emissions in Norway were consistent for 100 years at 17 kg per year, then there would be 1.7 tonnes emitted from one, relatively small country. To put this into context, a recent global emission inventory of PFHxS and PFDS has estimated that between 1958 and 2015, 120–1022 and 30–378 tons, respectively, have been emitted.39 To prevent landfills from contributing to future PFAS emissions, proper management strategies are key, such as developing low cost leachate treatment facilities, including PFAS in leachate monitoring, better understanding of leaching mechanisms from waste, and evaluating how concentrations of PFAS in leachate changes with PFAS levels in deposited waste.
from Norwegian landfills was in the range 3.2 to 110 kg per year (mean: 17 ± 29; median: 6.3 kg per year); though, it should be kept in mind there were several assumptions used to make this data, ranging from the (limited) sampling campaign to the assumption that the obtained data were representative of yearly emissions. Future sampling campaigns should address this. As hypothesized, due to the shift towards short-chain PFAS chemistry, emissions of short-chain PFASs appear to dominate over emissions of long-chain PFASs. Short-chain PFASs are generally more mobile, as made evident by the difficulty in this study in obtaining sediment concentrations of PFBS, despite it being the most dominating PFAS in leachate. Future studies could confirm this trend, and relate emission levels with current production levels, uses and disposal of specific PFASs. Landfills are one of the many sources of PFAS emissions. Here we presented a very rough estimation based on a literature comparison that landfills contribute approximately 3.5% of all European per capita emissions of PFOS. A similar estimation for other PFASs could not be made. Future studies could seek to quantify these values better through comparison of different emission sources, e.g. households, fire training facilities, airports, and industries, to their respective contributions to wastewater treatment plants, groundwater, rivers, and other recipients. Even though the composition of PFASs in commerce and in landfills change over time, landfills can continue to release contaminants like PFASs for years to come. If the emissions in Norway were consistent for 100 years at 17 kg per year, then there would be 1.7 tonnes emitted from one, relatively small country. To put this into context, a recent global emission inventory of PFHxS and PFDS has estimated that between 1958 and 2015, 120–1022 and 30–378 tons, respectively, have been emitted.39 To prevent landfills from contributing to future PFAS emissions, proper management strategies are key, such as developing low cost leachate treatment facilities, including PFAS in leachate monitoring, better understanding of leaching mechanisms from waste, and evaluating how concentrations of PFAS in leachate changes with PFAS levels in deposited waste.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Norwegian Research Council through FANTOM (NFR project # 231736/F20), basis funding for the NGI (GBV) and NIBIO. The authors thank all landfill operators for their cooperation in this study.References
- S. Brendel, É. Fetter, C. Staude, L. Vierke and A. Biegel-Engler, Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH, Environ. Sci. Eur., 2018, 30, 9 CrossRef PubMed.
- T. Begley, K. White, P. Honigfort, M. Twaroski, R. Neches and R. Walker, Perfluorochemicals: potential sources of and migration from food packaging, Food Addit. Contam., 2005, 22, 1023–1031 CrossRef CAS PubMed.
- Y. Zushi, J. N. Hogarh and S. Masunaga, Progress and perspective of perfluorinated compound risk assessment and management in various countries and institutes, Clean Technol. Environ. Policy, 2012, 14, 9–20 CrossRef CAS.
- B. Bhhatarai and P. Gramatica, Prediction of aqueous solubility, vapor pressure and critical micelle concentration for aquatic partitioning of perfluorinated chemicals, Environ. Sci. Technol., 2010, 45, 8120–8128 CrossRef PubMed.
- A. B. Lindstrom, M. J. Strynar and E. L. Libelo, Polyfluorinated compounds: past, present, and future, Environ. Sci. Technol., 2011, 45, 7954–7961 CrossRef CAS PubMed.
- B. Bonnet, Master thesis, Swedish University of Agricultural Sciences, 2017.
- UNEP, Report of the Conference of the Parties of the Stockholm Convention on Persistent Organic Pollutants on the work of its fourth meeting, http://chm.pops.int/Portals/0/Repository/COP4/UNEP-POPS-COP.4-38.English.pdf, 2009 Search PubMed.
- USEPA, PFAS National Environmental Management Plan, https://www.epa.vic.gov.au/%7E/media/Files/Yourenvironment/Landandgroundwater/PFASinVictoria/PFASNEMP/FINAL_PFAS-NEMP-20180110.pdf, 2018 Search PubMed.
- OECD, OECD portal on perfluorinated chemicals, Organisation for Economic Co-operation and Development, http://www.oecd.org/site/0,3407,en_21571361_44787844_1_1_1_1_1,00.html, 2018 Search PubMed.
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. De Voogt, A. A. Jensen, K. Kannan, S. A. Mabury and S. P. van Leeuwen, Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins, Integr. Environ. Assess. Manage., 2011, 7, 513–541 CrossRef CAS PubMed.
- A. Möller, L. Ahrens, R. Surm, J. Westerveld, F. van der Wielen, R. Ebinghaus and P. de Voogt, Distribution and sources of polyfluoroalkyl substances (PFAS) in the River Rhine watershed, Environ. Pollut., 2010, 158, 3243–3250 CrossRef PubMed.
- Z. Wang, I. T. Cousins, M. Scheringer and K. Hungerbuehler, Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions, Environment International, 2015, 75, 172–179 CrossRef CAS PubMed.
- J. P. Benskin, B. Li, M. G. Ikonomou, J. R. Grace and L. Y. Li, Per-and polyfluoroalkyl substances in landfill leachate: patterns, time trends, and sources, Environ. Sci. Technol., 2012, 46, 11532–11540 CrossRef CAS PubMed.
- B. O. Clarke, T. Anumol, M. Barlaz and S. A. Snyder, Investigating landfill leachate as a source of trace organic pollutants, Chemosphere, 2015, 127, 269–275 CrossRef CAS PubMed.
- C. A. Huset, M. A. Barlaz, D. F. Barofsky and J. A. Field, Quantitative determination of fluorochemicals in municipal landfill leachates, Chemosphere, 2011, 82, 1380–1386 CrossRef CAS PubMed.
- J. R. Lang, B. M. Allred, J. A. Field, J. W. Levis and M. A. Barlaz, National estimate of per-and polyfluoroalkyl substance (PFAS) release to US municipal landfill leachate, Environ. Sci. Technol., 2017, 51, 2197–2205 CrossRef CAS PubMed.
- J. Busch, L. Ahrens, R. Sturm and R. Ebinghaus, Polyfluoroalkyl compounds in landfill leachates, Environ. Pollut., 2010, 158, 1467–1471 CrossRef CAS PubMed.
- B. Li, M. N. Danon-Schaffer, L. Y. Li, M. G. Ikonomou and J. R. Grace, Occurrence of PFCs and PBDEs in landfill leachates from across Canada, Water, Air, Soil Pollut., 2012, 223, 3365–3372 CrossRef CAS.
- T. Eggen, M. Moeder and A. Arukwe, Municipal landfill leachates: a significant source for new and emerging pollutants, Sci. Total Environ., 2010, 408, 5147–5157 CrossRef CAS PubMed.
- T. E. Økland and K. Skoog, Literature survey: Polybrominated diphenyl ethers and perfluorinated compounds in the Norwegian environment, Norwegian Pollution Control Authority, Oslo, http://www.miljodirektoratet.no/old/klif/publikasjoner/2450/ta2450.pdf, 2008 Search PubMed.
- R. Kallenborn, U. Berger, U. Järnberg, M. Dam, B. Hedlund, A. Lundgren, B. Bügel and S. Sigurdsson, Perfluorinated alkylated substances (PFAS) in the nordic environment, 2004 Search PubMed.
- H. Yan, I. T. Cousins, C. Zhang and Q. Zhou, Perfluoroalkyl acids in municipal landfill leachates from China: Occurrence, fate during leachate treatment and potential impact on groundwater, Sci. Total Environ., 2015, 524, 23–31 CrossRef PubMed.
- L. Gobelius, J. Hedlund, W. Dürig, R. Tröger, K. Lilja, K. Wiberg and L. Ahrens, Per-and Polyfluoroalkyl Substances in Swedish Groundwater and Surface Water: Implications for Environmental Quality Standards and Drinking Water Guidelines, Environ. Sci. Technol., 2018, 52, 4340–4349 CrossRef CAS PubMed.
- I. Fuertes, S. Gómez-Lavín, M. Elizalde and A. Urtiaga, Perfluorinated alkyl substances (PFASs) in northern Spain municipal solid waste landfill leachates, Chemosphere, 2017, 168, 399–407 CrossRef CAS PubMed.
- C. P. Higgins and R. G. Luthy, Sorption of perfluorinated surfactants on sediments, Environ. Sci. Technol., 2006, 40, 7251–7256 CrossRef CAS PubMed.
- V. Gellrich, T. Stahl and T. Knepper, Behavior of perfluorinated compounds in soils during leaching experiments, Chemosphere, 2012, 87, 1052–1056 CrossRef CAS PubMed.
- M. S. McLachlan, S. Felizeter, M. Klein, M. Kotthoff and P. De Voogt, Fate of a perfluoroalkyl acid mixture in an agricultural soil studied in lysimeters, Chemosphere, 2019, 223, 180–187 CrossRef CAS PubMed.
- C. Kwadijk, P. Korytar and A. Koelmans, Distribution of perfluorinated compounds in aquatic systems in the Netherlands, Environ. Sci. Technol., 2010, 44, 3746–3751 CrossRef CAS PubMed.
- P. Zareitalabad, J. Siemens, M. Hamer and W. Amelung, Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater – A review on concentrations and distribution coefficients, Chemosphere, 2013, 91, 725–732 CrossRef CAS PubMed.
- H. P. H. Arp and G. Okkenhaug, Miljøgifter i sigevann fra avfallsdeponier i Norge. Data fra perioden 2006–2010, Report NGI report 20110546-00-5-R, Norwegian Geotechnical Institute, Oslo, http://www.miljodirektoratet.no/old/klif/publikasjoner/2978/ta2978.pdf, 2012 Search PubMed.
- A. Pistocchi and R. Loos, A map of European emissions and concentrations of PFOS and PFOA, Environ. Sci. Technol., 2009, 43, 9237–9244 CrossRef CAS PubMed.
- M. Murakami, E. Imamura, H. Shinohara, K. Kiri, Y. Muramatsu, A. Harada and H. Takada, Occurrence and sources of perfluorinated surfactants in rivers in Japan, Environ. Sci. Technol., 2008, 42, 6566–6572 CrossRef CAS PubMed.
- E. Schedin, MSc Master thesis, Uppsala University, 2013.
- C. Gallen, D. Drage, G. Eaglesham, S. Grant, M. Bowman and J. Mueller, Australia-wide assessment of perfluoroalkyl substances (PFASs) in landfill leachates, J. Hazard. Mater., 2017, 331, 132–141 CrossRef CAS PubMed.
- J. K. Øygard, E. Gjengedal and H. J. Mobbs, Trace element exposure in the environment from MSW landfill leachate sediments measured by a sequential extraction technique, J. Hazard. Mater., 2008, 153, 751–758 CrossRef PubMed.
- F. Wang and K. Shih, Adsorption of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on alumina: Influence of solution pH and cations, Water Res., 2011, 45, 2925–2930 CrossRef CAS PubMed.
- F. Wang, C. Liu and K. Shih, Adsorption behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on boehmite, Chemosphere, 2012, 89, 1009–1014 CrossRef CAS PubMed.
- B. Li, L. Y. Li and J. R. Grace, Adsorption and hydraulic conductivity of landfill-leachate perfluorinated compounds in bentonite barrier mixtures, J. Environ. Manage., 2015, 156, 236–243 CrossRef CAS PubMed.
- J. M. Boucher, I. T. Cousins, M. Scheringer, K. Hungerbühler and Z. Wang, Toward a Comprehensive Global Emission Inventory of C4–C10 Perfluoroalkanesulfonic Acids (PFSAs) and Related Precursors: Focus on the Life Cycle of C6-and C10-Based Products, Environmental Science & Technology Letters, 2017 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9em00170k |
| This journal is © The Royal Society of Chemistry 2019 |