Spraying functional fibres by electrospinning
Chuan-Ling
Zhang
ab and
Shu-Hong
Yu
*b
aSchool of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei, Anhui 230009, P. R. China
bDivision of Nanomaterials and Chemistry, Hefei National Laboratory for Physical Sciences at Microscale, Collaborative Innovation Center of Suzhou Nano Science and Technology, CAS Center for Excellence in Nanoscience, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China. E-mail: shyu@ustc.edu.cn
First published on 7th April 2016
Abstract
Electrospinning is a very simple, robust, and versatile technique to process solutions or melts into continuous fibres with diameters down to the nanoscale, which has attracted much attention in both research and commerce. Up to now, a rich variety of electrospun fibres with controllable morphology, alignment and composition have been synthesized, and the materials composed of functional electrospun fibres have already been successfully applied in many technological areas. In particular for anisotropic nanoparticles (NPs), electrospinning shows its powerful capability to assemble them on a large scale. The goal of this brief focus article is to show you how to use the electrospinning technique for preparing the functional fibres you want.
One dimensional (1D) nanomaterials, especially composite 1D nanowires, have generated great interest in the field of materials science due to their unique properties and fascinating applications in many areas.1 Among the methods for fabricating and assembling 1D nanostructures in the form of wires, belts, rods, tubes and rings, electrospinning has its own unique advantages in terms of flexibility, versatility and ease of fibre production, especially it allows the fabrication of continuous fibres with diameters down to the range of a few nanometers. Electrospinning is a simple technique that utilizes high electrostatic forces for fibre production, and fibres with high specific area could be obtained. This technique is applicable to virtually every soluble or fusible polymer, and polymers can be chemically modified and can also be loaded with additives ranging from simple NPs to complex species (e.g. enzymes, viruses, and bacteria), which has offered electrospun nanofibres and membranes a significant expansion of the already broad scope of their applications in a variety of fields, including optoelectronics, sensor technology, catalysis, energy, mechanical enhancement, and biotechnology.2–4 Electrospinning is not only a focus of intense academic investigation, but it is also increasingly being applied in many technological areas. Up to now, great progress on the modification of the instrument has been made for the industrialization of electrospinning. Czech's Elmarco, for example, has successfully produced the world's first industrial spinning machine that can produce nanofibrous mats with a width of more than 1 m, which is an important milestone in the field of electrospinning. Other countries, such as the United States, Western Europe, Japan and South Korea, have also made great progress in electrospinning industrialization. Here, we will mainly demonstrate how to use a single needle electrospinning instrument for preparing functional fibres in the laboratory.
Before telling you how to prepare functional fibres, we should first introduce the basic setup and principles of electrospinning. In strong contrast to conventional fibre producing techniques, electrospinning utilizes high electrostatic forces for fibre production, and it may be considered as a variant of the electrostatic spraying (or electrospray) process. The laboratory setup for single needle electrospinning is schematically illustrated in Fig. 1. Four major components are required for electrospinning: a direct current high voltage power supply (typically 5 to 50 kV), a metallic needle with a blunt-tip, a syringe for containing the electrospun solution, and a grounded conductive collector (e.g. a sheet of copper screen).
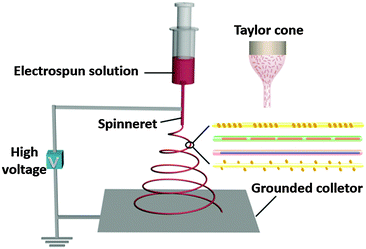 | ||
| Fig. 1 Schematic illustration of the laboratory setup for an electrospinning experiment with a vertical mode. The inset shows a sketch of the Taylor cone. | ||
The positive electrode of high voltage power supply is connected to the metallic needle, and the collector is connected to the ground. The reservoir containing the electrospun solution can be placed horizontally or vertically. Due to the confinement of surface tension, a droplet of the electrospun solution is formed at the tip of the needle. When a high voltage is applied, the droplet will become highly electrified and the induced charges are evenly built on the surface, leading to a force directly opposite to the surface tension. When the electrostatic repulsion among the charges is sufficiently strong to overcome the surface tension of the liquid, the liquid drop will be distorted into a conical object commonly known as the Taylor cone, followed by a jet of electrospun solution directed to the collector. This electrified jet then undergoes a stretching and whipping process, leading to the formation of a long and thin thread. As the liquid jet is continuously elongated by electrostatic repulsion and the solvent is evaporated at the same time, its diameter can be greatly reduced from hundreds of micrometers to as small as tens of nanometers. Finally, the jet will be solidified, leaving the bare charged polymer fibres on the collector. The basic electrospinning process has more recently been extended to compound co-electrospinning and precise deposition electrospinning to further broaden accessible fibre architectures and the potential areas of application. Besides, a few companies have started to develop electrospun nanofiber products on the basis of large-scale electrospinning setups.
Although the setup for electrospinning is very simple, there are several influencing factors to be considered during the fibre preparing process. In principle, nearly all soluble or fusible polymers can be processed into fibres by electrospinning, provided that a large set of parameters that influence electrospinning, for example, the parameters of the polymer (such as molecular weight, molecular-weight distribution, solubility, glass-transition temperature, melting point, and the pH value) and the electrospinning process (such as concentration, electrical conductivity, surface tension, feed rate, the work distance (the distance between the collector and the tip of the needle), temperature, and relative humidity) are correctly adjusted. Different electrospinning parameters can lead to fibres with very different morphologies (for example, cylindrical, belt, or porous) and dimensions, which are controlled by three main forces: the electrostatic force, the viscoelastic force, and the surface tension. High surface tensions tend to deform the liquid jet in droplets for minimizing the air/jet interface. To hinder the effect of surface tension, either large viscoelastic forces that resist rapid shape changes, and/or strong electrostatic repulsions among charges along the jet, are required to form smooth fibres without beads. Here we will tell you how to do spinning by introducing several important parameters.
The concentration of polymer is very important, as it will determine whether fibres can be electrospun or not. At lower concentrations, increasingly thinner fibres are formed, with additional beads along the fibre axis. Generally, increasing the polymer concentration or adding a pinch of surfactant to the electrospun solution could decrease the number of beads, so that fibres with diameters down to a few nanometers can be produced. It is observed that fibres become more uniform and form a cylindrical shape with increasing polymer concentration in solution; fibre diameters also increase significantly with increasing polymer concentration. At very high dilution, fibre formation no longer takes place because the surface tension will cause the polymer to break up into droplets before reaching the collector. The fibre morphology can also very often be cylindrical by the appropriate choice of solvent mixture (high-boiling/low-boiling solvents).
The viscosity of electrospun solution, which is affected by the molecular weight and concentration of polymer, and the temperature and relative humidity of the surroundings, has a similar effect on the electrospun fibres. In principle, for higher molecular weight and/or concentration, the viscosity of the solution increases accordingly. If the molecular weight of the polymer is too low, fibre formation no longer takes place as the viscosity of the solution cannot reach the electrospinning requirements even with a high polymer concentration. Conversely, if the viscosity of the solution is too high when excess polymer is added, it is also impossible to form electrospun fibres. Therefore, all polymers have an optimal molecular weight/concentration range in which they can be electrospun successfully. Within this optimal range, higher molecular weight/concentration leads to increased fibre diameters.
The flow rate of the polymer solution and the volatility of the solvent have an impact on fibre morphology, including diameter and porosity. At a higher flow rate, the diameter and pore size increase accordingly. Upon further increasing the follow rate, fibres may not be dried completely before reaching the collector, which may result in beaded morphology. Beaded morphology caused by inadequate fibre drying can also occur when the work distance is too short, or result in meshes with interconnected fibres. Preferably, quite volatile solvents are used in electrospinning; all solvents have to evaporate in order to get nice fibres with similar diameters. Highly volatile solvents tend to form a porous surface morphology, as they lead to the formation of fibres immediately surrounded by vapor.
It is not possible to make a general recommendation for particular concentrations and the resulting viscosities and conductivities, because the ideal values of these parameters vary considerably with the polymer–solvent system. As shown above, the variation of electrospinning parameters can lead to diverse possibilities for the physical and chemical properties of the electrospun materials. Further possibilities are provided by the choice of polymer–solvent system.
Besides the common circular shape for the cross-section of the solid electrospun nanofibres, complex architectures with higher specific surface area can also be obtained. Co-electrospinning is based on a spinneret consisting of two or more coaxial capillaries with different diameters. By co-electrospinning different fluids with this spinneret, fibres with hollow, multi-walled, or core–sheath structures could be fabricated. Besides, an emulsion electrospinning technique has been developed because it can encapsulate functional materials within fibres to form porous or core–shell structures without the need for a complex spinneret. In addition, porous fibres have also been prepared by adding appropriate amounts of other removable materials or electrospinning into a cryogenic liquid.
It is well known that the ability to control the arrangement of fibres is critical for enhancing the mechanical performance of the materials. For electrospinning, the charged fibre is often deposited onto the grounded collector and randomly oriented. Various methods have been tried to align the electrospun fibres, and several successful methods have been found based on external mechanical, electronic and/or magnetic fields. For example, by changing the grounded collector to two pieces of conductive strips (silicon or Au) separated by a void gap, the electrospun fibres could be parallelly aligned across the gap, and more complex architectures can be obtained when three or more conductive strips are used; by transferring uniaxially aligned nanofibres onto the same substrate in a layer-by-layer fashion, multi-layered films with controllable hierarchical structures could be obtained; by fixing two magnets in parallel in the collector region, fibres could be aligned across the gap between the two magnets, regardless of whether the fibres are magnetic or not; by using a rotating drum as a collector, the fibres could also be parallel aligned.
From the above discussion, you may know how to electrospin and control the morphology and alignment of the fibres at the same time. However, it is not the end. As pure polymer electrospun fibres/mats have no other important applications except their use as scaffolds or filters, a lot of research studies on how to prepare functional fibres have been done to give full play to the advantages of electrospinning.5 Among the kinds of fibres prepared by electrospinning, attention is focused on the NP–electrospun fibres, which possess both the advantages of organic polymers (i.e. lightweight and flexibility) and the special functionality of inorganic species (i.e. excellent catalytic activity and unique optical performance). Broad areas of applications exist in Materials and life sciences for such nanofibres, including not only mechanical enhancement, sensing, catalysis, and energy, but also tissue engineering, biomedicine, and wound healing.5–8 Herein, we will demonstrate how to fabricate NP–electrospun fibres in the following sections.
Up to now, composite fibres have been mainly fabricated via combining the electrospinning technique with some other methods or by directly adding NPs into the electrospun solution.6 If the NPs are unable to well disperse within the electrospun solution or cannot be fabricated on a large scale, NP–electrospun fibres can be indirectly fabricated by post-treatment of the electrospun fibres, such as calcination, surface treatment, hydrothermal, or gas–solid reaction. Based on different post-treatment methods, NPs can be formed on the surfaces or within the fibres.
Composite fibres could also be easily prepared by a one-step electrospinning technique: by directly adding as-synthesized NPs into the polymer solution. This is the most straightforward strategy to fabricate composite electrospun fibres as long as the NPs are uniformly distributed within the electrospun solution. With years of development of electrospinning, various kinds of NPs with different morphologies and compositions have been directly electrospun, including 0D NPs, 1D NPs, 2D NPs, and other organic or biomolecules. It is notable that for materials with anisotropic structures, such as polymer chains, nanorods (NRs) and nanowires (NWs), electrospinning shows its potential ability to assemble with several advantages: the NPs are usually aligned along the long axial direction of the electrospun fibres on a large scale and a freestanding film can be prepared. In particular for those materials whose properties are determined by their aggregation or orientation state, such as AuNRs, besides the assembling effect, electrospinning could facilely control the spaces among particles at the same time, just by varying the amount of NPs added into the electrospun solution.9
However, the NPs are easily aggregated or cannot be well dispersed in the electrospun solution, and cluster-like structures form in the obtained composite fibres. To solve this problem, one effective solution is to deal with the surface of the NPs, and the other one is to mix two different solutions together that could dissolve the polymer and disperse the NPs separately. For example, when AuNPs that are fabricated with sodium citrate as a surfactant are electrospun, for preparing AuNP–poly(vinyl alcohol) (PVA) nanofibres, water can be chosen as the solvent because both AuNPs and PVA can well disperse or dissolve in water. However, when preparing AuNP–poly(acrylonitrile) (PAN) nanofibres, water is no longer used as solvent because neither AuNPs could disperse in N,N-dimethylformamide (DMF) nor PAN could dissolve in water. In this case, poly(vinyl pyrrolidone) (PVP) is treated on the surface of AuNPs to make the NPs well disperse in DMF.
Sometimes, bead formation may occur at high particle concentrations, which may lead to the aggregation of colloids in the bead structures and affect the properties of the fibres. A suitable solution to this problem is adding salt into the electrospun solution, which increases the net charge density, eliminates bead formation, and favours jet thinning, thus resulting in fibres with smaller diameters.
In summary, we have demonstrated how to prepare functional fibres by an electrospinning technique. Hopefully, you get a general idea now and can understand how to prepare a variety of fibres with special functionalities, especially multifunctional materials can be facilely prepared by electrospinning two or more kinds of NPs simultaneously. More importantly, you can use electrospinning to assemble NPs so that composite materials with better or novel properties could be achieved. Electrospinning is a straightforward, easily accessible technology that does not require huge expenditure or sophisticated equipment, thus you can set up a system yourself easily, and you will probably find that utilizing NPs in electrospinning will be one of the most powerful tools for fabricating functional fibres and composite materials.
Acknowledgements
We acknowledge the funding support from the National Natural Science Foundation of China (Grants 51403195, 21521001, and 21431006), the National Basic Research Program of China (Grants 2014CB931800 and 2013CB933900), and the Users with Excellence of Hefei Science Center of Chinese Academy of Sciences (Grant 2015HSC-UE007).References
- J. W. Liu, H. W. Liang and S. H. Yu, Chem. Rev., 2012, 112, 4770 CrossRef CAS PubMed.
- A. Greiner and J. Wendorff, Angew. Chem., Int. Ed., 2007, 46, 5670 CrossRef CAS PubMed.
- D. Li and Y. Xia, Adv. Mater., 2004, 16, 1151 CrossRef CAS.
- C. J. Luo, S. D. Stoyanov, E. Stride, E. Pelan and M. Edirisinghe, Chem. Soc. Rev., 2012, 41, 4708 RSC.
- C. L. Zhang and S. H. Yu, Chem. Soc. Rev., 2014, 43, 4423 RSC.
- X. Lu, C. Wang and Y. Wei, Small, 2009, 5, 2349 CrossRef CAS PubMed.
- C. Huang, S. J. Soenen, J. Rejman, B. Lucas, K. Braeckmans, J. Demeester and S. C. De Smedt, Chem. Soc. Rev., 2011, 40, 2417 RSC.
- S. Agarwal, J. H. Wendorff and A. Greiner, Adv. Mater., 2009, 21, 3343 CrossRef CAS PubMed.
- C. L. Zhang, K. P. Lv, H. P. Cong and S. H. Yu, Small, 2012, 8, 648 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |
