Open Access Article
Open Access ArticleTraceless linkers used for reversible protein–polymer conjugations
Douglas A. Rose†
ab,
Zihuan Fu† ab,
Mikayla F. Tanab,
Daniele Vinciguerra
ab,
Mikayla F. Tanab,
Daniele Vinciguerra ab,
Priera H. Panescuab and
Heather D. Maynard
ab,
Priera H. Panescuab and
Heather D. Maynard *ab
*ab
aDepartment of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, California 90095, USA. E-mail: hmaynard@ucla.edu
bCalifornia NanoSystems Institute, University of California, Los Angeles, Los Angeles, California 90095, USA
First published on 30th January 2026
Abstract
Proteins and peptides are an important class of biomolecules employed as therapeutics. Polymer conjugation to therapeutic proteins and peptides can improve their stability and circulation time, as well as reduce aggregation compared to the native biomolecule. However, the steric effect of a large polymer has the potential to drastically reduce or even completely inhibit the bioactivity of the protein. In these cases, traceless and reversible protein–polymer conjugation, in which native protein is released upon exposure to specific stimuli, can be utilized to both mitigate the undesirable effect of conjugation, while also taking advantage of the benefits prior to the cargo delivery. In this review, various linkers used in the reversible conjugations of polymers onto proteins are discussed.
Introduction
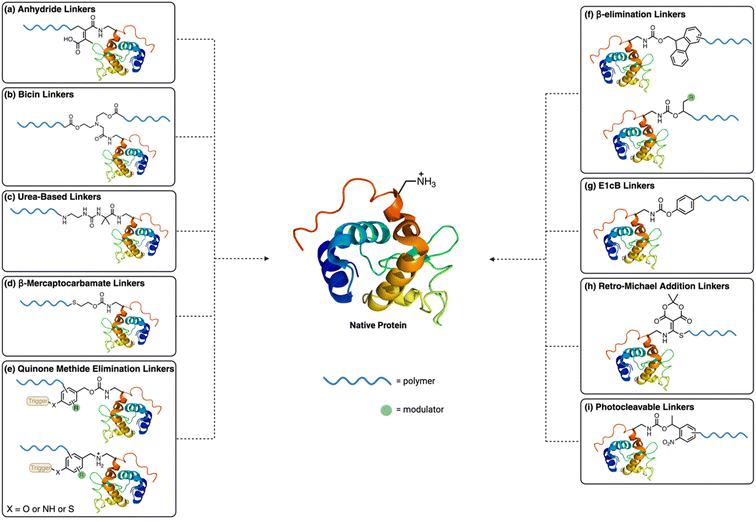
Protein conjugation is a versatile tool that allows for the alteration of protein stability, activity, and functionality.1 Specifically, polymer conjugation to therapeutically relevant proteins often results in an increased stability and circulation time in vivo.2,3 As a result, there are over 30 protein–polymer conjugates approved by FDA used in the clinic and many more in various stages of clinical trials, all of which are protein-poly(ethylene glycol) (PEG) conjugates.4 However, the addition of polymers can lead to a significant or complete loss of activity compared to the native proteins, especially when amino acid residues near a protein binding site are modified.5–7 To minimize such undesired effects, site-specific conjugation techniques can be employed to ensure that placement of the polymer is distant from the active site.8 However, this is not broadly applicable to all proteins of interest and requires a tailor-made strategy for each, resulting in a significant investment of time and resources.Another approach to circumvent this loss of activity is to place unstable linkages between the protein and the polymer substrate to create a reversible conjugate. These reversible linkages are referred to as traceless linkers. The resulting conjugates are fundamentally different than degradable polymer–protein conjugates, as the result is full release of the native proteins, rather than protein with attached small chemical fragments. This in turn allows for a stimulus-dependent recovery of protein activity (Fig. 1). Indeed, traceless linkers have made a significant impact on the field of protein–polymer conjugates over the past decade, during which two conjugates, Ascendis Pharmaceutical's palopegteriparatide9 and lonapegsomatropin,10 were approved by the FDA for clinical use.
Several aspects of traceless linkers are crucial to consider when designing protein conjugates including conjugation rate, selectivity, reversibility, and rate of release, especially when protein activity is of a high importance, or a long-acting therapeutic is needed. Several reviews have been published detailing traceless linkers and reversible conjugation in general.11–14 The reversible linkers detailed in this review have also been extensively utilized in the related fields such as antibody-drug conjugates (ADCs), prodrugs, and other bioconjugates. There are excellent reviews on these subjects.15–17 This review will focus on an overview for the various traceless linkers specifically used in the context of protein–polymer conjugation chemistries to help bioconjugation chemists choose a linker based on their desired application.
Lysine and N-terminus modification
Lysine residues are the most commonly used handles for bioconjugation reactions due to the high prevalence and reactivity of the amines across a wide array of proteins.18 This has led to the largest diversity in traceless linker conjugation strategies for lysine and N-terminal amines.Anhydride linkers
The first recorded demonstration of reversible protein conjugation employed maleic anhydride to modify the lysine residues prior to a tryptic digest or purification step (Fig. 2a).19–21 The linkers were subsequently reversed by hydrolysis under acidic conditions (pH 2–4). However, these reports showed little data on the rate of release because that was not the primary focus of the work. Once identified as a potential reversible strategy, it was quickly demonstrated that a substituted maleic anhydride with a variety of functionalities including sulfonates, sugars, fluorophores, and polymers could be used to reversibly modify protein solubility, stability, or functionality.22–24 Garman and Kalindjian demonstrated that urokinase-MA-PEG5000 conjugate was able to regenerate its activity upon incubation at pH 7.4 and 37 °C with a half-life of 6.1 hours presumably by PEG removal (Fig. 3).22 More recently, deeper examination into the anhydride class of linkers demonstrated release kinetics for a variety of different payloads across multiple proteins. It was shown that the rate at pH 5 is sufficient to achieve >80% protein release within 12 hours.25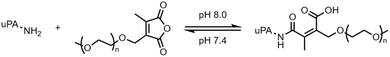 | ||
| Fig. 3 Synthesis and cleavage of urokinase-PEG conjugate.22 | ||
Tumors have more acidic endosomes than normal cells and they also develop acidic extracellular microenvironments with pH ≈ 6.5–7.0; therefore, acid sensitive substituted maleic anhydride linkers are intriguing candidates for anti-tumor applications.26 This feature was exploited to deliver antitumoral therapeutic RNase A. Specifically, RNase A was conjugated to a histidine-rich cationic oligomer for enhanced protein transduction. Whereupon entering the acidic environment of the endosome, the linker was cleaved, releasing native RNase A into the cytosol.27 Liu and coworkers designed a pH-responsive hyaluronidase (HAse) delivery system that used a 3-(bromomethyl)-4-methyl-2,5-furadione linker in the formation of a dextran-based HAse nanoparticle. Release of the protein in the extracellular matrix that is responsible for the irregular tumor microenvironment (TME) was evidenced by the rapid decrease in nanoparticle size when buffer exchanging from pH 7.4 to pH 6.0 (Fig. 4). Furthermore, a measured increase in enzymatic activity after exposure to weakly acidic conditions in vitro further confirmed the release of HAse. Lastly, in vivo fluorescence images indicated a decrease in tumor vasculature when treated with Dex-HAse.28 This research group then applied the same methylmaleic anhydride linker in the preparation of blood–brain-barrier (BBB) crossing antibody–polymer conjugates that would release upon entering glioma tumors. In vivo and ex vivo studies confirmed significant tumor growth suppression upon treatment with such conjugates.29 Other types of therapies such as anticancer immunotherapy or enhanced cancer combination therapies have also been recently developed using the same reversible conjugation of biocompatible polymers to various biologics.30,31
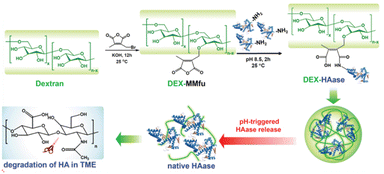 | ||
| Fig. 4 DEX-HAase conjugate releases HAase in tumor microenvironment. Reproduced from (ref. 28) with permission from John Wiley & Sons. | ||
In addition to cancer treatment applications, the anhydride linkers have been shown to help in the design and improvement of other types of drug delivery vehicles. Kandil et al. used a dimethylmaleic anhydride moiety to modify the peptide melittin in order to prepare an improved siRNA polyethylenimine (PEI) delivery system with enhanced control of drug release in endosomal compartments.32 Dynamic light scattering (DLS) and laser Doppler anemometry analyses confirmed that in acidic conditions, the zeta potential of the polyplexes increased as a result of the unmasking of lysine residues on the peptide surface. This further confirms the hypothesis that there would be successful cleavage upon entering the endosome where the pH is in the range of 4.5–6.0. Of particular interest is the synthesis of protein-containing nanoparticles or nanogels utilizing substituted maleic anhydride linkers. Nanosystems leveraging these linkers conjugated to model proteins myoglobin33 and BSA34 have been developed to demonstrate the potential of these drug delivery systems.
Chapman and coworkers have also demonstrated a maleic anhydride-furan Diels–Alder adduct linker.35 Instead of the conformation lock achieved with the maleic anhydride double bond, it was replaced with the rigid bicyclic Diels–Alder adduct moiety, resulting in a similar pH sensitivity. This linker was then used to conjugate PEG onto antibody to human serum antigen (a-HSA). The binding activity of the antibody was reduced significantly upon conjugating as little as 5 equivalents of PEG. Then, after incubating at pH 5.5 buffer for 12 hours, almost 90% of the original activity was recovered.
Bicin linkers
Another cyclization-based linker, the bicin linker (Fig. 2b), was initially demonstrated by Greenwald et al., which allows for the hydrolysis of an amide linkage to the protein through the anchimeric assistance of two ethyl alcohol-based appendages.36 These ester-containing appendages once hydrolyzed then backbite on the amide, thus favoring release of the amine and the resulting 4-(2-hydroxyethyl)morpholin-2-one byproduct (Fig. 5). This was originally demonstrated through the conjugation of branched or linear PEG to lysozyme to form the mono-PEGylated conjugate. Upon incubation in rat plasma at 37 °C, native lysozyme was slowly released with varying half-lives depending on the linker structure.37 This strategy was then adapted for the delivery of SS1P, a recombinant anti-mesothelin immunotoxin. The SS1P was PEGylated with either linear or branched PEG-bicin derivatives. These conjugates showed a 10-fold increased half-life compared to native SS1P in vivo, and the linear and branched conjugates reduced tumor size by 68 and 92%, respectively using a xenografted tumor mouse model.38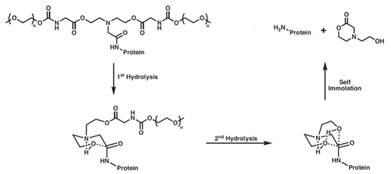 | ||
| Fig. 5 Traceless hydrolytic release of native protein from a PEGylated conjugate through use of a bicin linker.36 | ||
Urea-based cyclization linkers
Similar to the bicin linkers, urea-based linkers release native protein payloads via a cyclization mechanism (Fig. 2c).39 Ascendis Pharmaceutical utilized this type of linker in the successful development of Yorvipath (Palopegteriparatide) for the treatment of hypoparathyroidism (Fig. 6a). Hypoparathyroidism is an endocrine-deficiency disease characterized by low serum calcium levels, elevated serum phosphorus levels, and lack of parathyroid hormone (PTH) in the circulation.40 Full-sized parathyroid hormone, PTH(1-84), has a short half-life of approximately 3 hours following subcutaneous administrations, thus limiting its effectiveness. Palopegteriparatide is a PEGylated inactive prodrug of the N-terminal fragment of parathyroid hormone, PTH(1-34). The peptide is conjugated with two PEG20000 chains using the urea-based linker. After injection, the β-amino group can assist the backbiting and subsequent hydrolysis of the amides by the urea, resulting in the release of native peptide, as well as the cyclization byproduct 2,4-imidazolidinedione (Fig. 6b). The PEG increases the circulation time of PTH and gradually releases active PTH with a half-life of approximately 2.5 days, thus maintaining a steady concentration of PTH in the bloodstream.41 In 2024, the FDA approved Palopegteriparatide as a long-acting, daily injection treatment of adult hypoparathyroidism.9 | ||
| Fig. 6 (a) Ascendis pharmaceutical's palopegteriparatide long-acting PTH-PEG conjugate. (b) Traceless release release of native proteins via urea-based linker. | ||
β-Mercaptocarbamate linkers
The β-mercaptocarbamate linkage was initially developed as an alternative stimuli-responsive traceless protein linker by Chen et al. to include a thiol specific triggering mechanism (Fig. 2d).42 In the presence of 5 mM glutathione (GSH), the β-mercaptocarbamate would undergo a thiol–thioester exchange which unmasked the thiol, resulting in an intramolecular cyclization to release the native protein along with carbon dioxide and ethylene sulfide byproducts (Fig. 7). This strategy was demonstrated through the mono-PEGylation of lysozyme, which had a half-life of 0.73 hours in PBS at 37 °C in the presence of 5 mM GSH, simulating cytosolic conditions. Following lysozyme release, protein activity was recovered showing no significant difference when compared to fresh lysozyme. However, no release or recovery of activity was observed following incubation in the absence of GSH, signifying its importance in the release mechanism.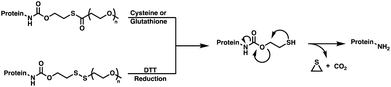 | ||
| Fig. 7 Thioester (top) or disulfide (bottom) triggered mechanism of protein release for the β-mercaptocarbamate traceless linkers. | ||
Following this report, researchers began using disulfides as an alternative chemical trigger to the thioesters (Fig. 7). Dutta et al. demonstrated this switch by using cytochrome C (CytC) to crosslink poly(methyl methacrylate) functionalized with the a β-mercaptocarbonate side chain.43 The resulting CytC nanogels were shown to display near quantitative recovery of protein activity after incubation under reducing conditions for 4 hours at 37 °C. Recently, Scherger et al. demonstrated the ability to carry out a post-polymerization modification of a RAFT-polymerization-based thiocarbonylthio end-group to an activated β-mercaptocarbonate.44 The same team then optimized the reactive handle to install a imidazole carbamate moiety for more facile conjugation to lysine residues.45 This chemistry was then used to conjugate poly(N,N-dimethylacrylamide) (pDMA) polymers to lysozyme and a nanobody against the mannose receptor of macrophages (α-MMR Nb). It was demonstrated that both protein–polymer hybrids with traceless linkers can be released upon exposure to reducing conditions with near full restoration. In addition, this disulfide strategy was employed in the preparation of pDMA-Toll-like receptor agonist conjugate, resulting in a prodrug that releases the cargo in a controlled manner.46
In another paper, stimuli-responsive protein nanogels were fabricated such that upon conjugation of the polymer to the protein, dithioethylcarbamate bonds were be formed, which were cleaved when exposed to glutathione or dithiothreitol (Fig. 8).47 After introduction of the nanogel to a 10 mM solution of glutathione, DLS measurements were taken at regular time intervals. The size of the nanogel decreased over time, indicating release of the protein. Additionally, SDS PAGE analysis confirmed release of protein upon addition of DTT to the sample. Similarly, Thayumanavan and coworkers prepared a dithioethylcarbonate-containing drug nanoassembly that tracelessly releases the attached drug molecule bortezomib.48
 | ||
| Fig. 8 Traceless releases of proteins from stimuli-responsive nanogel. Reproduced from (ref. 47) with permission from the Royal Society of Chemistry. | ||
Quinone methide elimination linkers
The quinone methide elimination class of linkers is the most prevalent and diverse set of traceless linkers for lysine modifications (Fig. 2e). The first demonstration of this chemistry was performed using a small molecule model system wherein an aniline-based linker was masked with a lysine residue. Upon cleavage of the lysine residue using trypsin, the aniline underwent a 1,6-elimination to release p-nitroaniline as a colorimetric reporter molecule.49Following the seminal publication, the applicability of this linker to protein–polymer conjugation was quickly identified and diversified using a variety of strategies to unmask protecting groups, including disulfide reduction,50–55 ester hydrolysis,56,57 and boronate oxidation.58–65 All of these examples result in the deprotection of either a phenol, thiophenol, or aniline head group that subsequently undergoes a 1,6-elimination reaction to release the protein cargo, as well as quinone methide and CO2 byproducts.
In 2021, the FDA approved Ascendis Pharmaceutical's Skytrofa (lonapegsomatropin) for use as a human growth hormone (hGH) replacement in pediatric patients.10,66,67 And in 2025, the FDA approval was expanded to the treatment of adult growth hormone deficiency.68 hGH is an ideal protein for the use of a traceless linker due to its short half-life of 20 minutes and its extreme activity loss, with less than 10% of its native activity, when PEGylated. Lonapegsomatropin is a PEGylated version of hGH containing a 4-arm PEG group attached to the hGH through a carbamate masked 1,6-benzyl carbamate linkage (Fig. 9). The release of native hGH was monitored in vitro and shown to have a half-life of 75 hours in PBS at 37 °C. Further studies in cynomogus monkeys showed that a single administration of the PEGylated hGH prodrug resulted in higher levels of IGF-1, a pharmacodynamic marker for hGH, when compared to daily hGH administration. This was confirmed in phase 3 clinical trial comparing a once weekly subcutaneous administration of the lonapegsomatropin prodrug at 0.24 mg kg−1, which outperformed daily hGH injections in pediatric patients with human growth hormone deficiency.69 This particular example further demonstrates the usefulness of traceless linkers when applied to protein–polymer therapeutics, in reducing the frequency of therapeutic injections, as well as improving patient outcomes.
Our group with Houk reported a new class of traceless linkers based on ortho- or para-hydroxybenzylamines.70 These linkers are stable in aqueous environments and can be conjugated to amines via reductive aminations on lysine and the N-terminal amine residue in a wide range of pH values. Compared to benzyl carbamates, these linkers produce a secondary amine, thus maintaining the isoelectric point of the protein. Once the protection group on the phenol is unmasked, the amine containing payload was slowly released (Fig. 10) via either 1,6- or 1,4-elimination. Importantly, the rate of release could be tuned by altering the electronics of the linkers substituents. In this work, the model enzyme lysozyme was used. After conjugation, it was determined that mono-PEGylated lysozyme lost approximately 30% of its original activity. However, upon release of the linker, near full recovery of lysozyme activity was seen. Recently, we demonstrated that using polycyclic aromatic cores instead of a benzene core, allows the rate of release to be increased significantly.71 Together, these two reports demonstrate half-lives ranging from 18 minutes to 14 days were achieved, further extending the applicability of these linkers.
 | ||
| Fig. 10 Hydroxybenzylamine traceless linkers releases lysozyme from lysozyme-PEG conjugate. Reprinted with permission from (ref. 70). Copyright 2022 American Chemical Society. | ||
β-elimination linkers
The fluorenylmethyloxycarbonyl (Fmoc) based linkers are another prevalent class of traceless linkers used in the preparation of protein conjugates (Fig. 2f). Small molecule Fmoc groups were first used for reversibly modifying any surface accessible lysines present on the protein of interest to toggle activity on and off.72–74 This was later advanced to using a sulfonated Fmoc-containing PEG species to modify the lysine residues of various therapeutically relevant proteins including interferon α,75 human growth hormone,76 insulin,77 and enkephalin.78 These traceless protein conjugates all demonstrated an increased circulation time in vivo with a steady release of native protein over time.The versatility of this linker has led Nektar therapeutics to develop a long acting IL2 protein polymer conjugate relying on an Fmoc derived β-elimination linker. The traceless protein–polymer conjugate consists of 6 PEG chains conjugated to IL2 resulting in a 4-fold increase in the half-life in vivo compared to the non-PEGylated control. The PEG chains slowly release at physiological pH, creating conjugated-IL2 species with fewer PEG chains and increased bioactivity. Sustained signaling through the heterodimeric IL2 receptor pathway (IL2Rβγ) preferentially activates and expands effector CD8 T and NK cells over Tregs.79–81 Unfortunately, it failed its Phase III clinical trial in 2022 because its benefit in combination with other anti-cancer therapeutics was found to be statistically insignificant.82
An alternative β-elimination traceless linker was recently developed by Santi et al. who created a tunable set of traceless linkers by modulating the pKa of the β-proton to the carbamate linkage (Fig. 2f).83 This was controlled by modulating the electronics of the substituents, which in turn control acidity of the C–H bond on the linker. This approach allowed the creation of a set of linkers with half-lives ranging from 14 to 10500 hours at pH 7.4 and 37 °C. This technology was applied to exenatide, a glucagon-like peptide-1 agonist, by conjugation to a traceless PEG or incorporation into a degradable hydrogel material.84–86 In both instances, the peptide was linked to the polymeric material through the aforementioned β-elimination linkers. The resulting release of the peptide was monitored in vivo and demonstrated a 56-fold increase to the half-life compared to that of the unmodified peptide.
E1cB linkers
Phenyl carbamate linkages (Fig. 2g) attached to lysine residues can be degraded via an E1cB mechanism. In neutral to basic aqueous conditions, the carbamate undergoes elimination to form an isocyanate intermediate, which is rapidly hydrolyzed to release the primary amine and CO2 byproduct. This strategy was initially demonstrated by Brandl et al., who modified the end-group of a 4-arm PEG species to contain an activated aryl carbonate.87 Lysozyme and BSA was then used to crosslink the multi-arm PEG species to form a hydrogel. Degradation of the resulting hydrogel was observed at pH 9.0 and 50 °C over a week, after an initial 24 hour onset period where almost no protein was released. A similar strategy to modify lysozyme was carried out by the same group using a linear PEG derivative rather than the multi-arm PEG.88 The structure of the linker was also diversified by altering the electronics of the aromatic core, which in turn displayed different rates of lysozyme release varying from 63% lysozyme released in 24 hours to 44% released over 28 days. This linker demonstrated a maximum release of 63%, which the authors hypothesized was due a side reaction between the amines on the protein reacting with the isocyanate intermediate produced, resulting in protein dimerization; this side product was observed via SDS-PAGE.Retro-Michael addition linkers
Retro-Michael addition has recently been investigated as a mechanism of release for lysine based traceless linkers (Fig. 2h). This concept was initially demonstrated by Diehl et al., who showed that the Michael addition of a primary amine onto a derivative of Meldrum's acid could be reversed in the presence of a thiol containing “decoupling agent” like dithiothreitol, cysteine, or ethanedithiol (Fig. 11).89 To demonstrate the ability of this linker to function as a traceless linker, the researchers functionalized the lysine residues on myoglobin, followed by a secondary addition of PEG-thiol. This PEGylated protein was then incubated with the decoupling agent for 36 hours, and quantitative release of the native myoglobin was observed by LCMS. | ||
| Fig. 11 Meldrums acid derived traceless linker relying on a retro-Michael addition to facilitate protein release. | ||
Zhuang et al. more recently demonstrated an alternative approach through the use of a functionalized α,β-unsaturated carbonyl compound.90 These linkers were demonstrated as traceless through their initial modification of β-lactoglobulin B and subsequent displacement by 2-(2-methoxyethoxy)ethanethiol over the course of two hours. This reaction is not, however, specific for the lysine residue and does simultaneously modify cysteine residues, which are also reversible.
Photocleavable linkers
Photocleavable linkers have long been used as an orthogonal protecting group strategy towards the protection of amines; typically, an o-nitrobenzyl group has been employed for this purpose (Fig. 2i). This concept was initially brought into the field of protein polymer conjugates by Georgianna et al. by modifying a PEG end-group with an o-nitrobenzyl group.91 This PEG was then used to modify the lysine side chains of lysozyme producing an average of 4 PEG chains per protein. Irradiation of the conjugate with 365 nm light resulted in the full restoration of lysozyme activity. A similar strategy was employed by Karas et al. in the synthesis of an amyloid-β peptide fragment.92 The peptide was highly prone to aggregation, making solid-phase peptide synthesis and the subsequent HPLC purification challenging. The incorporation of a triethylene glycol tag through a o-nitrobenzyl group to a lysine residue on the peptide reduced aggregation prior to cleavage from the resin and during HPLC purification. The triethylene glycol tag was readily removed via irradiation at 365 nm without significant degradation, allowing for the study of the aggregated fibril formation.Our group also published the synthesis of noncovalent enzyme nanogels utilizing a heterobifunctional, photocleavable linker. Initial covalent modification with this linker established a polymerizable handle at the surface of phenylalanine ammonia lyase (PAL), followed by radical polymerization with poly(ethylene glycol) methacrylate (PEGMA) monomer and ethylene glycol dimethacrylate crosslinker in solution. Final photoirradiation cleaved the linkage between the polymer and PAL to afford the noncovalent enzyme nanogels with enhanced enzymatic activity. Furthermore, the stability of PAL after exposure to trypsin or low pH was assessed and was found to be more stable in the noncovalent nanogel compared to the enzyme alone.93 We also used this chemistry to prepare poly(polyethylene glycol) acrylate (PEGA)-lysozyme conjugates with both grafting-to and grafting-from strategies. Following UV irradiation, 83% of the lysozyme activity was recovered.94
Cysteine modifications
Polymer conjugation to cysteine residues is also prevalent in the bioconjugation field due to their numerous advantages over other residues. For instance, due to its natural low abundance in proteins, cysteine residues are often targeted when site-selective conjugation is desired. Additionally, the unique nucleophilicity of the thiol containing cysteine residue allows for relatively quick and efficient conjugation reactions.95,96 Nonetheless, some proteins do not contain any cysteine residues, or the few present are hidden in hydrophobic pockets and/or involved in disulfide linkages vital to protein structure. In these cases, cysteine can be introduced using protein engineering techniques such as mutagenesis.97,98Importantly, due to their unique reactivity, cysteines are often structural components of the protein active site and their modification can greatly diminish protein activity.99,100 Therefore, developing conjugation strategies able to reversibly bind to cysteine and subsequently release the native protein in a traceless fashion is of particular significance for turning “off–on” protein activity. In this section, we will explore reversible conjugation reactions that specifically target cysteines or disulfide bridges.
Retro-Michael addition linkers
The gold standard in reversible cysteine conjugation has been the use of pyridyl disulfide to form a disulfide bond.101–103 This strategy has been discussed thoroughly elsewhere and will not be covered in detail in this review.Another common cysteine bioconjugation strategy is conjugation to maleimides which are known for their reactivity towards thiols as Michael acceptors. Generally, they are regarded as stable and non-reversible linkages, however, it has been demonstrated that in specific conditions they can undergo retro and exchange reactions in the presence of other thiols.104 An important factor when designing these linkers is that this reversibility can be eliminated by a hydrolytic ring-opening reaction.104–107
Oppositely, to make the thioether bond more readily reversible, maleimide analogues carrying leaving groups, such as bromine atoms have been designed. The resulting vinyl sulfide adduct has a higher propensity to undergo thiol-exchange reactions, which in turn regenerates the native peptide.108–113 For instance, the Grb2 adaptor protein containing a single cysteine mutation (L111C) was reacted with 1 equivalent of N-methyl bromomaleimide, resulting in complete conversion within one hour.108 The reaction was selective for the cysteine residue over the 8 surface accessible lysine residues present on the protein. When the conjugate was treated with an excess of TCEP, 85% of the native protein was released in 3 hours at 0 °C. Using a dibromo-substituted maleimide, a second functionalization can be performed, as was demonstrated with glutathione or thioglucose.109 In each case, an excess of 2-mercaptoethanol, TCEP, or glutathione was required in order to release the native protein. It was observed that native protein was released within 4 hours under conditions mimicking the cell cytoplasm. Interestingly, the incorporation of an electron withdrawing N-substituent, e.g. N-phenylmaleimides, increased the occurrence of an undesirable ring-opening side reaction, thus creating an irreversible conjugate unable to release the protein.110 The reversible chemistry was applied through a chemical vapor deposition polymerization of 4-(3,4-dibromomaleimide)[2.2]paracyclophane to form a polymer coating able to react with and release thiolated peptides. This strategy could be applied for the functionalization of biomedical sensing and diagnostic materials.113
Other classes of Michael acceptors exist and are utilized as reversible and traceless linkers, often with improved properties over maleimides. For instance, 5-methylene pyrrolones (5 MPs) exhibit high thiol specificity, improved stability under physiological conditions and traceless release at basic pH or by thiol exchange. 5 MPs were synthesized bearing different moieties as N-substituents including biotin, fluorescein or doxorubicin. These were subsequently conjugated to a histone H4 mutant (H4-R45C) containing a single cysteine. The conjugation was selective for cysteine and proceeded almost quantitively in two hours at a pH 7.5. The protein was then incubated in either a pH 9.5 solution or in the presence of glutathione at pH 7.5, both of which led to release of the unmodified protein.114
Similar to maleimide, pyridazinedione and its brominated derivatives were also designed to reversibly bind cysteine, while also demonstrating lower rates of hydrolysis compared to maleimides, with up to four possible points of chemical attachment.115–117 The Chudasama group reported that in the case of pyridazinedione, thiol addition and release rate can be tuned by modulating the electrophilicity of the pyridazinedione.115 Tripeptide CGY conjugated hydrogel was used as an example of this reversible cysteine modification. CGY was first conjugated to a bicyclononyne (BCN) functionalized pyridazinedione. This conjugate was then reacted with a 4-arm PEG azide via strain-promoted azide–alkyne cycloaddition (SPAAC) to achieve one CGY peptide per PEG chain on average. The resulting 4-arm PEG-CGY azide was again reacted with a 4-arm PEG-BCN via SPACC to achieve the hydrogel material. After incubating this hydrogel material in pH 7.4 PBS buffer, an increase in free CGY concentration was observed by HPLC, showing the release of cargo from the hydrogel (Fig. 12).117 The chemical reversibility of dibromopyridazinedione conjugation was also demonstrated upon incubation in the presence of a large excess of 2-mercaptoethanol or cytosolic glutathione concentrations, which released native protein within one hour.115 The high versatility of this scaffold was later shown when three different functionalities were incorporated within a single pyridazinedione–protein conjugate. A dual clickable dibromo pyridazinedione bearing an azide and a tetrazine was designed, synthesized and reacted initially with the protein, and subsequently with bicyclo[6.1.0]nonyne (BCN)-fluorescein and dibenzocyclooctyl-(DBCO)-biotin, to attach the tetrazine and the azide, respectively. The remaining bromo group was subsequently displaced by either a cysteine-containing peptide or an azide functionalized aniline, which was later used to incorporate PEG. Neither of the conjugates released the protein at normal blood concentrations of glutathione over 24 hours and were stable in serum for 7 days. Only the conjugates incorporating the cysteine-containing peptides released native protein in the presence of high glutathione concentrations, while the aniline conjugates showed no cleavage of the pyridazinedione. The increased stability of the aniline conjugate was attributed to decreased electrophilicity of the resulting linker, preventing the thiol displacement necessary to release native protein.118
 | ||
| Fig. 12 Pyridazinedione-based hydrogel releases CGY peptide. Adapted from (ref. 117). Copyright 2023 Royal Society of Chemistry. | ||
In 2020, Zhuang et al. developed a chemical switch based on a Triggerable Michael Acceptor (TMAc) bearing a good leaving group at the β position.90 The acceptor is initially coupled to a nucleophile, resulting in the formation of an α,β-unsaturated carbonyl. The presence of a second, stronger nucleophile results in a Michael addition and subsequent release of the initial nucleophile. The unique structure of the TMAc allows for a modular electronic design of the linker to fine tune the kinetics of the system. The concept was applied to selectively modify β-lactoglobulin B (βLGb) on its free cysteine using the appropriate TMAc. After, myoglobin (Myo) was modified with another TMAc and subsequently released with an excess of thiol within 2 hours. A similar concept was employed using a 4-substituted cyclopentenone with fast kinetics. In the presence of a Michael donor, the conjugated protein underwent a traceless release with no observable impact on protein structure or functionality. UBXD protein was used as a model and reacted with the cyclopentenone. The reaction occurred within one hour with a high specificity for cysteine residues, while showing no impact on the protein structure. The linker could be removed in 3 hours with an excess of mercaptoethanol.119
Maity et al. reported a light-controlled strategy for the reversible modification of Michael addition-linkers to the cysteine residue of a protein.120 Human Cellular Retinol Binding Protein II (hCRBPII) was labelled with a fluorescent ligand. Upon irradiation with UV light, the conjugation was reversed, presumably through a retro-Michael addition process, yielding a fluorescent species. This photoswitchable system enables better development of imaging and labeling of various proteins and can potentially be used in the design of future polymeric conjugates.
Unfortunately, there are very few examples of polymer conjugation reported using these strategies, largely because model studies are easier to carry out with small molecules. Although still uncommon, we believe most of the presented strategies could be applied to the polymer–protein conjugate field. Moreover, much of the currently published work does not investigate how these chemical modifications affect protein activity before and after traceless release. Assessment of protein activity before and after conjugation, as well as after release should become more widespread to verify that the reversible release is indeed traceless and does not negatively impact the protein function.
Pyridinium linkers
Recently, Wan et al. reported a pyridinium-based traceless linker for cysteine modification.121 Electron-deficient halo-pyridiniums can undergo rapid SNAr reactions with cysteine to form thiol-pyridinium adducts (Fig. 13). The rate of the reaction could be predicted and fine-tuned by adjusting the electronics of the substituents. Using bovine serum albumin (BSA) as a model protein, the authors demonstrated quantitative conjugation using a PEG3-alkyne bearing pyridiniums in under 10 minutes. The cleavability of this linker was examined by adding excess GSH. BSA recovery ranged from 35% to quantitative after 2 hours depending on the substitution position on the pyridinium ring. | ||
| Fig. 13 BSA protein releases tracelessly via pyridinium linkers. Reprinted with permission from (ref. 121). Copyright 2024 American Chemical Society. | ||
Disulfide bridging linkers
Disulfide bridges are found naturally in proteins and are often surface-exposed. Their primary function is to impart stability to the protein tertiary structure and therefore need to be preserved to maintain protein functionality.122 Brocchini et al. pioneered site-specific modification of various protein's disulfide bridges using bis-thiol alkylating PEG reagent.123,124 These first reports used irreversible conjugation, which can be detrimental to protein activity. For instance, in the case of human interferon α-2b, it led to a 92% loss of activity in vitro.124More recently, new linkers have been developed in an effort to achieve reversible conjugates, the most prevalent being the maleimide derivatives. The first example was provided by Smith et al. who developed dibromomaleimide as a new class of reversible bridging maleimide linker.108 TCEP was used to reduce the disulfide present in somatostatin, a 14 amino acid cyclic peptide, analogues of which are used in the treatment of acromegaly and gastroenteropancreatic tumors. Treatment of the reduced peptide with dibromomaleimide resulted in complete conversion to the disulfide bridged one. Researchers also demonstrated rapid conjugation of a fluorescein-maleimide to somatostatin. Exposure of the conjugate to another nucleophilic thiol, such as 2-mercaptoethanol, regenerated the reduced somatostatin. Once identified as a viable approach, this strategy was quickly extended to other peptides and explored for polymer conjugation. For instance, dibromomaleimide was functionalized with PEG via a modified Mitsunobu reaction and rapidly conjugated to salmon calcitonin (sCT), a 32 amino acid peptide used in the clinic for the treatment of various bone conditions. The authors then proceeded to demonstrate their method using an ATRP generated pPEGA. The initial attempt of employing a dibromomaleimide-functionalized initiator proved unsuccessful due to retardation of the polymerization by the maleimide moiety. To circumvent this issue, a strategy involving a postpolymerization modification of the initiator via a copper catalyzed azide–alkyne cycloaddition (CuAAC) click reaction or condensation reaction with dibromomaleic anhydride, was used to introduce the maleimide functionality. The conjugation reactions between ATRP polymer and sCT proceeded smoothly in less than 30 minutes (Fig. 14).125 The same strategy has been used for PEGylation of other proteins such as an antibody,126 BID, and RNase S variant peptide.127 This work has many advantages, such as low reaction time and no purification required due to the use of stoichiometric or near stoichiometric amount of reagents. While promising, the release and retention of activity was not investigated. Additionally, an initial reduction step is required, which could lead to protein unfolding, aggregation128 or disulfide scrambling129 due to the presence of free thiols before the conjugation.
 | ||
| Fig. 14 sCT disulfide bridging vith ATRP generated pPEGA via dibromomaleimide linker. Reprinted with permission from (ref. 125). Copyright 2012 American Chemical Society. | ||
To solve this issue, dithiophenolmaleimides, another class of maleimide derivatives, were developed for use in one-pot reactions with a reducing agent to limit any disulfide scrambling.130–132 The approach was demonstrated first by conjugating PEG to somatostatin. Initially, the two-step reaction, (reduction with TCEP followed by conjugation) led to complete bridging in 10 minutes with an equimolar amount of linker, which was a considerable improvement compared to aliphatic dithiomaleimide, requiring 10 equivalents and 1 hour to achieve complete functionalization. Later, one-pot in situ reduction of disulfides proved to be possible and quantitatively completed in 20 minutes, whereas use of dibromomaleimide resulted in only 60% functionalization due to its side reactions with TCEP.130 The conjugate showed retention of activity, but the release of native peptide was not demonstrated at this stage. Due to the higher tolerance of dithiophenolmaleimide to TCEP, the authors explored the possibility of using the linker as an ATRP initiator without any protecting groups. The PEGMA polymerization proceeded with acceptable linear first order kinetics, but similarly no release data was shown.131 Later, Collins et al. studied the reversibility of this strategy by conjugating a dithiophenolmalemide-functionalized pPEGMA to oxytocin, a cyclic peptide used to prevent postpartum hemorrhaging which has very limited stability in solution.132 The peptide disulfide was first reduced with TCEP, followed by reaction with the functional polymer overnight at 10 °C. After purification, the resulting conjugate was tested for stability which was greatly improved compared to the native peptide: after 28 days in accelerated conditions, only 2.5% of native oxytocin remained while 93.9% of oxytocin-p(PEGA480)20, and 86.5% of p(PEGA480)100 remained respectively. Finally, when the conjugate was exposed to an excess of glutathione (GSH) in the biorelevant range the native peptide was quantitatively released over 4 days.
Aryloxymaleimides are another class of maleimide derivatives with attenuated reactivity; this results in a higher selectivity for disulfide bridging rather than formation of the bis-adduct seen frequently when using dibromomaleimide. They are also resistant to TCEP, allowing for a one step in situ conjugation. Depending upon the peptide chosen, the conjugation reaction was shown to be reversible. Additionally, due to the intrinsic equilibrium of the reaction, the bridged peptide could be treated with another functionalized bromomaleimide, leading to reversible dual functionalization. The resulting dual-functionalized peptide could then be quantitatively released upon treatment with 2-mercaptoethanol.133
Other than maleimide derivatives, bis-sulfone reagents can be used to bridge disulfides. The resulting bis-sulfide bond was reversible in vitro in MCF-7 breast cancer cells, where GSH concentration is higher (10 mM), but not at the lower GSH concentrations present in plasma (20 µM), making this system ideal for cancer targeting delivery systems.134 Moreover, dibromopyridazinedione was utilized to bridge the somatostatin disulfide, followed by the release of reduced somatostatin over 72 hours when exposed to an excess of 2-mercaptoethanol.115
Interestingly, the high affinity between metals and disulfides can also be exploited as a bridging system. Arylarsenous acid was conjugated to sCT in less than 2 minutes via either an in situ or a two-step reduction-conjugation approach. The bond was cleavable by exposure to an excess of ethanedithiol (EDT). Notably, the selectivity for disulfide bonds compared to free thiols present in the same protein was higher than dibromomaleimides. The arsenic moiety could also be used as initiator for SET-LRP to obtain pPEGA. The resulting polymer had negligible toxicity across multiple cell lines compared to the arsenical small molecules. Quantitative conjugation of the polymer to sCT was achieved by treating the polymer with GSH to stabilize the arsenous acid As(III) and by increasing the amount of polymer used. The native peptide could be released following treatment with excess chelating agents, such as EDT or reduced lipoic acid in 107 or 30 minutes, respectively (Fig. 15).135
 | ||
| Fig. 15 (a) sCT conjugation to pPEGA via arylarsenous linker. (b) sCT releases in the presence of chelating agent such as EDT. Reprinted with permission from (ref. 135). Copyright 2015 American Chemical Society. | ||
Unfortunately, very few of these papers explore the possible loss of activity of the peptide or protein conjugate due to the modification of disulfide bonds. Moreover, most of these approaches provide the reduced peptide after release, which would require a further oxidation step to reform their disulfide linkages necessary to maintain the natural conformation and avoid loss of activity. Therefore, the development of a method affording the disulfide without the need for a subsequent oxidation reaction, which could be harmful for other protein residues, would be of great importance.
Briefly because this work is outside the main scope of this review, a similar approach was employed in the area of reversible peptide stapling, which consists of the constraint of peptides for various applications, such as avoiding or inhibiting protein–protein interactions, increasing stability, enhancing cell-uptake or improving target binding affinity.136 The already thoroughly discussed dibromomaleimides127,137 and pyridiniums121 were applied also in this field; whereas examples of new approaches include the use of dithioaryl(TCEP)pyridazinedione as a 2 in 1 reagent with both reducing and re-bridging function,138 a photocleavable s-tetrazine linker,139 UV-cleavable bis(bromomethyl)nitrobenzene linker,140 or 1,3,5-tris((pyridin-2-yldisulfanyl)methyl)benzene (TPSMB) as a planar, trivalent, thiol-specific linker.141
Methionine modifications
Lysine and cysteine have been the main focus in the field of traceless, reversible protein–polymer conjugation due to their nucleophilicity and ease of functionalization. However, the abundance of lysine in proteins limits the possibility of site-selective conjugation, while cysteine mostly exists in the oxidized disulfide form that often requires reduction prior to conjugation. Chai et al. recently reported a new traceless approach for reversible conjugation to methionine, the other sulfur-containing amino acid.142 Similar to the quinone methide elimination linkers detailed in the lysine modification section, this linker utilizes the 1,6 or 1,4-elimination of masked hydroxy- or amino-benzylsulfoniums to release the methionine thioether upon exposure to stimuli. The conjugation was conducted using benzyl bromide species at pH under 5.5. In these conditions, methionine was selectively modified in the presence of lysine, cysteine, and histidine due to their protonation and thus diminished nucleophilicity. To demonstrate the potential of this new linker, the authors chose Macropin, a naturally occurring peptide as a model system. It exhibits potent antimicrobial properties but also causes hemolysis.143 An alkyne-functionalized azido-benzyl bromide linker was used to selectively modify the methionine at pH 3. Then mPEG2000-N3 was conjugated via copper catalyzed azide–alkyne cycloaddition (CuAAC) reaction. In contrast to native Macropin, the Macropin-PEG conjugate displayed negligible cytotoxicity and antimicrobial properties (Fig. 16). Upon treatment of reducing agent tris(hydroxypropyl)-phosphine (THPP) to reveal the aniline, the antimicrobial activity of Macropin was largely recovered. | ||
| Fig. 16 Macropin-PEG conjugate using methionine 1,6-benzyl linker shows reduced antimicrobial activity and cytotoxicity. Adapted with permission from (ref. 142). Copyright 2025 American Chemical Society. | ||
Noncovalent traceless linkages
While covalent traceless strategies can effectively mitigate loss of biological activity, they still present synthetic challenges. Covalent linkers require chemical reactions between the polymer and protein (or peptide) followed by purification, which can be cumbersome and reduce scalability and yield. Noncovalent conjugation strategies, which rely on physical polymer-protein interactions, can circumvent these issues.144 Through selective and thermodynamically stable noncovalent complexes, polymers can strongly interact with proteins and peptides without chemically modifying their structures. These high-affinity complexes are what distinguish noncovalent conjugates from typical protein excipients. For example, lectin-specific complexation of a fucose-capped PEG with fucose-binding lectin has yielded noncovalent, multivalent protein–polymer complexes with micromolar binding dissociation.145 For protein bioconjugations, molecular recognition usually occurs via host–guest, hydrophobic, metal coordination, or ionic interactions (Table 1). Noncovalent linkages through these interactions are considered traceless if they demonstrate reversibility in relevant physiological conditions.| Interaction type | Noncovalent linkage | Affinity (Ka) (M−1) | Ref. |
|---|---|---|---|
| Host–guest | CB[7] + N-terminal aromatic amino acids (e.g. tryptophan and phenylalanine) | 106 | 146 |
| CB[7] + midchain aromatic or cationic ammonium residue | 103 to 104 | 146 | |
| Hydrophobic interactions | Insulin + cholesterol-PEG | 1.14 × 105 | 147 |
| Insulin + cholane-PEG | 3.98 × 104 | 147 | |
| Metal coordination complexes | G-CSF + 8-arm PEG-(NTA)8 | 2.1 × 109 | 148 |
| Ionic interactions | Keratinocyte growth Factor-2 + pentosane polysulfate-PEG20 | 1.1 × 107 | 149 |
| Bovine serum albumin + hyaluronic acid | 4 × 102 | 150 |
Host–guest complexes
Most host–guest bioconjugations apply the protein as the guest and polymer as the host molecule. For example, insulin has been coordinated and stabilized with cyclodextrin (CD)- or cucurbit[7]uril (CB[7])-functionalized polymeric host molecules.151–155 Webber and Appel et al. modified PEG with CB[7] and demonstrated binding with the B-chain N-terminal aromatic phenylalanine residue of insulin, as well as weaker interactions with midchain residues of glucagon, and an antibody for human CD20 (Fig. 17).152 The resulting conjugates improved the in vitro stability and function of the proteins. For insulin, the polymeric bioconjugate preserved stability and activity for 100 days in physiological conditions (pH 7.4, 37 °C) with agitation. This was greatly improved compared to free insulin and insulin + CB[7] (non-polymeric), which both aggregated and lost significant activity within ∼14 hours. Insulin with CB[7]-PEG was further evaluated and demonstrated extended in vivo activity (with PEG10000)152 as well as increased occurrence of fast-acting, monomeric insulin.154 Moreover, CB[7]-PEG binding did not affect insulin diffusivity or its association state. In a different approach, Meudom et al. engineered a B1-alanine insulin and installed a benzoic acid moiety on the N-terminus which has lower binding affinity to CB[7]-PEG. This insulin-CB[7]-PEG conjugate dissociated rapidly in subcutaneous space, thus enabling stabilization in formulation, while not increasing the duration of action, reducing the risk of a undesirable subcutaneous depot effect.155 Furthermore, stimulus–responsive properties were introduced to the N-terminus of the peptides to impart pH- or sugar-dependent host–guest binding.156 | ||
| Fig. 17 Strategy for supramolecular PEGylation. (a) Copper-free click reaction between cucurbit[7]uril (CB[7]) supramolecular host molecule bearing a single azide moiety (CB[7]-N3) and PEG-DBCO (Mn = 5, 10, or 30 kDa) yields CB[7]-functionalized PEG. (b) Cartoon depicting supramolecular PEGylation of the insulin protein through strong noncovalent binding of the CB[7] moiety to the B-chain N-terminal phenylalanine residue. Reprinted with permission from (ref. 152). Copyright 2016 PNAS. | ||
Kramer et al. also demonstrated that instead of PEGylation, a zwitterionic polypeptide monofunctionalized with CB inhibited aggregation when complexed to peptides insulin and calcitonin.157 These conjugates exhibit good biocompatibility, as they were nontoxic and could be degraded by protease. In another work, Webber and coworkers synthesized multifunctional dendrimers in which one side of the molecule was modified with PEG-linked cholesterol to facilitate cell membrane integration and the other side of the molecule was modified with CB.158
Hydrophobic interactions
Proteins are amphiphilic and will oftentimes unfold to present non-polar patches in solution. These hydrophobic areas are then available to interact with other non-polar moieties through hydrophobic interactions. Hydrophobic interactions are hypothesized to block surface absorption-induced protein aggregation and denaturation. As a result, studies which create PEG modifications with hydrophobic dansyl-,159,160 tryptophan-,160,161 phenylbutylamine-,160 benzyl-,160 cholesteryl-,147,160,162 and cholane-147 groups have examined the efficacy of stabilizing proteins and peptides. Asayama et al. synthesized a cholesteryl-PEG polymer attached through a urethane linkage.162 The polymer associated with insulin through cholesterol interaction with non-polar amino acids in insulin, such as alanine, valine, leucine, isoleucine, and phenylalanine (Fig. 18). The urethane linkage promoted additional noncovalent interactions via hydrogen bonding with hydrogen-bond-forming amino acids, such as serine, threonine, tyrosine, glutamine, and asparagine. The authors demonstrated that the cholesteryl-PEG interaction with insulin improved protein stability to protease digestion and enhanced in vivo activity as demonstrated through suppressed levels of glucose in mice. | ||
| Fig. 18 Design concept of the cholesterol end-modified poly(ethylene glycol) (Chol-U-Pr-mPEG) for by-product-free intact modification of insulin. Reprinted with permission from (ref. 162). Copyright 2025 American Chemical Society. | ||
Metal coordination complexes
Metal coordination complexes applied for protein conjugation typically leverage the strong interactions of metal ions (such as Ni2+ or Cu2+) with nitrilotriacetic acid (NTA), and histidine. There are expressed hexahistidine (His6)-tagged proteins that have been complexed with Ni2+ and NTA-polystyrene,163 NTA-PEG,164 or NTA-poly(N-acryloylmorpholine-stat-N-acryloxysuccinimide).165 While effective for site-specific labelling and protein stabilization, these examples require native protein modification and are therefore not considered truly “traceless”. However, there is an example of a naturally histidine-rich protein, GCSF, which was able to bind to Cu2+ and flexible, multi-arm 8-arm PEG-(NTA)8 polymer.148 Unfortunately, the in vivo half-life of GCSF was unaffected by the addition of polymer. The authors hypothesized that this was due to dissociation through dilution and/or competition with plasma proteins, which reduce the stability of the complex. Another study demonstrated metal coordination of native transferrin protein via Werner complexation.166 Here, a PEG-amine and transferrin, specifically the protein amines, were coordinated with Co2+. In vitro studies demonstrated ligand exchange with competitive binder ethanolamine, but the in vivo complexation and reversibility of bioconjugates created through metal coordination complexes must be further investigated.Ionic interactions
Ionic interactions, which leverages the electrostatic attraction between charged polymers and oppositely charged proteins, have also been explored as an option for traceless noncovalent conjugates. Mono-ion complexation of a diethylaminoethyl end-modified PEG to catalase has been studied.167 This noncovalent bioconjugate preserved protein activity, including in the presence of protease, trypsin or 10% fetal bovine serum, with similar efficacy compared to a covalent PEG-catalase conjugate, while keeping the native protein conformation more intact. Most other examples of ionic interactions used for protein stabilization are via polyelectrolytes.149,168–173 These are important multivalent, reversible complexes, but will not be discussed further in this review; polyelectrolyte-protein interactions have been recently reviewed in detail elsewhere.174 Their specificity decreases with increased ionic strength of the experimental media,175 making these interactions potentially less practical for in vivo studies. Hydrophobic groups, specifically ethyl, 1-hydroxyethyl, and benzyl, have been utilized to overcome these competing ionic interactions by reacting with poly(N,N-dimethylaminoethylmethacrylate)-block-PEG polymer to form cationic quaternary amine polyelectrolytes with enhanced affinity toward α-amylase in saline conditions.176As seen in Table 1, ionic interaction binding affinities vary widely, likely due to the many possible experimental and material variations. With the exception of insulin, there is a lack of studies that demonstrate the efficacy of noncovalent complexes in vivo. Noncovalent traceless strategies require physical interactions that are specific and thermodynamically- and kinetically favored, even in dilute solutions with competitive interactions. It follows that in vivo application is challenging due to the many additional proteins and small molecules that can disrupt molecular recognition events, thus lowering the complex binding affinity. However, this could be exploited to prepare a non-covalent conjugate that stabilizes the protein and then immediately releases it upon injection. Alternatively, if the noncovalent interaction is too robust, it may be difficult for the biomolecule to release within a therapeutically relevant timeframe. More experiments need to be conducted to confirm the ideal binding affinity for efficacious noncovalent, traceless bioconjugates.
Guidance for linker selection
This review highlights the diversity and breadth of traceless linkers currently in the field of reversible conjugation of polymers to peptides and proteins. The purpose of this review is to outline the different strategies for each conjugation handle, in order to allow readers to decide which linker design would fit best for their specific application. The first point of selection is to decide between covalent and non-covalent linkages. Non-covalent linkers have the advantage of synthetic and purification ease as well as high scalability. They require specific properties of the target proteins such as N-terminal aromatic residues for CB[7] host–guest interactions, and highly charged proteins for ionic interactions. Covalent linkages on the other hand can be more general and stable. If covalent linkers are to be used, the amino acid residues targeted should be carefully considered based on the amino acids present in the protein of interest, and how many polymers are desired. Lysine is abundant in proteins, and is often surface exposed, making it a popular conjugation site. Due to its abundance, the site selectivity and number of conjugations must be considered. Cysteine is the other common conjugation target due to its unique reactivity. However, most proteins lack surface exposed free cysteine, making engineered cysteine required in these cases. Disulfide bonds can also be exploited using various disulfide stapling methods. The main drawback is that disulfide linkages are usually important in protein conformation; modification can sometimes reduce the activity of the released proteins. Another consideration is what trigger is desired to release the polymer; this will decide what exact linker to utilize for a specific amino acid target. Furthermore, practical challenges during large-scale production and clinical translation may also need to be deliberated. For example, many traceless linkers mentioned in this review involve multi-step synthesis with low overall yield and air or moisture-sensitive intermediates which may not be economically and practically viable for manufacturing large-scale therapeutics. Some traceless linkers also exhibit premature release and degradation during synthesis, conjugation, and storage due to the inherent lability of these linkers. This instability can lead to reduced conjugation efficiency or potential safety issue with unwanted released protein present. Therefore, no one linker design is a perfect fit across all applications, but rather the specific constraints around each protein and application can inform the bioconjugation chemist which linker is the most appropriate, using the specific examples in this review as a guide.Future outlook
As traceless linkers are adapted towards increasingly specific applications, the specificity of the linker design is likely to increase. This includes the design of traceless linkers that are primarily cleaved under tissue specific conditions, which would require more precise triggering conditions, as compared to the more commonly used hydrolysis and reductive triggering functionalities.Several other traceless linkers have been used in reversible modification of small molecules and proteins, but could be employed as interesting new traceless linkers for protein–polymer conjugates. For example, trimethyl lock is comprised of a masked phenol, which upon unmasking undergos rapid intramolecular cyclization with the pendant amide bond to form the released amine product and dihydrocoumarin derivative byproduct.177,178 Various phenol masks have been explored, such as esters179 and phosphoric esters180 for esterase and phosphatase mediated releases, quinones for reduction-responsiveness,181 and acrylate for Michael addition trigger.182 Another exciting example, Tauton and coworker developed an inverted cyanoacrylamide-based Michael acceptor for reversible cysteine conjugation.183 This conjugate can undergo β-elimination to release the native protein. The release rate of this traceless linker can also be tuned by modulating the substituent of the cyanoacrylamide, resulting in release rate from minutes to days. It seems straightforward to apply these to polymer conjugates. Additionally, as the field of site-selective protein conjugation grows, the number of residue-specific chemistries has begun to broaden to serine and threonine,184 as well as aspartic acid and glutamic acid.185–187 However, to our knowledge, these new conjugation chemistries have also remain unexplored in the application of protein–polymer conjugation.
Author contributions
All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Acknowledgements
The authors acknowledge the U. S. National Science Foundation under grant No. CHE-2404202, UCLA Health Jonsson Comprehensive Cancer Center (JCCC) and the Dr Myung Ki Hong Chair in Polymer Sciences for funding support. Z. F. thanks the National Institute of Health Chemistry–Biology Interface Training Grant (T32GM136614) for support. M. F. T. thanks the UCLA Dissertation Year Fellowship for support. The authors thank Dr Tong Zhang and Dr Jeonghun Lee for reviewing the paper.Notes and references
- S. B. Gunnoo and A. Madder, Bioconjugation – using selective chemistry to enhance the properties of proteins and peptides as therapeutics and carriers, Org. Biomol. Chem., 2016, 14, 8002–8013 RSC.
- R. P. Sivasankaran, K. Snell, G. Kunkel, P. G. Georgiou, E. G. Puente and H. D. Maynard, Polymer-mediated protein/peptide therapeutic stabilization: Current progress and future directions, Prog. Polym. Sci., 2024, 156, 101867 CrossRef CAS.
- E. M. Pelegri-O’Day, E.-W. Lin and H. D. Maynard, Therapeutic Protein–Polymer Conjugates: Advancing Beyond PEGylation, J. Am. Chem. Soc., 2014, 136, 14323–14332 CrossRef PubMed.
- Y. Gao, M. Joshi, Z. Zhao and S. Mitragotri, PEGylated therapeutics in the clinic, Bioeng. Transl. Med., 2024, 9, e10600 CrossRef CAS PubMed.
- J. Morgenstern, G. Gil Alvaradejo, N. Bluthardt, A. Beloqui, G. Delaittre and J. Hubbuch, Impact of Polymer Bioconjugation on Protein Stability and Activity Investigated with Discrete Conjugates: Alternatives to PEGylation, Biomacromolecules, 2018, 19, 4250–4262 CrossRef CAS PubMed.
- M. Lucius, R. Falatach, C. McGlone, K. Makaroff, A. Danielson, C. Williams, J. C. Nix, D. Konkolewicz, R. C. Page and J. A. Berberich, Investigating the Impact of Polymer Functional Groups on the Stability and Activity of Lysozyme–Polymer Conjugates, Biomacromolecules, 2016, 17, 1123–1134 CrossRef CAS PubMed.
- C. S. Fishburn, The Pharmacology of PEGylation: Balancing PD with PK to Generate Novel Therapeutics, J. Pharm. Sci., 2008, 97, 4167–4183 CrossRef CAS PubMed.
- Y. Wang and C. Wu, Site-Specific Conjugation of Polymers to Proteins, Biomacromolecules, 2018, 19, 1804–1825 CrossRef CAS PubMed.
- C. for D. E. and Research, FDA approves new drug for hypoparathyroidism, a rare disorder, FDA, https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-new-drug-hypoparathyroidism-rare-disorder, accessed Nov. 2025.
- SKYTROFA (lonapegsomatropin-tcgd) for injection, for subcutaneous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761177lbl.pdf, accessed Nov. 2025.
- C. A. Blencowe, A. T. Russell, F. Greco, W. Hayes and D. W. Thornthwaite, Self-immolative linkers in polymeric delivery systems, Polym. Chem., 2011, 2, 773–790 RSC.
- A. G. Gavriel, M. R. Sambrook, A. T. Russell and W. Hayes, Recent advances in self-immolative linkers and their applications in polymeric reporting systems, Polym. Chem., 2022, 13, 3188–3269 RSC.
- C.-S. Wu and L. Cheng, Recent Advances towards the Reversible Chemical Modification of Proteins, ChemBioChem, 2023, 24, e202200468 CrossRef CAS PubMed.
- Y. D. Petri, F. G. FitzGerald and R. T. Raines, Chemoselective Reagents for the Traceless Bioreversible Modification of Native Proteins, Bioconjugate Chem., 2024, 35, 1300–1308 CrossRef CAS PubMed.
- J. D. Bargh, A. Isidro-Llobet, J. S. Parker and D. R. Spring, Cleavable linkers in antibody–drug conjugates, Chem. Soc. Rev., 2019, 48, 4361–4374 RSC.
- V. V. S. R. Edupuganti, J. D. A. Tyndall and A. B. Gamble, Self-immolative Linkers in Prodrugs and Antibody Drug Conjugates in Cancer Treatment, Recent Pat. Anti-Cancer Drug Discovery, 2021, 16, 479–497 CrossRef CAS PubMed.
- A. Nguyen, R. Böttger and S.-D. Li, Recent trends in bioresponsive linker technologies of Prodrug-Based Self-Assembling nanomaterials, Biomaterials, 2021, 275, 120955 CrossRef CAS PubMed.
- S. M. Hacker, K. M. Backus, M. R. Lazear, S. Forli, B. E. Correia and B. F. Cravatt, Global profiling of lysine reactivity and ligandability in the human proteome, Nat. Chem., 2017, 9, 1181–1190 CrossRef CAS PubMed.
- P. J. Butler, J. I. Harris, B. S. Hartley and R. Leberman, Reversible blocking of peptide amino groups by maleic anhydride, Biochem. J., 1967, 103, 78 Search PubMed.
- E. Palacián, A. López-Rivas, J. A. Pintor-Toro and F. Hernández, Dissociation of the protein components from chromatin by reversible modification with dimethylmaleic anhydride, Mol. Cell. Biochem., 1981, 36, 163–167 CrossRef PubMed.
- R. Hanai and J. C. Wang, Protein footprinting by the combined use of reversible and irreversible lysine modifications, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 11904–11908 CrossRef CAS.
- A. J. Garman and S. Barret Kalindjian, The preparation and properties of novel reversible polymer-protein conjugates 2-ω-Methoxypolyethylene (5000) glycoxymethylene-3-methylmaleyl conjugates of plasminogen activators, FEBS Lett., 1987, 223, 361–365 CrossRef CAS PubMed.
- K. Sakata, K. Hamase and K. Zaitsu, Reversible fluorescence labeling of amino groups of protein using dansylaminomethylmaleic anhydride, J. Chromatogr. B, 2002, 769, 47–54 CrossRef CAS.
- R. Schäfer, Synthesis and application of chemically reactive proteins by the reversible modification of protein amino groups with exo-cis-3,6-endo-epoxy-4,5-cis-epoxyhexahydrophthalic anhydride, Biochem. J., 1982, 203, 345–350 CrossRef PubMed.
- K. Maier and E. Wagner, Acid-Labile Traceless Click Linker for Protein Transduction, J. Am. Chem. Soc., 2012, 134, 10169–10173 CrossRef CAS PubMed.
- J.-Z. Du, H.-J. Li and J. Wang, Tumor-Acidity-Cleavable Maleic Acid Amide (TACMAA): A Powerful Tool for Designing Smart Nanoparticles To Overcome Delivery Barriers in Cancer Nanomedicine, Acc. Chem. Res., 2018, 51, 2848–2856 CrossRef CAS PubMed.
- X. Liu, P. Zhang, D. He, W. Rödl, T. Preiß, J. O. Rädler, E. Wagner and U. Lächelt, pH-Reversible Cationic RNase A Conjugates for Enhanced Cellular Delivery and Tumor Cell Killing, Biomacromolecules, 2016, 17, 173–182 CrossRef CAS PubMed.
- H. Wang, X. Han, Z. Dong, J. Xu, J. Wang and Z. Liu, Hyaluronidase with pH-responsive Dextran Modification as an Adjuvant Nanomedicine for Enhanced Photodynamic-Immunotherapy of Cancer, Adv. Funct. Mater., 2019, 29, 1902440 CrossRef.
- H. Wang, Y. Chao, H. Zhao, X. Zhou, F. Zhang, Z. Zhang, Z. Li, J. Pan, J. Wang, Q. Chen and Z. Liu, Smart Nanomedicine to Enable Crossing Blood–Brain Barrier Delivery of Checkpoint Blockade Antibody for Immunotherapy of Glioma, ACS Nano, 2022, 16, 664–674 CrossRef CAS PubMed.
- Y. Zhao, Y.-Q. Xie, S. Van Herck, S. Nassiri, M. Gao, Y. Guo and L. Tang, Switchable immune modulator for tumor-specific activation of anticancer immunity, Sci. Adv., 2021, 7, eabg7291 CrossRef CAS PubMed.
- M. Lyu, M. Yazdi, Y. Lin, M. Höhn, U. Lächelt and E. Wagner, Receptor-Targeted Dual pH-Triggered Intracellular Protein Transfer, ACS Biomater. Sci. Eng., 2024, 10, 99–114 CrossRef CAS PubMed.
- R. Kandil, Y. Xie, R. Heermann, L. Isert, K. Jung, A. Mehta and O. M. Merkel, Coming in and Finding Out: Blending Receptor-Targeted Delivery and Efficient Endosomal Escape in a Novel Bio-Responsive siRNA Delivery System for Gene Knockdown in Pulmonary T Cells, Adv. Therapeut., 2019, 2, 1900047 CrossRef PubMed.
- A. Tao, G. L. Huang, K. Igarashi, T. Hong, S. Liao, F. Stellacci, Y. Matsumoto, T. Yamasoba, K. Kataoka and H. Cabral, Polymeric Micelles Loading Proteins through Concurrent Ion Complexation and pH-Cleavable Covalent Bonding for In Vivo Delivery, Macromol. Biosci., 2020, 20, 1900161 CrossRef CAS.
- X. Zhang, P. Zhou, S. Zhuo, F. Zhang, J. Yu and X. Liu, Protein nanogels with enhanced pH-responsive dynamics triggered by remote NIR for systemic protein delivery and programmable controlled release, Int. J. Pharm., 2021, 605, 120833 CrossRef CAS PubMed.
- D. Melodia, Z. Di Pietro, C. Cao, M. H. Stenzel and R. Chapman, Traceless pH-Sensitive Antibody Conjugation Inspired by Citraconic Anhydride, Biomacromolecules, 2022, 23, 5322–5329 CrossRef CAS PubMed.
- R. B. Greenwald, H. Zhao, K. Yang, P. Reddy and A. Martinez, A New Aliphatic Amino Prodrug System for the Delivery of Small Molecules and Proteins Utilizing Novel PEG Derivatives, J. Med. Chem., 2004, 47, 726–734 Search PubMed.
- H. Zhao, K. Yang, A. Martinez, A. Basu, R. Chintala, H.-C. Liu, A. Janjua, M. Wang and D. Filpula, Linear and Branched Bicin Linkers for Releasable PEGylation of Macromolecules: Controlled Release in Vivo and in Vitro from Mono- and Multi-PEGylated Proteins, Bioconjugate Chem., 2006, 17, 341–351 CrossRef CAS PubMed.
- D. Filpula, K. Yang, A. Basu, R. Hassan, L. Xiang, Z. Zhang, M. Wang, Q. Wang, M. Ho, R. Beers, H. Zhao, P. Peng, J. Zhou, X. Li, G. Petti, A. Janjua, J. Liu, D. Wu, D. Yu, Z. Zhang, C. Longley, D. FitzGerald, R. J. Kreitman and I. Pastan, Releasable PEGylation of Mesothelin Targeted Immunotoxin SS1P Achieves Single Dosage Complete Regression of a Human Carcinoma in Mice, Bioconjugate Chem., 2007, 18, 773–784 CrossRef CAS PubMed.
- United States, US8906847B2, 2014.
- J. P. Bilezikian, A. Khan, J. T. Potts, M. L. Brandi, B. L. Clarke, D. Shoback, H. Jüppner, P. D'Amour, J. Fox, L. Rejnmark, L. Mosekilde, M. R. Rubin, D. Dempster, R. Gafni, M. T. Collins, J. Sliney and J. Sanders, Hypoparathyroidism in the adult: Epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research, J. Bone Miner. Res., 2011, 26, 2317–2337 CrossRef CAS PubMed.
- L. Holten-Andersen, S. Pihl, C. E. Rasmussen, J. Zettler, G. Maitro, J. Baron, S. Heinig, E. Hoffmann, T. Wegge, M. Krusch, F. Faltinger, S. Killian, K. Sprogoe, D. B. Karpf, V. M. Breinholt and F. Cleemann, Design and Preclinical Development of TransCon PTH, an Investigational Sustained-Release PTH Replacement Therapy for Hypoparathyroidism, J. Bone Miner. Res., 2019, 34, 2075–2086 CrossRef CAS PubMed.
- J. Chen, M. Zhao, F. Feng, A. Sizovs and J. Wang, Tunable Thioesters as “Reduction” Responsive Functionality for Traceless Reversible Protein PEGylation, J. Am. Chem. Soc., 2013, 135, 10938–10941 CrossRef CAS PubMed.
- K. Dutta, D. Hu, B. Zhao, A. E. Ribbe, J. Zhuang and S. Thayumanavan, Templated Self-Assembly of a Covalent Polymer Network for Intracellular Protein Delivery and Traceless Release, J. Am. Chem. Soc., 2017, 139, 5676–5679 CrossRef CAS PubMed.
- M. Scherger, H. J. Räder and L. Nuhn, Self-Immolative RAFT-Polymer End Group Modification, Macromol. Rapid Commun., 2021, 42, 2000752 CrossRef CAS PubMed.
- M. Scherger, Y. A. Pilger, P. Komforth, H.-J. Räder and L. Nuhn, Reversible Polymer–Protein Functionalization by Stepwise Introduction of Amine-Reactive, Reductive-Responsive Self-Immolative End Groups onto RAFT-Derived Polymers, ACS Biomater. Sci. Eng., 2024, 10, 129–138 CrossRef CAS PubMed.
- M. Scherger, Y. A. Pilger, J. Stickdorn, P. Komforth, S. Schmitt, K. Koynov, H. J. Räder and L. Nuhn, Efficient Self-Immolative RAFT End Group Modification for Macromolecular Immunodrug Delivery, Biomacromolecules, 2023, 24, 2380–2391 CrossRef CAS PubMed.
- Y. Zhang, D. Zhang, J.-T. Wang, X. Zhang and Y. Yang, Fabrication of stimuli-responsive nanogels for protein encapsulation and traceless release without introducing organic solvents, surfactants, or small-molecule cross-linkers, Polym. Chem., 2021, 12, 554–563 Search PubMed.
- B. Liu, R. Wu, S. Gong, H. Xiao and S. Thayumanavan, In Situ Formation of Polymeric Nanoassemblies Using an Efficient Reversible Click Reaction, Angew. Chem., Int. Ed., 2020, 59, 15135–15140 CrossRef CAS PubMed.
- P. L. Carl, P. K. Chakravarty and J. A. Katzenellenbogen, A novel connector linkage applicable in prodrug design, J. Med. Chem., 1981, 24, 479–480 Search PubMed.
- S. Zalipsky, N. Mullah, C. Engbers, M. U. Hutchins and R. Kiwan, Thiolytically Cleavable Dithiobenzyl Urethane-Linked Polymer–Protein Conjugates as Macromolecular Prodrugs: Reversible PEGylation of Proteins, Bioconjugate Chem., 2007, 18, 1869–1878 CrossRef CAS PubMed.
- T. Suma, J. Cui, M. Müllner, S. Fu, J. Tran, K. F. Noi, Y. Ju and F. Caruso, Modulated Fragmentation of Proapoptotic Peptide Nanoparticles Regulates Cytotoxicity, J. Am. Chem. Soc., 2017, 139, 4009–4018 CrossRef CAS PubMed.
- L. Qian, J. Fu, P. Yuan, S. Du, W. Huang, L. Li and S. Q. Yao, Intracellular Delivery of Native Proteins Facilitated by Cell-Penetrating Poly(disulfide)s, Angew. Chem., Int. Ed., 2018, 57, 1532–1536 CrossRef CAS PubMed.
- M. He, J. Li, H. Han, C. A. Borges, G. Neiman, J. J. Røise, P. Hadaczek, R. Mendonsa, V. R. Holm, R. C. Wilson, K. Bankiewicz, Y. Zhang, C. M. Sadlowski, K. Healy, L. W. Riley and N. Murthy, A traceless linker for aliphatic amines that rapidly and quantitatively fragments after reduction, Chem. Sci., 2020, 11, 8973–8980 RSC.
- H. Ren, J. Li, G. Liu, Y. Sun, X. Yang, Z. Jiang, J. Zhang, J. F. Lovell and Y. Zhang, Anticancer Vaccination with Immunogenic Micelles That Capture and Release Pristine CD8+ T-Cell Epitopes and Adjuvants, ACS Appl. Mater. Interfaces, 2022, 14, 2510–2521 CrossRef CAS PubMed.
- E. R. Hebels, S. Dietl, M. Timmers, J. Hak, A. van den Dikkenberg, C. J. F. Rijcken, W. E. Hennink, R. M. J. Liskamp and T. Vermonden, Versatile Click Linker Enabling Native Peptide Release from Nanocarriers upon Redox Trigger, Bioconjugate Chem., 2023, 34, 2375–2386 CrossRef CAS PubMed.
- S. Lee, R. B. Greenwald, J. McGuire, K. Yang and C. Shi, Drug Delivery Systems Employing 1,6-Elimination: Releasable Poly(ethylene glycol) Conjugates of Proteins, Bioconjugate Chem., 2001, 12, 163–169 Search PubMed.
- R. B. Greenwald, K. Yang, H. Zhao, C. D. Conover, S. Lee and D. Filpula, Controlled Release of Proteins from Their Poly(Ethylene Glycol) Conjugates: Drug Delivery Systems Employing 1,6-Elimination, Bioconjugate Chem., 2003, 14, 395–403 CrossRef CAS PubMed.
- M. Wang, S. Sun, C. I. Neufeld, B. Perez-Ramirez and Q. Xu, Reactive Oxygen Species-Responsive Protein Modification and Its Intracellular Delivery for Targeted Cancer Therapy, Angew. Chem., Int. Ed., 2014, 53, 13444–13448 CrossRef CAS PubMed.
- B. Liu, M. Ianosi-Irimie and S. Thayumanavan, Reversible Click Chemistry for Ultrafast and Quantitative Formation of Protein–Polymer Nanoassembly and Intracellular Protein Delivery, ACS Nano, 2019, 13, 9408–9420 Search PubMed.
- X. Liu, Z. Zhao, F. Wu, Y. Chen and L. Yin, Tailoring Hyperbranched Poly(β-amino ester) as a Robust and Universal Platform for Cytosolic Protein Delivery, Adv. Mater., 2022, 34, 2108116 CrossRef CAS PubMed.
- E. Tan, T. Wan, C. Yu, Q. Fan, W. Liu, H. Chang, J. Lv, H. Wang, D. Li, Y. Ping and Y. Cheng, ROS-responsive polypeptides for intracellular protein delivery and CRISPR/Cas9 gene editing, Nano Today, 2022, 46, 101617 Search PubMed.
- R. Lu, Y. Zheng, M. Wang, J. Lin, Z. Zhao, L. Chen, J. Zhang, X. Liu, L. Yin and Y. Chen, Reactive oxygen species-responsive branched poly (β-amino ester) with robust efficiency for cytosolic protein delivery, Acta Biomater., 2022, 152, 355–366 CrossRef CAS PubMed.
- G. Rong, L. Chen, F. Zhu, E. Tan and Y. Cheng, Polycatechols with Robust Efficiency in Cytosolic Peptide Delivery via Catechol-Boronate Chemistry, Nano Lett., 2022, 22, 6245–6253 CrossRef CAS PubMed.
- X. Gao, C. Yuan, E. Tan, Z. Li, Y. Cheng, J. Xiao and G. Rong, Dual-responsive bioconjugates bearing a bifunctional adaptor for robust cytosolic peptide delivery, J. Controlled Release, 2023, 355, 675–684 CrossRef CAS PubMed.
- X. Xu, Y. Ran, C. Huang and Z. Yin, Glucose and H2O2 Dual-Responsive Nanocomplex Grafted with Insulin Prodrug for Blood Glucose Regulation, Biomacromolecules, 2022, 23, 1765–1776 CrossRef CAS PubMed.
- United States, US9272048B2, 2016.
- United States, US20170312342A1, 2017.
- FDA Approves SKYTROFA® (Lonapegsomatropin-tcgd) for the Once-Weekly Treatment of Adults with Growth Hormone Deficiency | Ascendis Pharma, https://investors.ascendispharma.com/news-releases/news-release-details/fda-approves-skytrofar-lonapegsomatropin-tcgd-once-weekly, accessed 28 July 2025.
- B. S. Miller and K. C. Yuen, Spotlight on Lonapegsomatropin Once-Weekly Injection and Its Potential in the Treatment of Growth Hormone Deficiency in Pediatric Patients, Drug Des. Dev. Ther., 2022, 16, 2055–2066 CrossRef PubMed.
- D. A. Rose, J. W. Treacy, Z. J. Yang, J. H. Ko, K. N. Houk and H. D. Maynard, Self-Immolative Hydroxybenzylamine Linkers for Traceless Protein Modification, J. Am. Chem. Soc., 2022, 144, 6050–6058 CrossRef CAS PubMed.
- Z. Fu, J. W. Treacy, B. M. Hosier, K. N. Houk and H. D. Maynard, Controlling rates and reversibilities of elimination reactions of hydroxybenzylammoniums by tuning dearomatization energies, Chem. Sci., 2024, 15, 10448–10454 RSC.
- E. Gershonov, Y. Shechter and M. Fridkin, New concept for long-acting insulin: spontaneous conversion of an inactive modified insulin to the active hormone in circulation: 9-fluorenylmethoxycarbonyl derivative of insulin, Diabetes, 1999, 48, 1437–1442 CrossRef CAS PubMed.
- E. Gershonov, I. Goldwaser, M. Fridkin and Y. Shechter, A Novel Approach for a Water-Soluble Long-Acting Insulin Prodrug: Design, Preparation, and Analysis of [(2-Sulfo)-9-fluorenylmethoxycarbonyl]3-insulin, J. Med. Chem., 2000, 43, 2530–2537 CrossRef CAS PubMed.
- Y. Shechter, I. Goldwaser, I. Lavon, E. Gershonov, B. Mester, M. Mironchik, L. P. Pratt and M. Fridkin, A new approach for prolonging the half-life of peptides, proteins and low-molecular-weight drugs in vivo, Drugs Future, 2001, 26, 0669 CrossRef CAS.
- T. Peleg-Shulman, H. Tsubery, M. Mironchik, M. Fridkin, G. Schreiber and Y. Shechter, Reversible PEGylation: A Novel Technology To Release Native Interferon α2 over a Prolonged Time Period, J. Med. Chem., 2004, 47, 4897–4904 CrossRef CAS PubMed.
- H. Tsubery, M. Mironchik, M. Fridkin and Y. Shechter, Prolonging the Action of Protein and Peptide Drugs by a Novel Approach of Reversible Polyethylene Glycol Modification, J. Biol. Chem., 2004, 279, 38118–38124 Search PubMed.
- Y. Shechter, M. Mironchik, S. Rubinraut, H. Tsubery, K. Sasson, Y. Marcus and M. Fridkin, Reversible pegylation of insulin facilitates its prolonged action in vivo, Eur. J. Pharm. Biopharm., 2008, 70, 19–28 CrossRef CAS PubMed.
- Y. Shechter, E. Heldman, K. Sasson, T. Bachar, M. Popov and M. Fridkin, Delivery of Neuropeptides from the Periphery to the Brain: Studies with Enkephalin, ACS Chem. Neurosci., 2010, 1, 399–406 CrossRef CAS PubMed.
- D. Charych, S. Khalili, V. Dixit, P. Kirk, T. Chang, J. Langowski, W. Rubas, S. K. Doberstein, M. Eldon, U. Hoch and J. Zalevsky, Modeling the receptor pharmacology, pharmacokinetics, and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy, PLoS One, 2017, 12, e0179431 CrossRef PubMed.
- World Intellectual Property Organization, WO2015125159A1, 2015.
- United States, US20190008978A1, 2019.
- A. Diab, H. Gogas, S. Sandhu, G. V. Long, P. A. Ascierto, J. Larkin, M. Sznol, F. Franke, T. E. Ciuleanu, C. Pereira, E. Muñoz Couselo, F. Bronzon Damian, M. Schenker, A. Perfetti, C. Lebbe, G. Quéreux, F. Meier, B. D. Curti, C. Rojas, Y. Arriaga, H. Yang, M. Zhou, S. Ravimohan, P. Statkevich, M. A. Tagliaferri and N. I. Khushalani, Bempegaldesleukin Plus Nivolumab in Untreated Advanced Melanoma: The Open-Label, Phase III PIVOT IO 001 Trial Results, J. Clin. Orthod., 2023, 41, 4756–4767 CAS.
- D. V. Santi, E. L. Schneider, R. Reid, L. Robinson and G. W. Ashley, Predictable and tunable half-life extension of therapeutic agents by controlled chemical release from macromolecular conjugates, Proceedings of the National Academy of Sciences, 2012, 109, 6211–6216 Search PubMed.
- E. L. Schneider, J. Henise, R. Reid, G. W. Ashley and D. V. Santi, Hydrogel Drug Delivery System Using Self-Cleaving Covalent Linkers for Once-a-Week Administration of Exenatide, Bioconjugate Chem., 2016, 27, 1210–1215 Search PubMed.
- E. L. Schneider, B. R. Hearn, S. J. Pfaff, R. Reid, D. G. Parkes, N. Vrang, G. W. Ashley and D. V. Santi, A Hydrogel-Microsphere Drug Delivery System That Supports Once-Monthly Administration of a GLP-1 Receptor Agonist, ACS Chem. Biol., 2017, 12, 2107–2116 Search PubMed.
- E. L. Schneider, R. Reid, D. G. Parkes, T. A. Lutz, G. W. Ashley and D. V. Santi, A once-monthly GLP-1 receptor agonist for treatment of diabetic cats, Domest. Anim. Endocrinol., 2020, 70, 106373 Search PubMed.
- F. Brandl, N. Hammer, T. Blunk, J. Tessmar and A. Goepferich, Biodegradable Hydrogels for Time-Controlled Release of Tethered Peptides or Proteins, Biomacromolecules, 2010, 11, 496–504 Search PubMed.
- N. Hammer, F. P. Brandl, S. Kirchhof and A. M. Goepferich, Cleavable carbamate linkers for controlled protein delivery from hydrogels, J. Controlled Release, 2014, 183, 67–76 Search PubMed.
- K. L. Diehl, I. V. Kolesnichenko, S. A. Robotham, J. L. Bachman, Y. Zhong, J. S. Brodbelt and E. V. Anslyn, Click and chemically triggered declick reactions through reversible amine and thiol coupling via a conjugate acceptor, Nat. Chem., 2016, 8, 968–973 Search PubMed.
- J. Zhuang, B. Zhao, X. Meng, J. D. Schiffman, S. L. Perry, R. W. Vachet and S. Thayumanavan, A programmable chemical switch based on triggerable Michael acceptors, Chem. Sci., 2020, 11, 2103–2111 Search PubMed.
- W. E. Georgianna, H. Lusic, A. L. McIver and A. Deiters, Photocleavable Polyethylene Glycol for the Light-Regulation of Protein Function, Bioconjugate Chem., 2010, 21, 1404–1407 CrossRef CAS PubMed.
- J. A. Karas, A. Noor, C. Schieber, T. U. Connell, F. Separovic and P. S. Donnelly, The efficient synthesis and purification of amyloid-β(1–42) using an oligoethylene glycol-containing photocleavable lysine tag, Chem. Commun., 2017, 53, 6903–6905 Search PubMed.
- N. L. Forsythe, M. F. Tan, D. Vinciguerra, J. Woodford, A. Z. Stieg and H. D. Maynard, Noncovalent Enzyme Nanogels via a Photocleavable Linkage, Macromolecules, 2022, 55, 9925–9933 CrossRef CAS PubMed.
- M. F. Tan, B. M. Hosier, N. L. Forsythe and H. D. Maynard, Enzyme-polymer conjugates with photocleavable linkers for control over protein activity, Polym. Chem., 2024, 15, 1085–1092 RSC.
- J. M. Chalker, G. J. L. Bernardes, Y. A. Lin and B. G. Davis, Chemical Modification of Proteins at Cysteine: Opportunities in Chemistry and Biology, Chem.–Asian J., 2009, 4, 630–640 Search PubMed.
- S. B. Gunnoo and A. Madder, Chemical Protein Modification through Cysteine, ChemBioChem, 2016, 17, 529–553 Search PubMed.
- S. J. Paluck, T. H. Nguyen and H. D. Maynard, Heparin-Mimicking Polymers: Synthesis and Biological Applications, Biomacromolecules, 2016, 17, 3417–3440 Search PubMed.
- Q. Li, J. B. White, N. C. Peterson, K. W. Rickert, C. O. Lloyd, K. L. Allen, K. Rosenthal, X. Gao, H. Wu, W. F. Dall'Acqua, M. J. Borrok and P. Tsui, Tumor uptake of pegylated diabodies: Balancing systemic clearance and vascular transport, J. Controlled Release, 2018, 279, 126–135 Search PubMed.
- A. J. Maurais and E. Weerapana, Reactive-cysteine profiling for drug discovery, Curr. Opin. Chem. Biol., 2019, 50, 29–36 Search PubMed.
- E. Weerapana, C. Wang, G. M. Simon, F. Richter, S. Khare, M. B. D. Dillon, D. A. Bachovchin, K. Mowen, D. Baker and B. F. Cravatt, Quantitative reactivity profiling predicts functional cysteines in proteomes, Nature, 2010, 468, 790–795 Search PubMed.
- D. Bontempo, K. L. Heredia, B. A. Fish and H. D. Maynard, Cysteine-Reactive Polymers Synthesized by Atom Transfer Radical Polymerization for Conjugation to Proteins, J. Am. Chem. Soc., 2004, 126, 15372–15373 Search PubMed.
- P. C. Nauka, J. Lee and H. D. Maynard, Enhancing the conjugation yield of brush polymer–protein conjugates by increasing the linker length at the polymer end-group, Polym. Chem., 2016, 7, 2352–2357 RSC.
- O. Gok, P. Erturk, B. Sumer Bolu, T. N. Gevrek, R. Sanyal and A. Sanyal, Dendrons and Multiarm Polymers with Thiol-Exchangeable Cores: A Reversible Conjugation Platform for Delivery, Biomacromolecules, 2017, 18, 2463–2477 Search PubMed.
- R. P. Lyon, J. R. Setter, T. D. Bovee, S. O. Doronina, J. H. Hunter, M. E. Anderson, C. L. Balasubramanian, S. M. Duniho, C. I. Leiske, F. Li and P. D. Senter, Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates, Nat. Biotechnol., 2014, 32, 1059–1062 CrossRef CAS PubMed.
- A. D. Baldwin and K. L. Kiick, Tunable Degradation of Maleimide–Thiol Adducts in Reducing Environments, Bioconjugate Chem., 2011, 22, 1946–1953 Search PubMed.
- S. D. Fontaine, R. Reid, L. Robinson, G. W. Ashley and D. V. Santi, Long-Term Stabilization of Maleimide–Thiol Conjugates, Bioconjugate Chem., 2015, 26, 145–152 Search PubMed.
- H. Wu, P. J. LeValley, T. Luo, A. M. Kloxin and K. L. Kiick, Manipulation of Glutathione-Mediated Degradation of Thiol–Maleimide Conjugates, Bioconjugate Chem., 2018, 29, 3595–3605 Search PubMed.
- M. E. B. Smith, F. F. Schumacher, C. P. Ryan, L. M. Tedaldi, D. Papaioannou, G. Waksman, S. Caddick and J. R. Baker, Protein Modification, Bioconjugation, and Disulfide Bridging Using Bromomaleimides, J. Am. Chem. Soc., 2010, 132, 1960–1965 Search PubMed.
- L. M. Tedaldi, M. E. B. Smith, R. I. Nathani and J. R. Baker, Bromomaleimides: new reagents for the selective and reversible modification of cysteine, Chem. Commun., 2009, 6583–6585 RSC.
- C. P. Ryan, M. E. B. Smith, F. F. Schumacher, D. Grohmann, D. Papaioannou, G. Waksman, F. Werner, J. R. Baker and S. Caddick, Tunable reagents for multi-functional bioconjugation: reversible or permanent chemical modification of proteins and peptides by control of maleimide hydrolysis, Chem. Commun., 2011, 47, 5452–5454 RSC.
- P. Moody, M. E. B. Smith, C. P. Ryan, V. Chudasama, J. R. Baker, J. Molloy and S. Caddick, Bromomaleimide-Linked Bioconjugates Are Cleavable in Mammalian Cells, ChemBioChem, 2012, 13, 39–41 Search PubMed.
- R. I. Nathani, V. Chudasama, C. P. Ryan, P. R. Moody, R. E. Morgan, R. J. Fitzmaurice, M. E. B. Smith, J. R. Baker and S. Caddick, Reversible protein affinity-labelling using bromomaleimide-based reagents, Org. Biomol. Chem., 2013, 11, 2408–2411 RSC.
- A. Ross, H. Durmaz, K. Cheng, X. Deng, Y. Liu, J. Oh, Z. Chen and J. Lahann, Selective and Reversible Binding of Thiol-Functionalized Biomolecules on Polymers Prepared via Chemical Vapor Deposition Polymerization, Langmuir, 2015, 31, 5123–5129 CrossRef CAS PubMed.
- Y. Zhang, X. Zhou, Y. Xie, M. M. Greenberg, Z. Xi and C. Zhou, Thiol Specific and Tracelessly Removable Bioconjugation via Michael Addition to 5-Methylene Pyrrolones, J. Am. Chem. Soc., 2017, 139, 6146–6151 Search PubMed.
- V. Chudasama, M. E. B. Smith, F. F. Schumacher, D. Papaioannou, G. Waksman, J. R. Baker and S. Caddick, Bromopyridazinedione-mediated protein and peptide bioconjugation, Chem. Commun., 2011, 47, 8781–8783 Search PubMed.
- C. Bahou, R. J. Spears, A. E. Aliev, A. Maruani, M. Fernandez, F. Javaid, P. A. Szijj, J. R. Baker and V. Chudasama, Use of pyridazinediones as extracellular cleavable linkers through reversible cysteine conjugation, Chem. Commun., 2019, 55, 14829–14832 Search PubMed.
- L. N. C. Rochet, C. Bahou, J. P. Wojciechowski, I. Koutsopetras, P. Britton, R. J. Spears, I. A. Thanasi, B. Shao, L. Zhong, D.-K. Bučar, A. E. Aliev, M. J. Porter, M. M. Stevens, J. R. Baker and V. Chudasama, Use of pyridazinediones for tuneable and reversible covalent cysteine modification applied to peptides, proteins and hydrogels, Chem. Sci., 2023, 14, 13743–13754 RSC.
- C. Bahou, P. A. Szijj, R. J. Spears, A. Wall, F. Javaid, A. Sattikar, E. A. Love, J. R. Baker and V. Chudasama, A Plug-and-Play Platform for the Formation of Trifunctional Cysteine Bioconjugates that also Offers Control over Thiol Cleavability, Bioconjugate Chem., 2021, 32, 672–679 Search PubMed.
- J. Yu, X. Yang, Y. Sun and Z. Yin, Highly Reactive and Tracelessly Cleavable Cysteine-Specific Modification of Proteins via 4-Substituted Cyclopentenone, Angew. Chem., Int. Ed., 2018, 57, 11598–11602 Search PubMed.
- S. Maity, C. Bingham, W. Sheng, N. Ehyaei, D. Chakraborty, S. Tahmasebi-Nick, T. E. Kimmel, C. Vasileiou, J. H. Geiger and B. Borhan, Light controlled reversible Michael addition of cysteine: a new tool for dynamic site-specific labeling of proteins, Analyst, 2023, 148, 1085–1092 Search PubMed.
- C. Wan, Y. Zhang, J. Wang, Y. Xing, D. Yang, Q. Luo, J. Liu, Y. Ye, Z. Liu, F. Yin, R. Wang and Z. Li, Traceless Peptide and Protein Modification via Rational Tuning of Pyridiniums, J. Am. Chem. Soc., 2024, 146, 2624–2633 Search PubMed.
- P. J. Hogg, Disulfide bonds as switches for protein function, Trends Biochem. Sci., 2003, 28, 210–214 Search PubMed.
- S. Brocchini, S. Balan, A. Godwin, J.-W. Choi, M. Zloh and S. Shaunak, PEGylation of native disulfide bonds in proteins, Nat. Protoc., 2006, 1, 2241–2252 Search PubMed.
- S. Shaunak, A. Godwin, J.-W. Choi, S. Balan, E. Pedone, D. Vijayarangam, S. Heidelberger, I. Teo, M. Zloh and S. Brocchini, Site-specific PEGylation of native disulfide bonds in therapeutic proteins, Nat. Chem. Biol., 2006, 2, 312–313 Search PubMed.
- M. W. Jones, R. A. Strickland, F. F. Schumacher, S. Caddick, J. R. Baker, M. I. Gibson and D. M. Haddleton, Polymeric Dibromomaleimides As Extremely Efficient Disulfide Bridging Bioconjugation and Pegylation Agents, J. Am. Chem. Soc., 2012, 134, 1847–1852 Search PubMed.
- F. F. Schumacher, V. A. Sanchania, B. Tolner, Z. V. F. Wright, C. P. Ryan, M. E. B. Smith, J. M. Ward, S. Caddick, C. W. M. Kay, G. Aeppli, K. A. Chester and J. R. Baker, Homogeneous antibody fragment conjugation by disulfide bridging introduces spinostics, Sci. Rep., 2013, 3, 1525 Search PubMed.
- C. M. Grison, G. M. Burslem, J. A. Miles, L. K. A. Pilsl, D. J. Yeo, Z. Imani, S. L. Warriner, M. E. Webb and A. J. Wilson, Double quick, double click reversible peptide “stapling”, Chem. Sci., 2017, 8, 5166–5171 RSC.
- M. T. N. Petersen, P. H. Jonson and S. B. Petersen, Amino acid neighbours and detailed conformational analysis of cysteines in proteins, Protein Eng., 1999, 12, 535–548 CrossRef CAS PubMed.
- L. Eldjarn and A. Pihl, The Equilibrium Constants and Oxidation-Reduction Potentials of Some Thiol-Disulfide Systems1, J. Am. Chem. Soc., 1957, 79, 4589–4593 Search PubMed.
- F. F. Schumacher, M. Nobles, C. P. Ryan, M. E. B. Smith, A. Tinker, S. Caddick and J. R. Baker, In Situ Maleimide Bridging of Disulfides and a New Approach to Protein PEGylation, Bioconjugate Chem., 2011, 22, 132–136 Search PubMed.
- M. W. Jones, R. A. Strickland, F. F. Schumacher, S. Caddick, J. R. Baker, M. I. Gibson and D. M. Haddleton, Highly efficient disulfide bridging polymers for bioconjugates from radical-compatible dithiophenol maleimides, Chem. Commun., 2012, 48, 4064–4066 Search PubMed.
- J. Collins, J. Tanaka, P. Wilson, K. Kempe, T. P. Davis, M. P. McIntosh, M. R. Whittaker and D. M. Haddleton, In Situ Conjugation of Dithiophenol Maleimide Polymers and Oxytocin for Stable and Reversible Polymer–Peptide Conjugates, Bioconjugate Chem., 2015, 26, 633–638 Search PubMed.
- C. Marculescu, H. Kossen, R. E. Morgan, P. Mayer, S. A. Fletcher, B. Tolner, K. A. Chester, L. H. Jones and J. R. Baker, Aryloxymaleimides for cysteine modification, disulfide bridging and the dual functionalization of disulfide bonds, Chem. Commun., 2014, 50, 7139–7142 Search PubMed.
- T. Wang, D. Y. W. Ng, Y. Wu, J. Thomas, T. TamTran and T. Weil, Bis-sulfide bioconjugates for glutathione triggered tumor responsive drug release, Chem. Commun., 2013, 50, 1116–1118 RSC.
- P. Wilson, A. Anastasaki, M. R. Owen, K. Kempe, D. M. Haddleton, S. K. Mann, A. P. R. Johnston, J. F. Quinn, M. R. Whittaker, P. J. Hogg and T. P. Davis, Organic Arsenicals As Efficient and Highly Specific Linkers for Protein/Peptide–Polymer Conjugation, J. Am. Chem. Soc., 2015, 137, 4215–4222 CrossRef CAS PubMed.
- Y. Chen, C. Dai, J. Han, Y. Xing, F. Yin and Z. Li, Recent Chemical Biology Insights Towards Reversible Stapled Peptides, ChemBioChem, 2025, 26, e202500052 Search PubMed.
- A. Lindsey-Crosthwait, D. Rodriguez-Lema, M. Walko, C. M. Pask and A. J. Wilson, Structural optimization of reversible dibromomaleimide peptide stapling, Pept. Sci., 2021, 113, e24157 CrossRef CAS PubMed.
- M. T. W. Lee, A. Maruani, J. R. Baker, S. Caddick and V. Chudasama, Next-generation disulfide stapling: reduction and functional re-bridging all in one, Chem. Sci., 2015, 7, 799–802 Search PubMed.
- S. P. Brown and A. B. I. Smith, Peptide/Protein Stapling and Unstapling: Introduction of s-Tetrazine, Photochemical Release, and Regeneration of the Peptide/Protein, J. Am. Chem. Soc., 2015, 137, 4034–4037 CrossRef CAS PubMed.
- I. Takahashi, S. Kuroiwa, H. E. Lindfors, L. A. Ndamba, Y. Hiruma, T. Yajima, N. Okishio, M. Ubbink and S. Hirota, Modulation of protein–ligand interactions by photocleavage of a cyclic peptide using phosphatidylinositol 3-kinase SH3 domain as model system, J. Pept. Sci., 2009, 15, 411–416 Search PubMed.
- C. Ernst, J. Heidrich, C. Sessler, J. Sindlinger, D. Schwarzer, P. Koch and F. M. Boeckler, Switching Between Bicyclic and Linear Peptides — The Sulfhydryl-Specific Linker TPSMB Enables Reversible Cyclization of Peptides, Front. Chem., 2018, 6 DOI:10.3389/fchem.2018.00484.
- Y. Chai, C. Yu, Z. Chen, W. Duan, H. Chen, X. Qiu, Z. Xu, S. Liu, A. Danilenko, G. Frison, V. Alezra, E. Miclet, X. Li and Y. Wan, Rapid C–S+ Bond Cleavage via 1,6-Benzyl Elimination for Traceless Modification of Bioactive Peptides, J. Am. Chem. Soc., 2025, 147, 20807–20818 Search PubMed.
- Macropis fulvipes Venom component Macropin Exerts its Antibacterial and Anti-Biofilm Properties by Damaging the Plasma Membranes of Drug Resistant Bacteria | Sci. Rep., https://www.nature.com/articles/s41598-017-16784-6, accessed 8 September 2025.
- C. Reichert, G. Borchard and N. PEGylation, An Innovative Subchapter in the Field of Protein Modification, J. Pharm. Sci., 2016, 105, 386–390 Search PubMed.
- P. M. Antonik, A. M. Eissa, A. R. Round, N. R. Cameron and P. B. Crowley, Noncovalent PEGylation via Lectin–Glycopolymer Interactions, Biomacromolecules, 2016, 17, 2719–2725 CrossRef CAS PubMed.
- A. R. Urbach and V. Ramalingam, Molecular Recognition of Amino Acids, Peptides, and Proteins by Cucurbit[n]uril Receptors, Isr. J. Chem., 2011, 51, 664–678 Search PubMed.
- F. Mastrotto, F. Bellato, V. Andretto, A. Malfanti, M. Garofalo, S. Salmaso and P. Caliceti, Physical PEGylation to Prevent Insulin Fibrillation, J. Pharm. Sci., 2020, 109, 900–910 Search PubMed.
- A. Mero, T. Ishino, I. Chaiken, F. M. Veronese and G. Pasut, Multivalent and Flexible PEG-Nitrilotriacetic Acid Derivatives for Non-covalent Protein Pegylation, Pharm. Res., 2011, 28, 2412–2421 Search PubMed.
- S. Khondee, C. M. Olsen, Y. Zeng, C. R. Middaugh and C. Berkland, Noncovalent PEGylation by Polyanion Complexation as a Means To Stabilize Keratinocyte Growth Factor-2 (KGF-2), Biomacromolecules, 2011, 12, 3880–3894 Search PubMed.
- X. Du, P. L. Dubin, D. A. Hoagland and L. Sun, Protein-Selective Coacervation with Hyaluronic Acid, Biomacromolecules, 2014, 15, 726–734 Search PubMed.
- L. Wang, Y.-W. Yang, M. Zhu, G. Qiu, G. Wu and H. Gao, β-Cyclodextrin-conjugated amino poly(glycerol methacrylate)s for efficient insulin delivery, RSC Adv., 2014, 4, 6478–6485 Search PubMed.
- M. J. Webber, E. A. Appel, B. Vinciguerra, A. B. Cortinas, L. S. Thapa, S. Jhunjhunwala, L. Isaacs, R. Langer and D. G. Anderson, Supramolecular PEGylation of biopharmaceuticals, Proceedings of the National Academy of Sciences, 2016, 113, 14189–14194 Search PubMed.
- C. L. Maikawa, A. A. A. Smith, L. Zou, C. M. Meis, J. L. Mann, M. J. Webber and E. A. Appel, Stable Monomeric Insulin Formulations Enabled by Supramolecular PEGylation of Insulin Analogues, Adv. Therapeut., 2020, 3, 1900094 Search PubMed.
- C. L. Maikawa, A. A. A. Smith, L. Zou, G. A. Roth, E. C. Gale, L. M. Stapleton, S. W. Baker, J. L. Mann, A. C. Yu, S. Correa, A. K. Grosskopf, C. S. Liong, C. M. Meis, D. Chan, M. Troxell, D. M. Maahs, B. A. Buckingham, M. J. Webber and E. A. Appel, A co-formulation of supramolecularly stabilized insulin and pramlintide enhances mealtime glucagon suppression in diabetic pigs, Nat. Biomed. Eng., 2020, 4, 507–517 Search PubMed.
- R. Meudom, Y. Zhang, M. A. VandenBerg, L. Zou, Y. W. Zhang, M. J. Webber and D. H.-C. Chou, Supramolecular approaches for insulin stabilization without prolonged duration of action, Acta Pharm. Sin. B, 2023, 13, 2281–2290 CrossRef CAS PubMed.
- R. Meudom, N. Zheng, S. Zhu, M. T. Jacobsen, L. Cao and D. H.-C. Chou, Expanding peptide-cucurbit[7]uril interactions through selective N-terminal reductive alkylation, Curr. Res. Chem. Biol., 2022, 2, 100013 Search PubMed.
- Z. S. Clauss, R. Meudom, B. Su, M. A. VandenBerg, S. S. Saini, M. J. Webber, D. H.-C. Chou and J. R. Kramer, Supramolecular Protein Stabilization with Zwitterionic Polypeptide–Cucurbit[7]uril Conjugates, Biomacromolecules, 2023, 24, 481–488 Search PubMed.
- B. D. Gates, J. B. Vyletel, L. Zou and M. J. Webber, Multivalent Cucurbituril Dendrons for Cell Membrane Engineering with Supramolecular Receptors, Bioconjugate Chem., 2022, 33, 2262–2268 Search PubMed.
- C. Mueller, M. A. H. Capelle, T. Arvinte, E. Seyrek and G. Borchard, Noncovalent Pegylation by Dansyl-Poly(ethylene Glycol)s as a New Means Against Aggregation of Salmon Calcitonin, J. Pharm. Sci., 2011, 100, 1648–1662 Search PubMed.
- C. Mueller, M. A. H. Capelle, E. Seyrek, S. Martel, P.-A. Carrupt, T. Arvinte and G. Borchard, Noncovalent PEGylation: Different Effects of Dansyl-, l-Tryptophan–, Phenylbutylamino-, Benzyl- and Cholesteryl-PEGs on the Aggregation of Salmon Calcitonin and Lysozyme, J. Pharm. Sci., 2012, 101, 1995–2008 Search PubMed.
- C. Mueller, M. A. H. Capelle, T. Arvinte, E. Seyrek and G. Borchard, Tryptophan-mPEGs: Novel excipients that stabilize salmon calcitonin against aggregation by non-covalent PEGylation, Eur. J. Pharm. Biopharm., 2011, 79, 646–657 Search PubMed.
- S. Asayama, K. Nagashima, Y. Negishi and H. Kawakami, Byproduct-Free Intact Modification of Insulin by Cholesterol End-Modified Poly(ethylene glycol) for in Vivo Protein Delivery, Bioconjugate Chem., 2018, 29, 67–73 CrossRef CAS PubMed.
- H. Y. Cho, M. A. Kadir, B.-S. Kim, H. S. Han, S. Nagasundarapandian, Y.-R. Kim, S. B. Ko, S.-G. Lee and H. Paik, Synthesis of Well-Defined (Nitrilotriacetic Acid)-End-Functionalized Polystyrenes and Their Bioconjugation with Histidine-Tagged Green Fluorescent Proteins, Macromolecules, 2011, 44, 4672–4680 Search PubMed.
- T. H. Kim, M. Swierczewska, Y. Oh, A. Kim, D. G. Jo, J. H. Park, Y. Byun, S. Sadegh-Nasseri, M. G. Pomper, K. C. Lee and S. Lee, Mix to Validate: A Facile, Reversible PEGylation for Fast Screening of Potential Therapeutic Proteins In Vivo, Angew. Chem., Int. Ed., 2013, 52, 6880–6884 Search PubMed.
- D. Duret, Z. Haftek-Terreau, M. Carretier, C. Ladavière, M.-T. Charreyre and A. Favier, Fluorescent RAFT polymers bearing a nitrilotriacetic acid (NTA) ligand at the α-chain-end for the site-specific labeling of histidine-tagged proteins, Polym. Chem., 2017, 8, 1611–1615 Search PubMed.
- D. T. Nguyen, R. J. Cavazos, A. N. Harris and R. A. Petros, Werner Complexes Viewed Anew: Utilizing Cobalt Coordination Chemistry for ‘Traceless’ Stimuli-Responsive Bioconjugation Involving Therapeutic Nanoparticles, Protein PEGylation, and Drug-(Bio)polymer Conjugates, Comments Inorg. Chem., 2014, 34, 59–77 Search PubMed.
- S. Asayama, K. Nagashima and H. Kawakami, Facile Method of Protein PEGylation by a Mono-Ion Complex, ACS Omega, 2017, 2, 2382–2386 Search PubMed.
- T. Kurinomaru, S. Tomita, S. Kudo, S. Ganguli, Y. Nagasaki and K. Shiraki, Improved Complementary Polymer Pair System: Switching for Enzyme Activity by PEGylated Polymers, Langmuir, 2012, 28, 4334–4338 Search PubMed.
- D. Tsiourvas, Z. Sideratou, N. Sterioti, A. Papadopoulos, G. Nounesis and C. M. Paleos, Insulin complexes with PEGylated basic oligopeptides, J. Colloid Interface Sci., 2012, 384, 61–72 CrossRef CAS PubMed.
- T. Kurinomaru and K. Shiraki, Noncovalent PEGylation of l-Asparaginase Using PEGylated Polyelectrolyte, J. Pharm. Sci., 2015, 104, 587–592 Search PubMed.
- T. Kurinomaru, K. Kuwada, S. Tomita, T. Kameda and K. Shiraki, Noncovalent PEGylation through Protein–Polyelectrolyte Interaction: Kinetic Experiment and Molecular Dynamics Simulation, J. Phys. Chem. B, 2017, 121, 6785–6791 Search PubMed.
- A. Nieto-Orellana, M. D. Antonio, C. Conte, F. H. Falcone, C. Bosquillon, N. Childerhouse, G. Mantovani and S. Stolnik, Effect of polymer topology on non-covalent polymer–protein complexation: miktoarm versus linear mPEG-poly(glutamic acid) copolymers, Polym. Chem., 2017, 8, 2210–2220 Search PubMed.
- A. Nieto-Orellana, D. Coghlan, M. Rothery, F. H. Falcone, C. Bosquillon, N. Childerhouse, G. Mantovani and S. Stolnik, Dry-powder formulations of non-covalent protein complexes with linear or miktoarm copolymers for pulmonary delivery, Int. J. Pharm., 2018, 540, 78–88 Search PubMed.
- S. Gao, A. Holkar and S. Srivastava, Protein–Polyelectrolyte Complexes and Micellar Assemblies, Polymers, 2019, 11, 1097 CrossRef CAS PubMed.
- E. Seyrek, P. L. Dubin, C. Tribet and E. A. Gamble, Ionic Strength Dependence of Protein-Polyelectrolyte Interactions, Biomacromolecules, 2003, 4, 273–282 CrossRef CAS PubMed.
- K. Kuwada, T. Kurinomaru, S. Tomita and K. Shiraki, Noncovalent PEGylation-based enzyme switch in physiological saline conditions using quaternized polyamines, Colloid Polym. Sci., 2016, 294, 1551–1556 Search PubMed.
- O. A. Okoh and P. Klahn, Trimethyl Lock: A Multifunctional Molecular Tool for Drug Delivery, Cellular Imaging, and Stimuli-Responsive Materials, ChemBioChem, 2018, 19, 1668–1694 CrossRef CAS PubMed.
- A. S. Skwarecki, J. Stefaniak-Skorupa and M. G. Nowak, Trimethyl Lock Based Tools for Drug Delivery and Cell Imaging – Synthesis and Properties, Chem.–Eur. J., 2025, 31, e202403486 Search PubMed.
- S. Milstien, L. A. Cohen and control. I. Stereopopulation, Rate enhancement in the lactonizations of o-hydroxyhydrocinnamic acids, J. Am. Chem. Soc., 1972, 94, 9158–9165 CrossRef CAS PubMed.
- D. M. Vyas, Y. Ueda, H. Wong, J. D. Matiskella, S. Hauck, A. B. Mikkilineni, V. Farina, W. C. Rose and A. M. Casazza, in Taxane Anticancer Agents, American Chemical Society, 1994, vol. 583, pp. 124–137 Search PubMed.
- B. Wang, S. Liu and R. T. Borchardt, Development of a Novel Redox-Sensitive Protecting Group for Amines Which Utilizes a Facilitated Lactonization Reaction, J. Org. Chem., 1995, 60, 539–543 CrossRef CAS.
- J. Zhou, J. Zhang, H. Ren, X. Dong, X. Zheng and W. Zhao, A turn-on fluorescent probe for selective detection of glutathione using trimethyl lock strategy, J. Photochem. Photobiol., A, 2018, 355, 94–100 CrossRef CAS.
- J. M. Bradshaw, J. M. McFarland, V. O. Paavilainen, A. Bisconte, D. Tam, V. T. Phan, S. Romanov, D. Finkle, J. Shu, V. Patel, T. Ton, X. Li, D. G. Loughhead, P. A. Nunn, D. E. Karr, M. E. Gerritsen, J. O. Funk, T. D. Owens, E. Verner, K. A. Brameld, R. J. Hill, D. M. Goldstein and J. Taunton, Prolonged and tunable residence time using reversible covalent kinase inhibitors, Nat. Chem. Biol., 2015, 11, 525–531 Search PubMed.
- R. J. Grams, W. L. Santos, I. R. Scorei, A. Abad-García, C. A. Rosenblum, A. Bita, H. Cerecetto, C. Viñas and M. A. Soriano-Ursúa, The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology, Chem. Rev., 2024, 124, 2441–2511 CrossRef CAS PubMed.
- N. A. McGrath, K. A. Andersen, A. K. F. Davis, J. E. Lomax and R. T. Raines, Diazo compounds for the bioreversible esterification of proteins, Chem. Sci., 2014, 6, 752–755 RSC.
- K. A. Mix, J. E. Lomax and R. T. Raines, Cytosolic Delivery of Proteins by Bioreversible Esterification, J. Am. Chem. Soc., 2017, 139, 14396–14398 CrossRef CAS PubMed.
- J. V. Jun, Y. D. Petri, L. W. Erickson and R. T. Raines, Modular Diazo Compound for the Bioreversible Late-Stage Modification of Proteins, J. Am. Chem. Soc., 2023, 145, 6615–6621 Search PubMed.
Footnote |
| † D. A. R and Z. F. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |