Strip electrodes: a novel, effective and minimally invasive therapeutic option for correcting DNS via electromechanical reshaping†
Mohamed Jameer Basha
Jahankir‡
 ab,
Harisharan
Ramesh‡
*abc,
Thilak
Chakaravarthi
*abc,
Ajay
Agarwal
d,
Amit
Goyal
e and
Gowri Manohari
Balachander
ab,
Harisharan
Ramesh‡
*abc,
Thilak
Chakaravarthi
*abc,
Ajay
Agarwal
d,
Amit
Goyal
e and
Gowri Manohari
Balachander
 *f
*f
aCaldor Health Technologies Pvt Ltd, Mannudaiyar Street, Kurumbapalayam, Coimbatore, Tamil Nadu – 641007, India
bAtal Incubation Centre – Centre for Cellular and Molecular Biology, IDA Uppal, Habsiguda, Hyderabad, Telangana – 500039, India
cInterdisciplinary Research Programme, Smart Healthcare, Indian Institute of Technology, Jodhpur, Rajasthan – 342030, India
dDepartment of Electrical Engineering, Indian Institute of Technology, Jodhpur, Rajasthan – 342030, India
eDepartment of Otorhinolaryngology, All Indian Institute of Medical Sciences, Jodhpur, Rajasthan – 342005, India
fSchool of Biomedical Engineering, Indian Institute of Technology (BHU), Varanasi, Uttar Pradesh – 221005, India. E-mail: gowribalachander.bme@iitbhu.ac.in; Tel: +91 542-7165134
First published on 15th November 2024
Abstract
Deviated nasal septum (DNS) is a common condition affecting nasal breathing, which is generally treated using septoplasty. However, this invasive surgical method carries potential risks of post-surgical complications. Alternatively, electromechanical reshaping (EMR) is a novel method that has evolved as a non-thermal, minimally invasive option to reshape the cartilage using mechanical pressure and direct current (DC) without significant tissue damage. However, the existing flat and needle electrodes tested in animal tissues have raised significant concerns due to their safety. Thus, herein, we aimed to develop a novel strip electrode configuration and optimize dosimetry to achieve efficient reshaping without compromising its safety. Electric field simulations showed that our novel 5-strip electrode configuration with a thickness of 0.5 mm achieved optimal electric field, requiring minimal current flow compared to flat electrodes. EMR was performed on ex vivo goat cartilage at various dosimetry groups to analyze four-day shape retention. The optimized strip electrode reshaped the ex vivo goat septal cartilage effectively at a dosimetry of 20 mA for 15 minutes, whereas the flat electrode needed 35 mA for 15 minutes. DMMB assay, ATR-FTIR spectroscopy, tensile testing, and histopathology analysis demonstrated reduced tissue damage while supporting increased efficiency and mechanical stability with the strip electrode configuration, emphasizing its safety. Thus, the optimized strip electrode-based EMR emerges as a viable non-invasive approach for reshaping the nasal septal cartilage, which can be used to treat DNS. Further in vivo studies are recommended to validate the long-term safety and efficacy of this technique.
1. Introduction
Deviated nasal septum (DNS) is a misalignment of the thin hyaline cartilage present between the two nasal valves. This misalignment leads to nasal congestion, breathing difficulty and, in some cases, nasal valve collapse, chronic sinusitis or rhinitis.1 Studies have reported that 80% of the population is affected by DNS.2 Congenitally, DNS can be due to the improper growth of the septum during fetal development or compression caused during vaginal birth.3 In some cases, DNS can also occur because of an acquired injury. Nasal congestions due to septal deviations can be temporarily managed with nasal steroid sprays or nasal irrigation.4 However, for permanent relief, a conventional invasive surgical procedure called nasal septoplasty is performed. Septoplasty is often performed alongside other procedures such as turbinoplasty, rhinoplasty, or sinus surgery. Typically, septoplasty involves five key steps: (1) incision of the mucosa and exposure of the septum, (2) release of the forces causing angulations or deviations, (3) realignment of the septum, (4) reimplantation of crushed cartilage, and (5) closure with absorbable sutures, followed by placement of splints in each side of the nasal cavity for 7 days, with packing for 24 hours.5 However, similar to invasive surgeries, septoplasty is associated with post-surgical complications, including postoperative pain and discomfort and excessive bleeding. In rare cases, studies have also reported incidents of septal perforations, risk of infection, followed by prolonged healing, cerebrospinal fluid rhinorrhea, ocular complications, toxic shock syndrome and cosmetic deformities.6 Among them, haemorrhage is the most common complication (16%), followed by recurrence of the deviation (15.5%).7,8 This has also led to adverse consideration among health policy makers and insurance companies given that the post-operative improvements are discrepant.9 The other modalities to treat DNS include laser-assisted and radiofrequency-based cartilage reshaping using heat-based mechanisms. These methods employ high temperature in the range of 50 °C to 88 °C, resulting in the loss of chondrocytes and compromised structural integrity.10,11 Considering all the above-mentioned challenges, electromechanical reshaping (EMR) has emerged as a promising alternative with the specific advantage of non-thermal-dependent reshaping modality for DNS treatment. Moreover, EMR is minimally invasive compared to septal correction surgeries. It can be performed in-office under local anesthesia without any post-procedural complications such as pain, discomfort, bleeding, risk for infections and delayed healing.EMR involves the use of a unique combination of mechanical pressure and low amount of direct current (DC) to reshape the cartilage. The mechanical pressure creates stress in the cartilage and DC relaxes the stress via the electrolysis of water in the cartilage matrix. Electrolysis results in the breakdown of water into H+ and OH− ions. This influx of ions disrupts the ionic bonds of the glycosaminoglycan (GAG) matrix, collagen, and proteoglycan layers. The ionic bonds between the layers are responsible for the structural rigidity of the cartilage and its shape memory. Thus, by disrupting the ionic bonds, EMR employs a biochemical way for permanently reshaping cartilage.12 To achieve the safe and effective reshaping of cartilage through EMR, factors such as electrode material, configuration, and dosimetry are crucial to be optimized.
Various electrode materials such as gold, aluminium, and platinum have been explored for EMR applications.13,14 However, aluminium and gold have been found to cause electrodeposition on tissues, while platinum is expensive.15 Different electrode configurations, including flat and needle types, have been tested in various animal models involving auricular, nasal septum, tracheal, and costal cartilages14,16–18 (Fig. 1). However, although these configurations have shown some promise, they often result in tissue damage and reduced chondrocyte viability. Alternatively, needle electrodes offer focused and localized current delivery, which minimize the area of tissue damage but require invasive insertion into the cartilage to induce EMR.19,20 Previous studies using ex vivo cartilage samples have several limitations that hinder their practical utility. These samples possessed a non-uniform thicknesses (typically 1 mm or 2 mm), absent of a mucosal layer, and limited in number with low reproducibility.20
 | ||
| Fig. 1 Key literature on EMR exploring different electrode materials, configurations and in vivo feasibility. | ||
The present study aimed to demonstrate the potential of EMR for reshaping nasal cartilage to correct DNS. This study is the first to innovate a novel approach for the design and deployment of a patented strip electrode configuration for EMR applications to overcome the disadvantages of the flat and needle electrode configurations.21 Electrodes must be designed to deliver a more focused electric field at lower dosimetry considering their practical usability. Thus, their design plays a major role in maximizing their efficacy and reducing tissue injury. In our study, titanium electrodes were used because of their biocompatibility, high corrosion resistance in the biological environment, inertness, and low cost compared to aluminum, gold and platinum.22 Moreover, titanium has low thermal conductivity, which is ideal for minimizing heat generation during EMR, and also maintains tissue viability. The strip electrode configuration serves as effective option given that it generates a focused electric field, allowing precise targeting of tissue with lower dosimetry. This minimizes the thermal damage and preserves the chondrocyte viability and extracellular matrix integrity, ensuring safer reshaping and promoting quicker recovery. Together, these factors enhance the precision and safety of the EMR procedure, addressing key challenges in current reshaping techniques.
In the current study, goat (Capra hircus) nasal septum was used due to its resemblance in terms of structural integrity and pathophysiology with the human nasal septum and only minor variations.23 Experiments were conducted directly on the extracted cartilage samples with mucosal layers with the actual cartilage thickness of 2 mm to 5 mm. The current study also discusses the resistance, structural integrity, and molecular and mechanical stability of cartilage after EMR, and thereby its implications on the safety and efficacy of reshaping. Incorporating the specific requirements in each step of experimentation, this study explored the possibility of utilizing non-invasive electrode configurations for DNS treatment through electromechanical reshaping technology.
2. Experimental methods
2.1. Simulation, design and fabrication of strip electrode
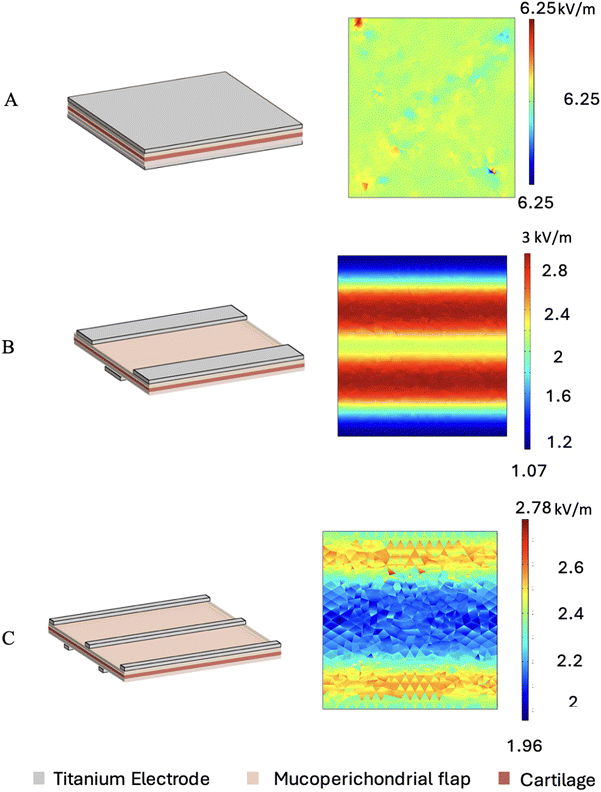
Different electrode configurations were simulated using the finite element analysis package (COMSOL Multiphysics, COMSOL, Palo Alto, CA) based on two assumptions, as follows: (i) efficient EMR would happen at the desired area, where the average electric field is greater than 1.8 kV m−1 and (ii) the electric field area of 4–15 kV m−1 has more viable cells and dead cell areas with an electric field of more than 15 kV m−1.20In the COMSOL Multiphysics simulation, the cartilage was modelled as a sandwich structure between the perichondrium and mucosal membrane. The electrodes were modelled over the mucosal membrane given that it touches the mucosa. The voltage was kept constant at 6 V. Three different configurations were designed as follows: (1) total surface flat electrode, (2) five strip electrodes and (3) three strip electrodes. The strip electrode in both configurations 2 and 3 were modelled with varying width from 2 mm to 0.3 mm. The cartilage with perichondrium and mucosa were modelled as a 10 × 10 mm slab. All the strip electrodes were positioned equidistant from each another and appeared to be inter digitated when overlapped from two sides. The polarity was kept opposite on both the sides. The electric field strength at the centre (area subjected to reshaping) was obtained from simulation and plotted against total electric field distribution to get the optimal configuration for effective reshaping.
A medical-grade titanium sheet with a thickness of 0.5 mm (Ti-6Al-4V) was procured from Coimbatore metals. The titanium strip and flat electrodes according to the optimised width (Fig. 2) were cut in the required dimensions using an electrical discharge machining (EDM) (Fig. 2B). For recurrent usage, the electrodes were ultrasonically cleansed in ethanol for 15 min, followed by drying before fixing them on a 3D-printed jig (mechanical pressure component for reshaping experiments) (Fig. 2C and D). The reshaping jig was designed as two right-angled triangles (base triangle – sides of 5 cm and hypotenuse of 7.07 cm; top triangle – sides of 6 cm and hypotenuse of 8.48 cm) and 3D printed using a fused deposition modelling (FDM) UltiMaker S5 (UltiMaker B.V.) 3D printer.
2.2. Sample preparation
The nasal septum of a goat was obtained from the abattoir located near Kacheguda, Hyderabad. The nasal septal cartilage was harvested within 2 h of slaughter and processed within an hour before using it for experiments (Fig. S1, ESI†). The specimens were cut to have dimensions of 1 cm wide and 3 cm long (Fig. 3A). Subsequently, the samples were immersed in 1× PBS until the EMR experiments.2.3. Electromechanical reshaping
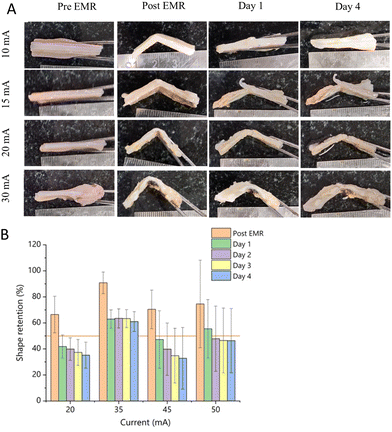
The samples were placed between two acrylonitrile butadiene styrene (ABS) 3D-printed jigs containing flat or strip electrodes according to the simulated configuration. The current was supplied using a Bio-Rad PowerPac Basic power supply. Dosimetry groups of 20 mA, 35 mA, 45 mA, and 50 mA was applied for 15 min in the flat titanium electrodes. Alternatively, for the titanium strip electrodes, 10 mA, 15 mA, 20 mA, and 30 mA were applied for 15 min. Each dosimetry was performed in quintets to compensate for the sample-to-sample variability. After completion of EMR, the tissue was immersed in 1× PBS along with the jig to restore the pH for 15 min at room temperature (Fig. 3E). The control samples were placed in the jig to exert mechanical pressure without supplying an electric current.2.4. Shape retention
Post-EMR, the samples were stored in 1× PBS for 4 days to analyse their shape retention. Images were captured pre-EMR, post-EMR, day 1, day 2, day 3, and day 4 for all configurations and dosimetry groups. Images were captured cross sectionally and shape retention (%) was assessed by measuring the bend angle using three point of angle measurement in the ImageJ software.24| Shape retention (%) = (γ init − γ relax/γ init − γ imp) × 100 |
2.5. Structural integrity analysis
The structural integrity of the samples was analysed through the dimethylmethylene blue assay (DMMB).25 DMMB solution (250 mL) was prepared with 4 mg DMMB (Sigma), 760 mg glycine, 400 mg NaCl, and 0.1 M acetic acid with the pH under 3.5. Standard solutions of chondroitin sulphate (Sigma) were prepared in concentrations ranging from 5 μg mL−1 to 200 μg mL−1 at the intervals of 10 μg mL−1 using PBS as the solvent. 20 μL of sample was mixed with 180 μL DMMB solution in a 96-well plate and observed in a spectrophotometer at 525 nm. The colour changes from blue to violet denoted the reaction of DMMB with sGAG. A standard curve was plotted with the R value. Then, the spectrophotometric readings were converted into w/v and the percentage of sGAG release was observed.2.6. Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy
The molecular structural constituent of the cartilage was analysed using ATR-FTIR spectroscopy. The control and experimental samples were deep frozen and lyophilized to remove the water, eliminating its interference and enhancing the signal to noise ratio during the analysis. The spectrum analysis was carried out on a FTIR spectrophotometer (SHIMADZU, IRSpiritSingle). FTIR spectra were recorded at a resolution of 4 cm−1 and 45 scans were averaged to obtain a good signal-to-noise ratio. The ATR measurement area was a circular spot with a diameter of approximately 1.5 mm. The spectrum was recorded in the wavenumber range of 400–4000 cm−1.2.7. Analysis of mechanical properties
For analysing the mechanical properties of the cartilage after EMR, uniaxial tensile deformation was applied with a universal testing machine (UTM) (DAK Systems Inc.). Cartilage samples, approximately 7 cm in length, were reshaped, and then cut into a ‘dog bone’ shape to ensure proper fitting and uniform stress distribution during testing. The exact dimensions of the ‘dog bone’ sample are provided in Fig. 4. The prepared cartilage samples were mounted on the testing machine and uniaxial tension was applied at a constant rate of 50 mm min−1 until the cartilage reached its breaking point. The tensile strength and elongation at break were recorded to assess the mechanical properties of the reshaped cartilage. | ||
| Fig. 4 (A) Schematic of cartilage dimensions for mechanical testing, and (B) dimensions of the cartilage cut into a “dog bone” shape for tensile testing. | ||
2.8. Histopathology
The cartilage samples were fixed in 10% neutral buffered formalin for 24 h to 48 h, followed by dehydration using increased concentrations of ethanol solutions. Subsequently, the tissues were infiltrated with molten paraffin wax and embedded in paraffin blocks. Thin sections of the samples were cut with a thickness of 1 μm using a microtome and mounted on glass slides. Then, the samples were deparaffinized by immersing the slides in xylene, followed by rehydration using alcohol and rinsing in tap water. The slides stained with haematoxylin solution for 5 min to stain the nuclei, followed by counterstaining with eosin solution to stain the cytoplasm and extracellular matrix (ECM). Coverslips were applied using a mounting medium and the slides were allowed to dry before microscopic evaluation. During the microscopic evaluation, the images were captured with a reference scale bar and the extent of damaged area was measured using ImageJ.2.9. Data analysis
The data was analysed in two-way analysis of variance (ANOVA), which compared the shape retention among 5 replicates in each group. The statistical significance was established through a two-tailed t-test (p ≤ 0.05), checking the total amount of sGAG between strip and flat electrode.3. Results and discussion
3.1. Finite element modelling (FEM) and simulation of electrode configurations showing uniform and non-uniform electric field distribution
The electric field distribution determines the effective and safe reshaping of tissue. To select the ideal electrode geometry for EMR, different electrode designs were modelled and simulated using the COMSOL finite element package for analysing the electric field distribution. In case of flat electrodes, an electric field of 6.25 kV m−1 was uniformly distributed throughout the cartilage area (Fig. 5A). A 3-strip configuration generated an electric field of >1.8 kV m−1, which was distributed over 70% of the tissue area (Fig. 5B). The electric field observed in the flat electrodes was due to the placement of electrodes completely on both sides of the tissue slab; hence, the electric field passing through the entire tissue area was uniform. Alternatively, in the case of the strip electrodes, a negative strip was placed opposite and in between two positive strips (as shown in Fig. 3E), allowing the electric field to pass through the tissue evenly in regions not in contact with the electrodes. Previous studies suggest that an electric field of >1.8 kV m−1 is required for efficient reshaping.18 The COMSOL simulation results showed that the 5-electrode configuration with an electrode width of 1 to 0.5 mm provided the optimal electric field of 1.96–3.75 kV m−1 (Fig. 6A) throughout the tissue area (Fig. 5C). The 5-strip configuration with a width of 0.5 mm generated an electric field of 1.96–2.3 kV m−1 and 2.3–2.78 kV m−1 in 40% and 60% of the tissue area, respectively (Fig. 5C). The 0.5 mm strip electrode design was selected because it satisfied both the conditions of requiring more than 1.8 kV m−1 throughout the cartilage area and minimum electric field (Fig. 6B). Although the flat electrode configuration produced a uniform electric field distribution of 6.25 kV m−1 (Fig. 6A), the strip electrodes produced the maximum electric field of 2.78 kV m−1 (Fig. 6A) with only 12.5% electrode contact area (Fig. 6B) and less than a 3 °C increase in the cartilage temperature during reshaping. Flat electrodes have been shown to reduce the chondrocyte viability due to their higher electric field and high surface contact with tissues.20 In contrast, strip electrodes have the minimum electric field and reduced surface contact with the tissue (Fig. 6B). The minimum electric field in the tissue was reported to significantly aid the survival of chondrocytes.26 The electrode geometry and electric field significantly impact the cartilage reshaping and cell viability. A higher electric field induces more tissue damage and chondrocyte loss.26 The simulation results confirmed this by showing that the narrower strip electrodes produce a more controlled electric field, reducing tissue injury.3.2. Lowered dosimetry requirement for efficient reshaping of tissues with novel strip electrodes
We aimed to validate the simulation results via reshaping experiments with ex vivo nasal septum. Accordingly, a comparative analysis of the shape retention between the strip and flat electrodes was performed. The reshaped nasal septum was stored at 4 °C for 4 days to analyse the shape retention of the tissue each day. The images were analysed using the ImageJ software and percentage shape retention was calculated and compared as mentioned in the Experimental methods. In general, an increase in applied dosimetry would increase the shape retention but also cause tissue damage.27 In the case of the flat electrodes, a dosimetry above 50 mA damaged the architecture of the cartilage and affected the ability of the reshaped cartilage to retain its shape. Furthermore, the tissue reshaped with a dosimetry above 50 mA was charred and torn after reshaping (Fig. S2, ESI†). This could be due to the loss of chondrocyte viability and loss of sulphated glycosaminoglycans (sGAG). Alternatively, the reshaped cartilages at lower dosimetry groups such as 20 mA in the flat electrode and 10 mA in the strip electrodes could not retain their shape, indicating insufficient EMR (Fig. 7 and 8, respectively).The ideal dosimetry for efficient shape retention is determined by two conditions, as follows: (1) cartilage should retain at least 50% of the shape imposed for at least 4 days and (2) the required dosimetry should be low to avoid tissue damage. The flat titanium electrodes at 35 mA retained 61% of the imposed shape (Fig. 7B) at the end of the 4th day and the strip electrodes at 20 mA effectively retained 54% at the end of the 4th day (Fig. 8B). The strip electrodes could achieve comparable reshaping effects even at much lower dosimetry groups (p = 0.18). Prior studies reported the allowable dosimetry range of 10 mA to 460 mA in nasal cartilages, signifying the potential risk of electrical injury and pain in clinical use cases.14 In contrast, our novel strip electrodes showed a lower current requirement (20 mA). In any electrical circuit, electrodes with a lower surface area draw a lower amount of current.28 Thus, this result validates the efficacy and potential clinical utility of the strip electrode configuration over the flat electrodes in achieving significant shape retention even at the lower dosimetry level of 20 mA. Thus, the strip electrode was selected as the effective configuration for further analysis in the present study give that it required 43% less dosimetry than the flat electrodes.
3.3. Increased resistance and voltage requirement in the case of EMR with novel strip electrodes
Voltage and resistance were monitored during EMR given that they can be potential determinants of efficacy and safety. We aimed to analyse the effect of different electrode configurations on the voltage and resistance during reshaping for understanding the mechanism of EMR and correlating it with the safety and efficacy results. A custom-made voltage monitor was used to record the voltage during the reshaping process and resistance was calculated from the voltage data. The flat electrodes at 35 mA and strip electrodes at 20 mA were observed to have an average resistance of 0.3 kΩ and 3.0 kΩ, respectively (Fig. 9A). Previous studies reported the tissue resistance of 1 kΩ.29 The high resistance in our study can be attributed to two reasons, as follows: (i) titanium is a poor conductor compared to platinum, aluminium, and gold, leading to higher resistance. However, we chose titanium given that there was no electrodeposition in the samples like that observed with other metals15,30 and its lower price compared to platinum and (ii) previous studies used a uniform cartilage thickness in the range of 0.6 mm to 2 mm without a mucosal layer in all the EMR experiments, and therefore observed much less resistance. We performed the experiments with the intact mucosal layer because it enabled the utility of the experimental data for preclinical and clinical studies. The thickness and layers of different tissues such as mucosa, perichondrium and cartilage increased the resistance of the tissue. The maximum resistance during the strip electrode experiments was found to be 8 times higher (4.7 kΩ) than the flat electrodes (0.58 kΩ) (Fig. 7A). This is due to the lower surface area of the electrodes where the current is localised, and the electric field is non-uniform with higher and lower electric field gradients (Fig. 5C). However, in the case of the flat electrodes, their larger surface area provided more pathways for current to travel through, significantly reducing the overall resistance with a uniform electric field.Similarly, the voltage of the strip electrodes peaked at around 120 V in 4 min, and subsequently saturated between 70 V and 80 V (Fig. 9B). However, the flat electrodes peaked at 58 V in 6 min, and subsequently saturated between 40 V and 50 V (Fig. 9B). The increased voltage was proportional to the resistance values. Despite the high voltages, the electric field of the strip electrode was low enough to enhance tissue viability and efficiently reshape the tissue. From another perspective, the water molecules in the tissue are the key determinant of its conductivity. These water molecules are consumed during EMR due to redox reactions, and hence over the time, the conductivity decreases and resistance increases. In the case of the flat electrodes, it has a larger surface area to access water molecules throughout the tissue, aiding in uniform redox reaction and resistance pattern. Accordingly, the strip will have a smaller area for its redox reaction, which will exhaust the locally available water molecules within 30 s. The exhaustion of water molecules increases the resistance and requirement of voltage to access the surrounding water molecules for electrical conductivity.31
3.4. Similar structural integrity and mechanical stability in both electrode configurations
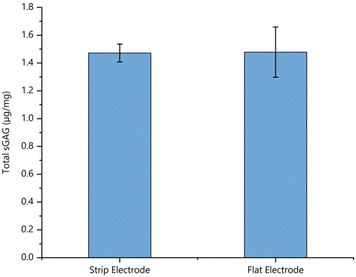
The sGAG is a key constituent of the ECM, supporting the physiology of the cartilage. It helps in maintaining the viscoelasticity and ensures mechanical stability.32 In the present study, the DMMB assay was utilized to evaluate the structural integrity of the reshaped cartilage by measuring the total amount of GAG present in the reshaped cartilage after EMR. DMMB dye is a positively charged cationic dye with strong affinity to negatively charged molecules. The sGAGs present in the cartilage have negatively charged sulphate groups, which bind to the DMMB dye through electrostatic interaction. This interaction leads to a spectral shift in the bound solution. Generally, DMMB dye has the maximum absorbance at approximately 620 nm but after the spectral shift, it appears at the lower wavelength of 525 nm, and the colour changes from blue to purple or pink based on the concentration of sGAGs.A standard curve was prepared using chondroitin-6-sulphate obtained from shark cartilage (r2 = 0.9825). The cartilages reshaped using both electrode configurations with an average volume of 1 cm3 and weight of 176.3 mg showed an sGAG concentration of 1.47 μg mg−1 (Fig. 10). The total sGAG concentration was found to be similar in both cartilages reshaped using flat and strip electrodes (p = 0.95). This indicates that the mechanical stability after reshaping is similar in both electrode configurations. A high precision tensile test can supplement the findings of the mechanical stability accurately, given that DMMB assays have lower sensitivity levels in detecting minor variations in cartilage structural integrity.33
3.5. Molecular structural retainability of flat and strip electrode configurations
Septal cartilage has extracellular matrix (ECM) components that are synthesized by chondrocytes. Collagen is the most abundant component of ECM and supports cartilage with structural integrity, tensile strength and stiffness.34,35 Proteoglycans, mainly aggrecan, are entrapped with collagen, providing resistance against compressive forces. There is also high content of sulphated glycosaminoglycans (S-GAGs), which attracts positive ions and water molecules with its negative charge, thereby maintaining an adequate level of water in the cartilage.36The fresh tissue control and cartilage treated with the flat and strip configurations were analysed with FTIR spectroscopy, with the spectra recorded in the range of 4000–750 cm−1 and the results revealed notable differences in the regions associated with water content, protein structure, lipids, and glycosaminoglycans37,38 (Fig. 11). The novel strip configuration only had minor shifts in peak intensities compared to the native cartilage biomolecular architecture, whereas the flat configuration showed significant changes, indicating the loss of the native architecture.
 | ||
| Fig. 11 FTIR spectra of control (black), cartilage reshaped with the flat electrode (pink) at 35 mA, and cartilage reshaped with the strip electrode (blue) at 20 mA. | ||
In the OH and NH stretching region (∼3500–3000 cm−1), intensity shifts were observed in the two treated samples, especially the flat configuration showed a notable reduction in intensity. The peaks at ∼3484 cm−1 and ∼3292 cm−1, which are ascribed to water molecules, polysaccharides (such as hyaluronic acid), and proteins, diminished substantially. This reduction indicates a loss of water content and possible degradation of key structural components such as hyaluronic acid. The changes were more severe in the flat electrode sample, which is likely due to the higher current intensity, which suggests that EMR with the flat configuration can lead to the greater disruption of the cartilage hydration and protein structures.
The lipid region (3000–2870 cm−1) exhibited an increase in peak intensity in the strip electrode sample. This region is associated with the CH3 and CH2 stretching in lipids and proteins. The observed increase suggests a shift toward a more lipophilic environment.
In the protein secondary structure region (∼1800–1500 cm−1), critical changes were noted in the amide I and II bands, which indicate the protein folding and hydrogen bonding patterns, respectively. A new peak at ∼1743 cm−1 emerged in both treated samples, signalling the presence of aldehyde groups due to lipid peroxidation, a sign of oxidative stress. The intensity of this peak was significantly higher in the flat sample, further supporting the notion of greater oxidative damage at higher current levels. Additionally, the shifts in the amide I (∼1650 cm−1) and amide II (∼1540 cm−1) bands toward lower frequencies reflected a structural transformation in proteins, likely from α-helix to random coil or β-sheet formations. This conformational shift, which was more pronounced in the flat electrode group, suggests a weakening of the mechanical strength and elasticity of the cartilage. Alternatively, the strip electrode did not show any differences in the amide I and amide II regions.
The regions of 1200 cm−1 and 1280 cm−1 indicates the collagen amide III vibration with significant mixing with the CH2 from the glycine backbone and proline sidechain. The alteration in this region and decrease in peak intensity indicate mild collagen structural changes in both the strip and flat configurations.
The glycosaminoglycan region (∼1250–900 cm−1) displayed a decreased intensity and peak broadening in the flat configuration, whereas the intensities increased in the strip configuration. The increased sGAG intensity in the strip configuration may be due to the mild denaturation and exposure of hidden functional groups and local pH changes. Glycosaminoglycans are vital for the ability of cartilage to retain water and resist compressive forces. The damage observed in the flat configuration highlights the potential risks of reducing the shock-absorbing and hydrating capacities of cartilage.
The FTIR spectra clearly indicate that electromechanical reshaping altered the structural integrity of the septal cartilage in a dosimetry-dependent manner. The novel strip electrode configuration (20 mA) led to less pronounced structural degradation compared to the flat electrode (35 mA), suggesting that the current intensity and configuration play a pivotal role in the extent of tissue alteration. Although both configurations demonstrated potential for cartilage reshaping, the strip electrode configuration offers a more controlled and less invasive option, minimizing structural damage.
3.6. Higher mechanical stability with novel strip electrode configuration
The tensile strength analysis of the cartilage treated with the strip and flat electrode configurations revealed notable differences in their mechanical properties. The strip electrode configuration exhibited a tensile strength of 3.882 MPa, with an elongation at break of 6.930%. This indicates that the strip electrode induced significant stiffening in the cartilage, potentially due to crosslinking or realignment of the collagen fibers within the tissue. The increased tensile strength suggests a more rigid structure, which can be advantageous in maintaining the structural integrity after reshaping. However, the reduced elongation at break indicates a more stable but less flexible final form. The stiffening of cartilage reshaped with strip electrodes can be critical in ensuring long-term stability post-procedure, preventing undesirable shape changes.In contrast, the flat electrode configuration produced a tensile strength of 1.662 MPa and a much higher elongation at break of 15.922%. This suggests a different mechanical response, with the cartilage becoming more flexible and less rigid. Although it offers increased flexibility, the lower tensile strength implies that the structure may be more prone to deformation over time. The inverse relationship between tensile strength and elongation at break observed in both configurations highlights a common biomechanical phenomenon, as reported by Huang et al. (2023),39 where stiffer, higher-strength materials break at lower strain, whereas more flexible materials exhibit higher elongation.
The stiffness, as measured by Young's modulus, followed a similar pattern as the tensile strength. The strip electrode configuration resulted in a Young's modulus of 0.4413 MPa, indicating a stiffer material compared to the flat electrode configuration, which showed a lower modulus of 0.1525 MPa. These findings are consistent with the overall trend, where the strip configuration yielded a more rigid cartilage structure, while the flat configuration reduced the stiffness of the cartilage. The reduced strength of the cartilage in the flat configuration may be due to the higher current and damage to the native collagen structure. According to the mechanical analysis, the novel strip electrode seems to have greater stiffness and strength compared to the flat electrode, favouring the efficacy of this technology. However, the samples were not hydrated before testing, which may affect the viscoelastic behaviour of cartilage, and future in vivo studies may address this limitation.
3.7. Conserved cartilage morphology in tissues reshaped with novel strip electrodes
In previous EMR studies, histopathological analysis was done only in the auricular cartilages.40–42 Alternatively, this study provides insights into the cellular arrangement, ECM integrity and morphological features of nasal septal cartilages after EMR. After EMR, the tissues were fixed in 10% buffered formalin and prepared for staining, as mentioned in the Experimental methods. Some tissues were found to be calcified, and the specimens were decalcified using 10% formic acid or 1% hydrochloric acid for 15–60 min and washed in water for one day to remove the decalcification solution. This decalcification step helped in clearing the tissue sections and preventing blade damage during sectioning. All the experiments were performed in triplicate and duplicate, as shown in Fig. S3–S5 (ESI†). The control sample was treated with just mechanical pressure without electric current. The control samples were normal with evenly placed lacunae and chondrocytes but with mild compression in the mucosal layer, as shown in the Fig. 13A. The sample reshaped with the flat electrode at 35 mA had significant loss of tissue architecture with shrunken, elongated and mosaic-like crowded chondrocytes. The cells were intensely crowded in the cartilage part and the salivary glands in the mucosal layer were completely compressed, as shown in Fig. 13D. The samples reshaped with the 0.5 mm strip electrode showed minimal tissue damage compared to the flat electrode samples. Minimal mucosal changes were observed only on the areas where the strip electrodes were in contact with the tissue. The ECM and cellular integrity were close to normal with a mildly altered tissue architecture. The chondrocytes remained intact and uncrowded. The mucosal layer was found to be patchy, and the salivary glands were partially altered, as shown in Fig. 13G. The extent of damage was calculated using the ImageJ software with the scalebar as a reference. Submucosal changes were only observed at 1.76 mm surrounding the strip electrode placement and the damage was localized. In the tissues reshaped with the novel strip electrodes, around 44% of their submucosal surface remained like fresh tissue, whereas with the flat electrodes, 100% of the tissue area was damaged.According to the literature, in vivo EMR studies in a New Zealand white rabbit model reported neo chondrogenesis after 3 months of EMR, in which needle electrodes were used.19 The damage caused in the reshaped cartilage using the strip electrodes was minimal in both the mucosa and cartilage. Histological evidence supports that the strip configuration-based EMR is safe and morphologically conservative as a septoplasty procedure and superior to flat electrode-based EMR. The dosimetry required for efficient reshaping also correlates well with the histopathological evidence.
4. Discussion
Our study demonstrated that reshaping nasal septal cartilage using titanium strip electrodes requires a lower dosimetry of 20 mA for 15 min compared to that of 30 mA needed for flat electrodes over the same period (Fig. 7 and 8, respectively). The thin (0.5 mm) strip electrode allowed a more focused and efficient current distribution (Fig. 6 and 9), minimizing tissue damage, as confirmed by the molecular (Fig. 10 and 11), mechanical (Fig. 12), and histopathological analyses (Fig. 13), respectively. This design preserved both the chondrocyte viability and ECM integrity, making it a safer, more efficient option for cartilage reshaping (Fig. 10 and 13), respectively. This configuration also generated the optimal electric field, while maintaining a lower overall current. The flat electrodes, while covering a larger area, required higher dosimetry for cartilage reshaping. This broad electric field could cause an uneven current distribution, leading to elevated temperatures and a greater risk of thermal damage to the extracellular matrix (ECM) and chondrocytes, potentially extending the recovery time and causing complications. In contrast, the strip electrode provided a more localized electric field, enabling effective reshaping at a lower dosimetry. This configuration reduced the tissue exposure and thermal injury risk, offering a more precise and controlled approach. It also supported quicker recovery and improved clinical outcomes. | ||
| Fig. 12 Stress/strain graph of the cartilage reshaped with the flat electrode at 35 mA (pink) and cartilage reshaped with the strip electrode at 20 mA (strip). | ||
The simulation results showed a temperature rise of less than 3 °C, necessitating experimental validation through continuous monitoring during the EMR process. The unique properties of titanium, including its non-adherence and biocompatibility, make it a viable option, especially when considering more costly alternatives such as passivated platinum. Although platinum can offer possibilities for EMR reshaping with lower dosimetry requirements, the use of titanium in this context has two important implications for clinical translation. Firstly, given that titanium does not leach during EMR, there is no risk of electrode deposition in tissues, which aids in meeting ISO 10993 standards for the biological evaluation of medical devices. Secondly, cost-effectiveness is essential for reducing economic burdens in healthcare. Titanium has exceptional corrosion resistance and chemical stability, forming a protective oxide layer that ensures durability even in extreme environments, such as acidic and oxidative conditions.43 Also, its low thermal conductivity (24.5 W m−1 K−1) compared to materials such as platinum (72.4 W m−1 K−1) is a critical factor in minimizing unwanted thermal effects during EMR.44 Additionally, the proven biocompatibility of titanium, as demonstrated by its ability to support human mesenchymal stem cell proliferation and maintain high cell viability (∼85%), makes it a viable choice for medical implants.45,46 However, considering the electrical properties of titanium, it is not a good conductor (4.2 μΩ m) compared with platinum (10.6 μΩ m). Nevertheless, titanium is more cost-effective than platinum, which is essential for ensuring the affordability of this procedure for patients.47 Thus, the choice of titanium in this study aimed to optimize the balance between minimizing heat generation and maintaining biocompatibility, making it a key contributor to the overall effectiveness of the EMR-based approach.
In this study, we assessed retention for four days, a first in this context. This ex vivo studies offer a controlled environment ideal for preliminary testing. However, they lack key in vivo factors, such as tissue vascularization, blood flow, immune and inflammatory responses, biomechanical forces, and chondrogenesis, all playing a crucial role in the long-term outcomes of cartilage reshaping.48 However, despite this limitation, the mechanical and histological analyses in our ex vivo results provide strong evidence that the strip electrodes can effectively reshape cartilage with minimal tissue damage. The mucosal damage by the strip electrodes was recoverable, similar to knife-based surgical procedures, as reported by Cukurova et al., but the damage in cartilage due to flat electrodes cannot be recovered, given that a similar degree of damage was observed in laser-based cartilage reshaping.49 The limitations of ex vivo studies further underscore the importance of in vivo validation. These studies will help assess the recovery times and post-procedure care protocols, such as the use of nasal washes and gels to prevent scarring and enhance healing. Understanding these factors in vivo is critical for optimizing the long-term functional outcomes for patients. However, the assessing treatment efficacy in in vivo models poses challenges, given that there is no direct animal model that accurately represents DNS. For example, in the FDA 510(k) summary, devices such as VivAer (Aerin Medical) that use radiofrequency for nasal valve collapse have only been tested for safety in canines.
The bio-molecular integrity of the cartilage after EMR was assessed using the DMMB assay, ATR-FTIR spectroscopy, and mechanical testing. The DMMB assay indicated similar levels of sGAGs in the cartilage reshaped with both the strip and flat titanium electrodes. However, ATR-FTIR spectroscopy revealed that the cartilage reshaped with the strip electrode exhibited a spectral profile closely aligned with the control cartilage, while the flat electrode demonstrated significant deviations, particularly at key peaks such as the glycosaminoglycan and polysaccharide regions, indicating structural damage. In mechanical testing, the cartilage reshaped using the strip electrode exhibited greater stiffness compared to the flat electrode. However, the results from ATR-FTIR and mechanical testing demonstrate a complementary relationship, indicating that the strip electrode configuration maintained the structural integrity of the cartilage with minimal degradation of its essential components such as collagens and glycosaminoglycans, while the sGAG quantification from the DMMB assay is anomalous. This anomaly may be attributed to the localized ionic concentration changes induced by the electrochemical environment during EMR. The electro-hydrolysis reactions likely altered the local pH and ionic balance around the cartilage matrix, affecting the binding affinity of sGAGs with the cationic dye (dimethylmethylene blue) used in the assay. Given that the DMMB assay is highly sensitive to changes in ionic strength and pH, these variations were likely more evident in the cartilage reshaped by the flat electrode, where the electric field is less uniform. Together, these findings emphasize the critical need for in vivo studies to validate the outcomes observed in the ex vivo analyses, ensuring the clinical relevance of the strip electrode performance in real physiological conditions. Hence, future in vivo studies in models such as goats will further validate the clinical utility by evaluating the safety and ensure long-term adoption.
Once the safety is evaluated in animal studies, efficacy would require prospective multicentric human trial with patients who are potential candidate for septoplasty. Here, patient selection also plays a major role in determining efficacy. Given that EMR can only make changes to cartilaginous area of the septum, patients with anterior cartilaginous DNS in the nasal valve area will benefit more from the procedure than other types of DNS. By evaluating the efficacy, post-operative care, recovery time, and cost benefit in comparison with traditional septoplasty, the proposed technology will move to clinical utility with widespread adoption.
The proposed EMR with novel strip electrode configuration is minimally invasive, thereby minimizing tissue damage, preserving the extracellular matrix (ECM) and posing fewer complications such as swelling, pain, and scarring. These benefits are expected to enhance the functional and aesthetic outcomes, contributing to a smoother recovery and higher patient satisfaction. Similarly, other minimally invasive techniques such as high-intensity focused ultrasound (HIFU) and balloon sinuplasty, have seen successful clinical adoption as replacements for traditional surgeries in their respective fields. Both HIFU and balloon sinuplasty have advantages such as reduced tissue trauma, shorter recovery times, and fewer complications, all driving their widespread clinical adoption. These examples showcase how innovative, minimally invasive techniques can be rapidly adopted over traditional surgical methods in clinical settings due to their high success rates and better patient outcomes.50
5. Conclusion
This study evaluated the potential of using a novel strip electrode configuration for reshaping nasal septal cartilage using EMR in comparison to the flat electrode configuration, which has been reported to cause tissue damage. The 0.5 mm 5-strip electrode configuration achieved efficient shape retention with minimal damage to both the mucosa and cartilage. The novel strip configuration required a lower current dosimetry of 20 mA compared to the flat electrode requirement of 35 mA, thereby minimizing the risk of tissue injury. Moreover, the strip electrodes showed conserved biomolecular integrity, higher mechanical stability and unaltered tissue architecture. The results support the feasibility of strip electrodes for clinical applications in DNS treatment, offering a promising alternative to traditional methods. Future in vivo clinical studies should focus on designing the treatment component for clinical usage and validating long-term outcomes to fully realize the clinical potential of this technology.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There is no conflict of interests to be declared.Acknowledgements
Support from the Startup India Seed Fund Scheme (SISFS), Government of India, (#18208) is gratefully acknowledged. Support from the Science and Engineering Research Board (SERB), Government of India, (SRG/2023/001183) is gratefully acknowledged.References
- A. Rehman, S. Hamid, M. Ahmad and A. F. Rashid, A Prospective Study of Nasal Septal Deformities in Kashmiri Population Attending a Tertiary Care Hospital, Int. J. Otolaryngol., 2012, 01(03), 77–84, DOI:10.4236/ijohns.2012.13016.
- D. G. Roblin and R. Eccles, What, if any, is the value of septal surgery?, Clin. Otolaryngol. Allied Sci., 2002, 27(2), 77–80, DOI:10.1046/j.1365-2273.2002.00531.x.
- A. Bhattacharjee, S. Uddin and P. Purkaystha, Deviated nasal septum in the newborn-A 1-year study, Indian J. Otolaryngol. Head Neck Surg., 2005, 57(4), 304–308, DOI:10.1007/BF02907694.
- M. M. H. T. Van Egmond, M. M. Rovers, G. Hannink, C. T. M. Hendriks and N. Van Heerbeek, Septoplasty with or without concurrent turbinate surgery versus non-surgical management for nasal obstruction in adults with a deviated septum: a pragmatic, randomised controlled trial, Lancet, 2019, 394(10195), 314–321, DOI:10.1016/S0140-6736(19)30354-X.
- S. P. Most and S. F. Rudy, Septoplasty, Facial Plast. Surg. Clin. North Am., 2017, 25(2), 161–169, DOI:10.1016/j.fsc.2016.12.002.
- J. Dąbrowska-Bień, P. H. Skarżyński, I. Gwizdalska, K. Łazęcka and H. Skarżyński, Complications in septoplasty based on a large group of 5639 patients, Eur. Arch. Oto-Rhino-Laryngol., 2018, 275(7), 1789–1794, DOI:10.1007/s00405-018-4990-8.
- Y. B. Chhatbar, S. Sharma, P. M. Patel, D. D. Bavarva and V. B. Patel, Complications and Management of Endoscopic Septoplasty at a Tertiary Care Center in India, Int. J. Sci. Res., 2020, 1, 82–84, DOI:10.36106/ijsr/6417928 , Published online.
- C. H. Shin and Y. J. Jang, Factors Affecting the Complication Rate of Septoplasty: Analysis of 1506 Consecutive Cases of Single Surgeon, Facial Plast. Surg., 2023, 39(04), 387–392, DOI:10.1055/a-1990-2818.
- F. Sommer and T. K. Hoffmann, Septoplasty—a surgical or political challenge?, Lancet, 2019, 394(10195), 276–278, DOI:10.1016/S0140-6736(19)31241-3.
- M. W. Keefe, A. Rasouli and S. A. Telenkov, et al., Radiofrequency Cartilage Reshaping: Efficacy, Biophysical Measurements, and Tissue Viability, Arch. Facial Plast. Surg., 2003, 5(1), 46–52, DOI:10.1001/archfaci.5.1.46.
- H. K. Kim, B. J. Wong, H. P. Benton, L. H. L. Liaw, J. S. Nelson and T. E. Milner, in Histology of porcine nasal cartilage grafts following Nd:YAG (1320 nm) laser-mediated reshaping: effects of sequential irradiation, ed. D. D. Duncan, S. L. Jacques and P. C. Johnson, 2001, p. 240 DOI:10.1117/12.434707.
- B. M. Hunter, J. Kallick and J. Kissel, et al., Controlled-Potential Electromechanical Reshaping of Cartilage, Angew. Chem., Int. Ed., 2016, 55(18), 5497–5500, DOI:10.1002/anie.201600856.
- S. J. Hong, M. Lee, C. J. Oh and S. Kim, Monitoring of Biological Changes in Electromechanical Reshaping of Cartilage Using Imaging Modalities, BioMed Res. Int., 2016, 2016, 1–7, DOI:10.1155/2016/7089017.
- A. Lim, D. E. Protsenko and B. J. F. Wong, Changes in the Tangent Modulus of Rabbit Septal and Auricular Cartilage Following Electromechanical Reshaping, J. Biomech. Eng., 2011, 133(9), 094502, DOI:10.1115/1.4004916.
- K. H. K. Ho, D. E. Protsenko, R. Wright and B. J. F. Wong in Effect of electrode composition on electromechanical cartilage reshaping, ed. L. S. Bass, N. Kollias, R. S. Malek, et al., 2003, p. 300 DOI:10.1117/12.485067.
- S. Hussain, C. T. Manuel, D. E. Protsenko and B. J. F. Wong, Electromechanical reshaping of ex vivo porcine trachea, Laryngoscope, 2015, 125(7), 1628–1632, DOI:10.1002/lary.25189.
- D. E. Protsenko, K. Ho and B. J. F. Wong, Stress Relaxation in Porcine Septal Cartilage During Electromechanical Reshaping: Mechanical and Electrical Responses, Ann. Biomed. Eng., 2006, 34(3), 455–464, DOI:10.1007/s10439-005-9051-y.
- K. Badran, C. Manuel, C. Waki, D. Protsenko and B. J. F. Wong, Ex Vivo Electromechanical Reshaping of Costal Cartilage in the New Zealand White Rabbit Model, Laryngoscope, 2013, 123(5), 1143–1148, DOI:10.1002/lary.23730.
- A. Y. Y. Yau, C. Manuel, S. F. Hussain, D. E. Protsenko and B. J. F. Wong, In Vivo Needle-Based Electromechanical Reshaping of Pinnae: New Zealand White Rabbit Model, JAMA Facial Plast. Surg., 2014, 16(4), 245–252, DOI:10.1001/jamafacial.2014.85.
- E. C. Wu, A. Khan, D. E. Protsenko, et al., in Electromechanical reshaping of rabbit septal cartilage: a six needle electrode geometric configuration, ed. N. Kollias, B. Choi, H. Zeng, et al., 2009, p. 716128 DOI:10.1117/12.820701.
- A Device for Reshaping a Cartilage.
- T. Hanawa, Titanium–Tissue Interface Reaction and Its Control With Surface Treatment, Front. Bioeng. Biotechnol., 2019, 7, 170, DOI:10.3389/fbioe.2019.00170.
- F. Apaydin, M. Saghir, R. F. F. P. Garcia, M. Daoud and A. Jaber, Lamb Head as a Training Model for Septoplasty and Rhinoplasty, Facial Plast. Surg., 2022, 38(05), 525–529, DOI:10.1055/a-1731-0332.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis, Nat. Methods, 2012, 9(7), 671–675, DOI:10.1038/nmeth.2089.
- R. Farndale, D. Buttle and A. Barrett, Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue, Biochim. Biophys. Acta, Gen. Subj., 1986, 883(2), 173–177, DOI:10.1016/0304-4165(86)90306-5.
- D. E. Protsenko, K. Ho and B. J. F. Wong, Survival of Chondrocytes in Rabbit Septal Cartilage After Electromechanical Reshaping, Ann. Biomed. Eng., 2011, 39(1), 66–74, DOI:10.1007/s10439-010-0139-7.
- A. Lim, D. Protsenko and B. Wong, Methods for evaluating changes in cartilage stiffness following electromechanical reshaping, Head Neck Oncol., 2010, 2(S1), O18, DOI:10.1186/1758-3284-2-S1-O18.
- E. K. Brunton, R. Rajan and A. J. Lowery, Optimising electrode surface area to minimize power consumption in a cortical penetrating prosthesis, In: 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER), IEEE, 2013, pp. 1477–1480 DOI:10.1109/NER.2013.6696224.
- D. E. Protsenko, K. Ho and B. J. F. Wong, in Monitoring of electrical peoperties during cartilage reshaping, ed. L. S. Bass, N. Kollias, R. S. Malek, et al., 2003, p. 307 DOI:10.1117/12.485076.
- K. K. Ho, S. H. D. Valdes, D. E. Protsenko, G. Aguilar and B. J. F. Wong, Electromechanical reshaping of septal cartilage, Laryngoscope, 2003, 113(11), 1916–1921, DOI:10.1097/00005537-200311000-00011.
- D. A. Dean, T. Ramanathan, D. Machado and R. Sundararajan, Electrical impedance spectroscopy study of biological tissues, J. Electrost., 2008, 66(3–4), 165–177, DOI:10.1016/j.elstat.2007.11.005.
- L. Nimeskern, L. Utomo and I. Lehtoviita, et al., Tissue composition regulates distinct viscoelastic responses in auricular and articular cartilage, J. Biomech., 2016, 49(3), 344–352, DOI:10.1016/j.jbiomech.2015.12.032.
- J. J. Stubendorff, E. Lammentausta, A. Struglics, L. Lindberg, D. Heinegård and L. E. Dahlberg, Is cartilage sGAG content related to early changes in cartilage disease? Implications for interpretation of dGEMRIC, Osteoarthritis Cartilage, 2012, 20(5), 396–404, DOI:10.1016/j.joca.2012.01.015.
- ed. Freeman M. A. R., Adult Articular Cartilage, Pitman Medical, 2nd edn, 1979 Search PubMed.
- A. K. Williamson and K. Masuda, Thonar EJMA, Sah RL. Growth of Immature Articular Cartilage in Vitro: Correlated Variation in Tensile Biomechanical and Collagen Network Properties, Tissue Eng., 2003, 9(4), 625–634, DOI:10.1089/107632703768247322.
- P. Baddam, F. Bayona-Rodriguez, S. M. Campbell, H. El-Hakim and D. Graf, Properties of the Nasal Cartilage, from Development to Adulthood: A Scoping Review, Cartilage, 2022, 13(1), 19476035221087696, DOI:10.1177/19476035221087696.
- M. Kyriakidou, A. F. Mavrogenis, S. Kyriazis, A. Markouizou, T. Theophanides and J. Anastassopoulou, An FT-IR Spectral Analysis of the Effects of γ-Radiation on Normal and Cancerous Cartilage, In vivo, 2016, 30(5), 599–604 CAS.
- L. Rieppo, S. Saarakkala, T. Närhi, H. J. Helminen, J. S. Jurvelin and J. Rieppo, Application of second derivative spectroscopy for increasing molecular specificity of Fourier transform infrared spectroscopic imaging of articular cartilage, Osteoarthritis Cartilage, 2012, 20(5), 451–459, DOI:10.1016/j.joca.2012.01.010.
- C. C. Huang, Tuning gelatin–alginate bioink properties by introducing new decellularized elastic cartilage scaffolds for bioinspired composite membranes in orthopedics, Polym. Bull., 2023, 80(3), 3279–3291, DOI:10.1007/s00289-022-04211-4.
- K. W. Badran, C. T. Manuel and A. C. Loy, et al., Long-term in vivo electromechanical reshaping for auricular reconstruction in the New Zealand white rabbit model, Laryngoscope, 2015, 125(9), 2058–2066, DOI:10.1002/lary.25237.
- C. T. Manuel, T. Tjoa, T. Nguyen, E. Su and B. J. F. Wong, Optimal Electromechanical Reshaping of the Auricular Ear and Long-term Outcomes in an In Vivo Rabbit Model, JAMA Facial Plast. Surg., 2016, 18(4), 277–284, DOI:10.1001/jamafacial.2016.0166.
- S. Oliaei, C. Manuel and B. Karam, et al., In Vivo Electromechanical Reshaping of Ear Cartilage in a Rabbit Model: A Minimally Invasive Approach for Otoplasty, JAMA Facial Plast. Surg., 2013, 15(1), 34, DOI:10.1001/2013.jamafacial.2.
- B. S. Gugelmin, L. S. Santos, H. D. A. Ponte and C. E. B. Marino, Electrochemical Stability and Bioactivity Evaluation of Ti6Al4V Surface Coated with Thin Oxide by EIS for Biomedical Applications, Mater. Res., 2015, 18(3), 602–607, DOI:10.1590/1516-1439.201514.
- The Engineering ToolBox 2005, Metals, Metallic Elements and Alloys – Thermal Conductivities, Metals, Metallic Elements and Alloys – Thermal Conductivities, 2005, https://www.engineeringtoolbox.com/thermal-conductivity-metals-d_858.html.
- J. S. Khaw, R. Xue, N. J. Cassidy and S. H. Cartmell, Electrical stimulation of titanium to promote stem cell orientation, elongation and osteogenesis, Acta Biomater., 2022, 139, 204–217, DOI:10.1016/j.actbio.2021.08.010.
- S. Sharma, R. Adalati and M. Sharma, et al., Single-step fabrication of di-titanium nitride thin-film flexible and biocompatible supercapacitor, Ceram. Int., 2022, 48(23), 34678–34687, DOI:10.1016/j.ceramint.2022.08.055.
- L. Verestiuc, M. C. Spataru and M. S. Baltatu, et al., New Ti–Mo–Si materials for bone prosthesis applications, J. Mech. Behav. Biomed. Mater., 2021, 113, 104198, DOI:10.1016/j.jmbbm.2020.104198.
- P. K. Nguyen, C. Hart, K. Hall, I. Holt and C. K. Kuo, Establishing in vivo and ex vivo chick embryo models to investigate fetal tendon healing, Sci. Rep., 2023, 13(1), 9600, DOI:10.1038/s41598-023-35408-w.
- İ. Çukurova, Seeking an innocent method for pediatric septoplasty: in vivo comparison of Cottle's method, radiofrequency and laser in rabbits, Turk. J. Ear Nose Throat, 2012, 22(6), 324–331, DOI:10.5606/kbbihtisas.2012.062.
- H. Levine and D. Rabago, Balloon Sinuplasty: A Minimally Invasive Option for Patients with Chronic Rhinosinusitis, Postgrad. Med., 2011, 123(2), 112–118, DOI:10.3810/pgm.2011.03.2269.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01306a |
| ‡ Equal contributions. |
| This journal is © The Royal Society of Chemistry 2025 |