Open Access Article
Open Access ArticleExploration of functional group effects on D2/H2 separation selectivity within the UiO-66 framework†
Xiufang
Li
a,
Yanxi
Tan
ab,
Zhanfeng
Ju
 b,
Wenjing
Wang
b,
Wenjing
Wang
 *ab and
Daqiang
Yuan
*ab and
Daqiang
Yuan
 *ab
*ab
aUniversity of the Chinese Academy of Sciences, Beijing 100049, China
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: wjwang@fjirsm.ac.cn; ydq@fjirsm.ac.cn
First published on 3rd December 2024
Abstract
The efficient separation of deuterium from hydrogen remains a significant challenge due to the limitations of conventional techniques, such as cryogenic distillation and the Girdler-sulfide process combined with electrolysis, which are char-acterized by substantial energy demands and relatively low separation coefficients. In contrast, the quantum sieving effect, based on porous materials, offers a promising approach to overcoming these challenges. This study presents a novel application of strong adsorption sites (μ3-OH group) within the nanoporous metal–organic framework of UiO-66 for hydrogen isotope separation. By incorporating diverse organic functional groups into UiO-66, we successfully synthesized four derivative materials: UiO-66–NH2, UiO-66–CH3, UiO-66–NO2, and UiO-66–Ph. Experimental data reveal that the introduction of these functional groups modulated the material's pore size and channel polarity, significantly impacting its adsorption and separation performance for hydrogen isotopes. Notably, UiO-66–NH2, with the smallest pore size and highest channel polarity, exhibited superior hydrogen isotope adsorption capacity and selectivity, highlighting its potential as an effective adsorbent for isotope separation.
Introduction
Deuterium (2H, D), a stable isotope of hydrogen (1H, H), has been widely utilized in modern industry, scientific research, and as a fuel for nuclear fusion.1–5 However, its extremely low natural abundance (0.0156%) and near-identical physicochemical properties with other hydrogen isotopes present significant challenges for enrichment. To date, only a few large-scale techniques have been employed for the industrial purification of deuterium. These include electrolysis of heavy water extracted via the Girdler-sulfide process and cryogenic distillation at 24 K. Both methods are known to be highly time-consuming and energy-intensive, with relatively low separation efficiencies. This necessitates the exploration of alternative approaches that can meet the requirements for sustainable deuterium production.6One of the most promising alternatives for D2/H2 separation is quantum sieving (QS) based on nanoporous materials. Kinetic quantum sieving (KQS) and chemical affinity quantum sieving (CAQS) effects are often used to achieve high selectivity in hydrogen isotope separation.7–20 KQS occurs when the difference between pore size and molecular size becomes comparable to the de Broglie wavelength under cryogenic conditions, leading to faster D2 diffusion through nanoporous material channels. However, the KQS effect demonstrates significant isotope selectivity only at cryogenic temperatures, limiting its practical application. To increase operating temperature, the CAQS effect has been developed. In this process, heavier isotopes are selectively adsorbed at strong binding sites due to differences in zero-point energy (ZPE).
The most extensively studied class of CAQS materials for D2/H2 separation is Metal–Organic Frameworks (MOFs).21–28 MOFs allow for flexible adjustment of pore size and open metal sites to achieve high D2/H2 selectivity. Some MOFs with a high density of metal open sites have successfully achieved efficient D2/H2 separation. However, most MOFs containing open metal sites suffer from limited moisture stability, as water readily attacks the metal active sites, leading to material degradation.
Therefore, effectively utilizing the CAQS effect to enhance D2/H2 separation performance while maintaining MOF structural stability is a critical issue that needs addressing. UiO-66, a well-known zirconium(IV)-containing MOF, has attracted considerable attention due to its exceptional stability, excellent adsorption properties, cost-effective regenerability, and good functional-group tolerance during synthesis. Despite the absence of open metal sites in UiO-66, the μ3-OH group of the Zr6O4(OH)4 inorganic cornerstones can serve as strong interaction sites, facilitating intense interactions between the material and hydrogen or deuterium.29
Current research on CAQS primarily focuses on open metal sites, while exploration of interactions between hydrogen isotope gases and non-metal active centers remains limited. This study reports the synthesis of UiO-66 and four of its derivatives (UiO-66–NH2, UiO-66–NO2, UiO-66–CH3, UiO-66–Ph), along with a detailed analysis of their D2/H2 separation capabilities (Scheme 1). Our findings indicate that the incorporation of functional groups into UiO-66 leads to improved separation performance.
Results and discussion
The synthesis of UiO-66 and its derivatives was conducted following a modified procedure based on previously published literature.30 As shown in Fig. 1a, the powder X-ray diffraction (PXRD) patterns of the synthesized materials were compared with simulated PXRD patterns, revealing good phase purity and functional-group tolerance for UiO-66 and its derivatives. Infrared spectroscopic analysis of the derivatives confirmed the successful incorporation of the intended functional groups, as evidenced by characteristic peaks (Fig. S1†). | ||
| Fig. 1 (a) Simulated and observed PXRD patterns of UiO-66 and its derivatives; (b) N2 isotherms of UiO-66 and its derivatives at 77 K. | ||
The porosity of UiO-66 and its derivatives was assessed using nitrogen adsorption isotherms at 77 K. Both UiO-66 and its derivatives displayed typical type I isotherms, indicating their microporous structures (Fig. 1b). The nitrogen adsorption capacities were measured as follows: 328 cm3 g−1 for UiO-66, 291 cm3 g−1 for UiO-66–NO2, 295 cm3 g−1 for UiO-66–NH2, 262 cm3 g−1 for UiO-66–CH3, and 252 cm3 g−1 for UiO-66–Ph. The corresponding specific surface areas determined by the Brunauer–Emmett–Teller (BET) method were found to be 1250 m2 g−1 for UiO-66, 985 m2 g−1 for UiO-66–NO2, 1036 m2 g−1 for UiO-66–NH2, 960 m2 g−1 for UiO-66–CH3, and 902 m2 g−1 for UiO-66–Ph. These data indicate that UiO-66 has the highest BET surface area, with the addition of different functional groups resulting in varying degrees of reduction in specific surface area.
The pore size distribution (PSD) was further analyzed using density functional theory (DFT) calculations. The results demonstrated that the derivatives (UiO-66–NO2, UiO-66–NH2, and UiO-66–CH3) possess smaller pore sizes than pristine UiO-66 (Fig. S2†). Contrary to expectations based on group size, UiO-66–Ph would be expected to have a smaller pore size than the other derivatives. However, the opposite result is observed. This discrepancy could be attributed to the orientation of the benzene rings and the presence of defects within the material, which together permit the formation of larger pore sizes.
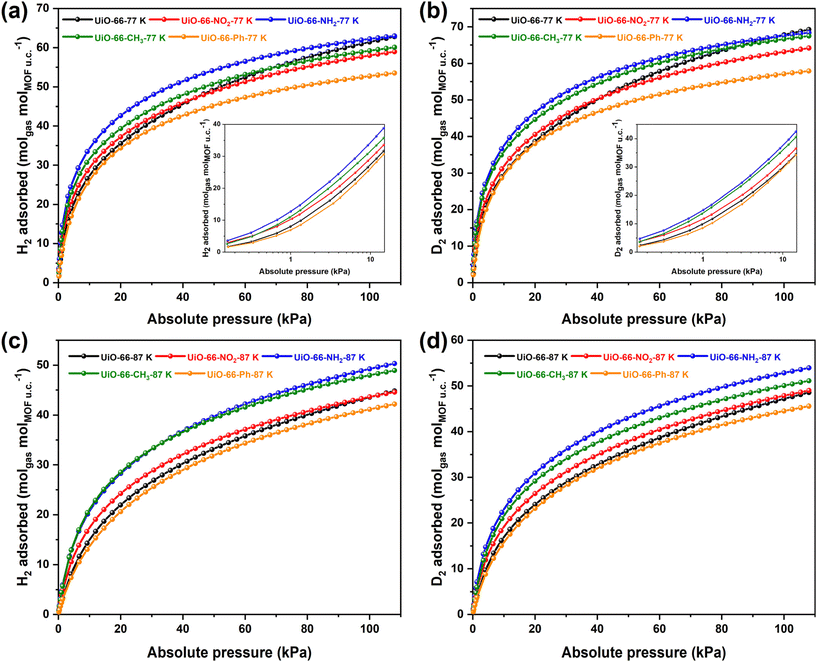
The high BET values and microporous structures of UiO-66 and its derivatives motivate our investigation into their adsorption capacities for hydrogen isotopes. The initial stage of the study involved analyzing H2 and D2 gas adsorption data at temperatures of 77 K and 87 K (Fig. 2 and Table S1†). Due to the change in mass induced by the introduction of functional groups, the amount of adsorbed gas per unit cell was selectively used as a measure of the material's adsorption performance.
At 77 K and 110 kPa, the D2 adsorption capacities of UiO-66–NH2 and UiO-66–CH3 were nearly equivalent to that of UiO-66, with UiO-66–NO2 showing a slight decrease and UiO-66–Ph exhibiting the most substantial reduction. For H2 adsorption under identical conditions, the H2 adsorption capacity of UiO-66–NH2 was comparable to that of UiO-66, while both UiO-66–CH3 and UiO-66–NO2 experienced minor decreases, with UiO-66–Ph demonstrating the most significant decline. Notably, at low pressures, the H2 and D2 gas adsorption capacities of UiO-66–NH2, UiO-66–NO2, and UiO-66–CH3 exceeded those of the pristine material UiO-66, indicating an enhanced interaction with hydrogen isotope gases despite the decrease in BET values.
A significant shift in isotope gas adsorption behavior was observed at 87 K. For D2 adsorption, UiO-66–NH2 and UiO-66–CH3 exhibited higher capacities across the entire pressure range compared to UiO-66. UiO-66–NO2 showed increased adsorption in the low-pressure region, with its capacity at 110 kPa nearly matching that of UiO-66, while UiO-66–Ph maintained a lower capacity throughout. The H2 adsorption behavior at 87 K followed a similar pattern to D2. As the temperature rose to 87 K, the decrease in H2 and D2 adsorption performance for UiO-66–NH2 and UiO-66–CH3 was less than that of UiO-66, suggesting that organic functional groups effectively enhanced the material's affinity for hydrogen isotopes. The reduced adsorption performance of UiO-66–Ph can be primarily attributed to its lower specific surface area and microporous volume (Table S1†).
Additionally, D2 adsorption capacities at both 77 K and 87 K were higher than those of H2, pointing to quantum effects. The isotherms for both H2 and D2 adsorption completely overlapped, indicating no diffusion barrier for hydrogen isotopes within the pores. The primary cause of the differential adsorption between H2 and D2 is attributed to strong adsorption sites, predominantly driven by the interaction between the hydrogen isotope gases and the μ3-OH group of the Zr6O4(OH)4 inorganic cornerstones. The incorporation of organic functional groups (–NH2, –CH3, –NO2) has effectively modulated pore size and polarity, further enhancing the material's interaction with isotope gases.
To gain further insight into the affinity of UiO-66 and its derivatives for hydrogen isotopes, the isosteric heats of adsorption (Qst) were calculated (Fig. S3–S14†). At zero loading, the Qst values for D2 in UiO-66–NH2 (9.3 kJ mol−1), UiO-66–NO2 (8.9 kJ mol−1), and UiO-66–CH3 (9.0 kJ mol−1) were higher than those of pristine UiO-66 (8.7 kJ mol−1). Notably, the Qst value for D2 in UiO-66–NH2 exceeds that of some porous materials reported in the literature, such as USTC-700 (4.0 kJ mol−1),31FJI-Y9 (6.2 kJ mol−1),32FJI-Y11 (7.9 kJ mol−1),33FIR-29 (6.1 kJ mol−1),32Co(pyz)[Pt(CN)4] (8.0 kJ mol−1),34 and Cu–BDC–NH2 (7.1 kJ mol−1).35 The high adsorption enthalpy of UiO-66–NH2 is predominantly attributed to its smaller pore size distribution and channel polarity. However, the Qst value for D2 in UiO-66–Ph (8.3 kJ mol−1) was lower than that in UiO-66 due to its low pore volume and reduced channel polarity. These findings are similarly applicable to the Qst values of H2. Compared with UiO-66 and other derivatives, a larger ΔQst (0.6 kJ mol−1) between D2 and H2 in UiO-66–NH2 suggests that this material may possess good D2/H2 separation properties.
To investigate the practical separation performance of UiO-66–NH2, breakthrough studies were conducted on D2/H2/Ne (3-3-94, v/v) ternary mixtures at 77 K (Fig. S15–S18†). The results showed that H2 broke through the adsorption bed first, followed by a time lag before D2 slowly eluted and reached equilibrium. This indicates that UiO-66–NH2 preferentially adsorbs D2 over H2. Additionally, a roll-up behavior for H2 was observed, likely due to the partial replacement of adsorbed H2 on the adsorption sites by more strongly adsorbing D2 in the gas mixture (Fig. 3a).
 | ||
| Fig. 3 (a) The dynamic breakthrough curves of UiO-66–NH2; (b) comparison of H2 uptake and separation factor for UiO-66 and its derivatives. | ||
Furthermore, the separation performance of UiO-66 and other derivatives has also been characterized. Breakthrough experiments suggest that materials modified with organic functional groups generally show improved separation performance. However, while UiO-66–CH3, UiO-66–NO2, and UiO-66–Ph exhibited increased separation coefficients compared to UiO-66, they experienced a decrease in H2 adsorption capacity to varying degrees. In contrast, UiO-66–NH2 maintained high D2 adsorption capacity while also enhancing selectivity. For an equimolar mixture of D2/H2, the calculated separation factor was 1.38 (Fig. 3b, Table S2†). For practical industrial application, it is crucial that the ideal adsorbent have good recycling performance. Multiple D2/H2 mixed-gas dynamic breakthrough experiments were performed under consistent operating conditions. The separation performance of D2/H2 did not significantly change within three continuous cycles, indicating that UiO-66–NH2 is robust and a promising candidate for D2/H2 separation (Fig. S19†).
Conclusions
This study delves into the influence of incorporating various organic functional groups into the UiO-66 framework on the adsorption–separation efficiency for hydrogen isotopes. The findings substantiate that by selectively modifying the pore size and channel polarity through the strategic introduction of organic functional groups, a substantial enhancement in the material's affinity for hydrogen isotopes can be achieved, thereby improving its overall separation performance. Notably, UiO-66–NH2 emerges as an outstanding candidate due to its high capacity for adsorbing hydrogen isotopes and superior selectivity. This research underscores that, aside from the presence of metal open sites, precise adjustment of pore size and channel polarity is a viable strategy to elevate the separation performance of hydrogen isotopes in CAQS studies. UiO-66–NH2 demonstrates exceptional potential for isotope separation applications, highlighting the importance of tailored material modifications in enhancing adsorption–separation efficiency.Experimental
Materials and general methods
Infrared (IR) spectra were collected using KBr pellets over a range of 3000–400 cm−1, recorded on a Nicolet Magna 750 FT-IR spectrometer. Powder X-ray diffraction (PXRD) patterns were obtained using Cu Kα radiation (λ = 1.5406 Å) on a Rigaku Mini 600 X-ray diffractometer. Gas adsorption–desorption measurements were conducted with an automatic volumetric adsorption apparatus (Micromeritics, ASAP2020 PLUS).Synthesis of UiO-66 and its derivatives
In a typical synthesis, terephthalic acid (9.3 mg, 0.08 mmol) and ZrCl4 (13.2 mg, 0.04 mmol) were ultrasonically dissolved in a 10 mL solution of N,N-dimethylformamide (DMF), followed by the addition of 2.3 mL of acetic acid. The resulting mixture was then transferred into a sealed vial and heated at 120 °C for 24 hours. After gradually cooling to room temperature, the white powders were collected. Yield: 7.5 mg, 80%. Synthesis of other derivatives followed a similar procedure, with substitutions on the terephthalic acid to introduce the corresponding functional groups on the organic ligand.Breakthrough measurements
The breakthrough experiments were carried out using a dynamic gas breakthrough equipment (BSD Instrument, BSD-MAB). Activated samples were carefully transferred into a 2 mL quartz column, which was sealed at both ends with quartz wool. The column was subsequently heated to 100 °C for 12 hours, with a flow of Ne gas at 8 mL min−1 passing through it. Prior to the measurement, the column was precooled by immersed in a Dewar flask containing liquid nitrogen for approximately 20 minutes. After this preparation, a gas mixture of H2/D2/Ne (3/3/94, vol%) was introduced into the column at a flow rate of 15 mL min−1. The inlet gas flow was precisely managed by a mass flow controller (MFC), and the outlet gas composition was continuously monitored using a mass spectrometer. To regenerate the sample, it was purged with Ne gas at 10 mL min−1 at a temperature of 100 °C for 120 minutes.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Nature Science Foundation of China (22275191, 22275186); the Self-deployment Project Research Program of Haixi Institutes, Chinese Academy of Sciences (CXZX-2022-GH01, CXZX-2022-JQ11); the Nature Science Foundation of Fujian Province (No. 2022I0037).References
- M. Glugla, R. Lässer, L. Dörr, D. K. Murdoch, R. Haange and H. Yoshida, The inner deuterium/tritium fuel cycle of ITER, Fusion Eng. Des., 2003, 69, 39–43 CrossRef CAS.
- R. Smith, D. A. J. Whittaker, B. Butler, A. Hollingsworth, R. E. Lawless, X. Lefebvre, S. A. Medley, A. I. Parracho and B. Wakeling, Hydrogen isotope separation for fusion power applications, J. Alloys Compd., 2015, 645, S51–S55 CrossRef CAS.
- I. V. Stiopkin, C. Weeraman, P. A. Pieniazek, F. Y. Shalhout, J. L. Skinner and A. V. Benderskii, Hydrogen bonding at the water surface revealed by isotopic dilution spectroscopy, Nature, 2011, 474, 192–195 CrossRef CAS PubMed.
- G. Zaccai, How Soft Is a Protein? A Protein Dynamics Force Constant Measured by Neutron Scattering, Science, 2000, 288, 1604–1607 CrossRef CAS PubMed.
- M. V. Ananyev, A. S. Farlenkov and E. K. Kurumchin, Isotopic exchange between hydrogen from the gas phase and proton-conducting oxides: Theory and experiment, Int. J. Hydrogen Energy, 2018, 43, 13373–13382 CrossRef CAS.
- L. Matei, C. Postolache, G. Bubueanu and C. Podina, Synthesis of Labeled Compounds Using Recovered Tritium from Expired Beta Light Sources, Fusion Sci. Technol., 2017, 54, 643–646 CrossRef.
- J. J. M. Beenakker, V. D. Borman and S. Y. Krylov, Molecular transport in subnanometer pores: zero-point energy, reduced dimensionality and quantum sieving, Chem. Phys. Lett., 1995, 232, 379–382 CrossRef CAS.
- S. A. FitzGerald, C. J. Pierce, J. L. Rowsell, E. D. Bloch and J. A. Mason, Highly selective quantum sieving of D2 from H2 by a metal-organic framework as determined by gas manometry and infrared spectroscopy, J. Am. Chem. Soc., 2013, 135, 9458–9464 CrossRef CAS PubMed.
- H. Oh and M. Hirscher, Quantum Sieving for Separation of Hydrogen Isotopes Using MOFs, Eur. J. Inorg. Chem., 2016, 2016, 4278–4289 CrossRef CAS.
- I. Krkljus, T. Steriotis, G. Charalambopoulou, A. Gotzias and M. Hirscher, H2/D2 adsorption and desorption studies on carbon molecular sieves with different pore structures, Carbon, 2013, 57, 239–247 CrossRef CAS.
- H. Oh, K. S. Park, S. B. Kalidindi, R. A. Fischer and M. Hirscher, Quantum cryo-sieving for hydrogen isotope separation in microporous frameworks: an experimental study on the correlation between effective quantum sieving and pore size, J. Mater. Chem. A, 2013, 1, 3244–3248 RSC.
- J. Y. Kim, L. Zhang, R. Balderas-Xicohténcatl, J. Park, M. Hirscher, H. R. Moon and H. Oh, Selective Hydrogen Isotope Separation via Breathing Transition in MIL-53(Al), J. Am. Chem. Soc., 2017, 139, 17743–17746 CrossRef CAS.
- D. He, L. Zhang, T. Liu, R. Clowes, M. A. Little, M. Liu, M. Hirscher and A. I. Cooper, Hydrogen Isotope Separation Using a Metal-Organic Cage Built from Macrocycles, Angew. Chem., Int. Ed., 2022, 61, e202202450 CrossRef CAS PubMed.
- H. Oh, S. B. Kalidindi, Y. Um, S. Bureekaew, R. Schmid, R. A. Fischer and M. Hirscher, A cryogenically flexible covalent organic framework for efficient hydrogen isotope separation by quantum sieving, Angew. Chem., Int. Ed., 2013, 52, 13219–13222 CrossRef CAS PubMed.
- M. Liu, L. Zhang, M. A. Little, V. Kapil, M. Ceriotti, S. Yang, L. Ding, D. L. Holden, R. Balderas-Xicohténcatl, D. He, R. Clowes, S. Y. Chong, G. Schütz, L. Chen, M. Hirscher and A. I. Cooper, Barely porous organic cages for hydrogen isotope separation, Science, 2019, 366, 613–620 CrossRef CAS.
- R. Xiong, J. Chen, L. Zhang, P. Li, X. Yan, Y. Song, W. Luo, T. Tang, G. Sang and M. Hirscher, Hydrogen isotopes separation in Ag(I) exchanged ZSM-5 zeolite through strong chemical affinity quantum sieving, Microporous Mesoporous Mater., 2021, 313, 110820 CrossRef CAS.
- J. Y. Kim, H. Oh and H. R. Moon, Hydrogen Isotope Separation in Confined Nanospaces: Carbons, Zeolites, Metal-Organic Frameworks, and Covalent Organic Frameworks, Adv. Mater., 2019, 31, e1805293 CrossRef.
- L. Bondorf, J. L. Fiorio, V. Bon, L. Zhang, M. Maliuta, S. Ehrling, I. Senkovska, J. D. Evans, J.-O. Joswig, S. Kaskel, T. Heine and M. Hirscher, Isotope-selective pore opening in a flexible metal-organic framework, Sci. Adv., 2022, 8, eabn7035 CrossRef CAS PubMed.
- R. Muhammad, S. Jee, M. Jung, J. Park, S. G. Kang, K. M. Choi and H. Oh, Exploiting the Specific Isotope-Selective Adsorption of Metal-Organic Framework for Hydrogen Isotope Separation, J. Am. Chem. Soc., 2021, 143, 8232–8236 CrossRef CAS PubMed.
- J. Teufel, H. Oh, M. Hirscher, M. Wahiduzzaman, L. Zhechkov, A. Kuc, T. Heine, D. Denysenko and D. Volkmer, MFU-4 - a metal-organic framework for highly effective H2/D2 separation, Adv. Mater., 2013, 25, 635–639 CrossRef CAS PubMed.
- J. Y. Kim, R. Balderas-Xicohtencatl, L. Zhang, S. G. Kang, M. Hirscher, H. Oh and H. R. Moon, Exploiting Diffusion Barrier and Chemical Affinity of Metal-Organic Frameworks for Efficient Hydrogen Isotope Separation, J. Am. Chem. Soc., 2017, 139, 15135–15141 CrossRef CAS.
- M. Jung, J. Park, R. Muhammad, J. Y. Kim, V. Grzimek, M. Russina, H. R. Moon, J. T. Park and H. Oh, Elucidation of Diffusivity of Hydrogen Isotopes in Flexible MOFs by Quasi-Elastic Neutron Scattering, Adv. Mater., 2021, 33, e2007412 CrossRef PubMed.
- L. Zhang, S. Jee, J. Park, M. Jung, D. Wallacher, A. Franz, W. Lee, M. Yoon, K. Choi, M. Hirscher and H. Oh, Exploiting Dynamic Opening of Apertures in a Partially Fluorinated MOF for Enhancing H2 Desorption Temperature and Isotope Separation, J. Am. Chem. Soc., 2019, 141, 19850–19858 CrossRef CAS.
- I. Savchenko, A. Mavrandonakis, T. Heine, H. Oh, J. Teufel and M. Hirscher, Hydrogen isotope separation in metal-organic frameworks: Kinetic or chemical affinity quantum-sieving?, Microporous Mesoporous Mater., 2015, 216, 133–137 CrossRef CAS.
- H. Oh, I. Savchenko, A. Mavrandonakis, T. Heine and M. Hirscher, Highly Effective Hydrogen Isotope Separation in Nanoporous Metal–Organic Frameworks with Open Metal Sites: Direct Measurement and Theoretical Analysis, ACS Nano, 2014, 8, 761–770 CrossRef CAS.
- X. Li, X. Wang, M. Li, J. Luo, Y. An, P. Li, J. Song, C. Chen, X. Feng and S. Wang, Highly selective adsorption of D2 from hydrogen isotopes mixture in a robust metal bistriazolate framework with open metal sites, Int. J. Hydrogen Energy, 2020, 45, 21547–21554 CrossRef CAS.
- I. Weinrauch, I. Savchenko, D. Denysenko, S. M. Souliou, H. H. Kim, M. Le Tacon, L. L. Daemen, Y. Cheng, A. Mavrandonakis, A. J. Ramirez-Cuesta, D. Volkmer, G. Schutz, M. Hirscher and T. Heine, Capture of heavy hydrogen isotopes in a metal-organic framework with active Cu(I) sites, Nat. Commun., 2017, 8, 14496 CrossRef CAS.
- I. Krkljus and M. Hirscher, Characterization of hydrogen/deuterium adsorption sites in nanoporous Cu–BTC by low-temperature thermal-desorption mass spectroscopy, Microporous Mesoporous Mater., 2011, 142, 725–729 CrossRef CAS.
- S. Chavan, J. G. Vitillo, D. Gianolio, O. Zavorotynska, B. Civalleri, S. Jakobsen, M. H. Nilsen, L. Valenzano, C. Lamberti, K. P. Lillerud and S. Bordiga, H2 storage in isostructural UiO-67 and UiO-66 MOFs, Phys. Chem. Chem. Phys., 2012, 14, 1614–1626 RSC.
- Y. Huang, W. Qin, Z. Li and Y. Li, Enhanced stability and CO2 affinity of a UiO-66 type metal-organic framework decorated with dimethyl groups, Dalton Trans., 2012, 41, 9283–9285 RSC.
- Q. Yan, J. Wang, L. Zhang, J. Liu, M. Wahiduzzaman, N. Yan, L. Yu, R. Dupuis, H. Wang, G. Maurin, M. Hirscher, P. Guo, S. Wang and J. Du, A squarate-pillared titanium oxide quantum sieve towards practical hydrogen isotope separation, Nat. Commun., 2023, 14, 4189 CrossRef CAS.
- F. J. Zhao, Y. X. Tan, W. Wang, Z. Ju and D. Yuan, Optimizing H2, D2, and C2H2 Sorption Properties by Tuning the Pore Apertures in Metal-Organic Frameworks, Inorg. Chem., 2018, 57, 13312–13317 CrossRef CAS.
- Y. Si, X. He, J. Jiang, Z. Duan, W. Wang and D. Yuan, Highly effective H2/D2 separation in a stable Cu-based metal-organic framework, Nano Res., 2019, 14, 518–525 CrossRef.
- J. Ha, M. Jung, J. Park, H. Oh and H. R. Moon, Thermodynamic Separation of Hydrogen Isotopes Using Hofmann-Type Metal-Organic Frameworks with High-Density Open Metal Sites, ACS Appl. Mater. Interfaces, 2022, 14, 30946–30951 CrossRef CAS.
- A. Dastbaz, J. Karimi-Sabet, Y. Amini and M. A. Moosavian, A comprehensive study on the kinetics and isotherms of D2/H2 adsorptive separation using pure and composite Cu-BDC-NH2 MOFs at 77 K, Int. J. Hydrogen Energy, 2024, 61, 893–900 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi02802c |
| This journal is © the Partner Organisations 2025 |