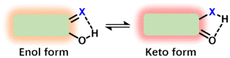Recent advances in fluorescent probes for fatty liver imaging by detecting lipid droplets
Long
He
ab,
Hang
Li
a,
Yao
Tang
b,
Tian-Bing
Ren
 *b and
Lin
Yuan
*b and
Lin
Yuan
 b
b
aKey Laboratory of Theoretical Organic Chemistry and Functional Molecule, Ministry of Education, School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan, 411201, P. R. China
bState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082, P. R. China. E-mail: rentianbing@hnu.edu.cn
First published on 4th September 2024
Abstract
Fatty liver, a major health problem worldwide, is closely associated with aberrant accumulation and alteration of energy storage organelles, lipid droplets (LDs), in the disease process. Fluorescent probes with excellent optical performance, high sensitivity/selectivity and real-time monitoring have emerged as an attractive tool for the detection of LDs used in the diagnosis of fatty liver. In this review, we summarize various probes based on different response mechanisms to image LDs in the fatty liver process using different excitation imaging modes and emission wavelengths, including the visible to the near-infrared, two/three-photon, and the second near-infrared region. The perspectives and barriers associated with the reported lipid droplet (LD) probes for future development are also discussed.
1. Introduction
Globally, fatty liver disease (FLD), including alcoholic and nonalcoholic FLD, has recently attracted extensive attention as the most common chronic liver disease affecting and threatening human life and health, and its prevalence is continuously increasing.1,2 In the early stages, FLD can be managed through appropriate dietary and physical activity modifications. However, in advanced stages, it can progress to steatohepatitis or cirrhosis and hepatocellular carcinoma in severe cases. In addition, FLD can increase the risk of other chronic diseases, such as atherosclerosis and type 2 diabetes.3,4 Hence, timely detection of FLD is of significant importance for early treatment.The primary pathological characteristic of FLD is excessive lipid accumulation in the liver, resulting in intracellular lipid droplet (LD) overload.4–7 Lipid droplets (LDs) are lipid-rich subcellular organelles in eukaryotic cells and comprise neutral lipids, primarily triacylglycerol and cholesterol esters, covered by a phospholipid monolayer.8 LDs play a crucial role in regulating energy storage and are closely correlated with lipid metabolism.9 LDs are also involved in numerous important physiological processes including membrane transport, cell activation and apoptosis, signal transduction and protein degradation.10 Recent studies have revealed that the highly dynamic characteristics of LDs correspond to their biological functions and that the variation in the number, size, and distribution of LDs can reflect the metabolic state of cells or tissues. LDs are highly associated with many common metabolic diseases, such as FLD.11 The dysregulation of LD metabolism in hepatocytes results in the intracellular accumulation of lipids, activation of pathogenic mechanisms, and the subsequent development of FLD.12 Therefore, tracking and monitoring the dynamic LD changes in living cells and tissues is of great value for investigating the physiology and pathology of FLD, as well as for effective clinical diagnosis.
In recent years, several analytical methods have been developed to track and view LDs in cells and in vivo.13 Among them, fluorescent probes are an important tool for the visualization and real-time imaging of LDs to assess FLD because of their high sensitivity, non-invasiveness, excellent selectivity, superior spatiotemporal resolution, and good biocompatibility (Fig. 1).14–17 Thus, numerous LD-specific fluorescent probes have been developed and successfully employed to visualize the distribution and dynamic behaviour of LDs in hepatocytes, facilitating the early diagnosis of FLD and enabling the elucidation of various pathological processes associated with FLD. In this review, we mainly summarize recent advances in organic fluorescent probes based on different excitation imaging modes and emission wavelengths for diagnosing FLD through LD detection. We present the different detection mechanisms of these probes and conclude with a summary of the achievements made in optimizing the properties of these LD probes, as well as an analysis of the present challenges and future development trends in this field.
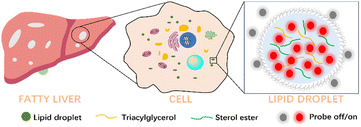 | ||
| Fig. 1 Schematic illustration of the architecture of LDs in the fatty liver process and LD probes for fatty liver imaging. | ||
2. Detection mechanisms for developing lipid droplet fluorescent probes
Owing to its advantages, fluorescence imaging technology has been increasingly used in pathological research. Consequently, various fluorescent probes have been devised for fluorescence imaging of LDs based on a plethora of dye skeletons, including coumarin, boron-dipyrromethene (BODIPY), triphenylamine-based, and hemicyanine.18,19 These probes can accurately identify changes in LD polarity and viscosity in fatty liver processes based on a variety of detection mechanisms, including intramolecular charge transfer (ICT), aggregation-induced emission (AIE), and excited-state intramolecular proton transfer (ESIPT), thus enabling accurate disease assessment.20,212.1. Intramolecular charge transfer mechanism
The fluorescence phenomenon of fluorophores involves the transfer of charge and energy.22 ICT is a well-known luminescence mechanism for fluorescent dyes. ICT dipolar molecules consist of an electron donating group (D) and an electron withdrawing group (A) conjugated to form a “push–pull” (D–A) system.23 Upon light excitation, this bipolar dye undergoes an intramolecular excited-state charge transfer from an electron donor to an acceptor, resulting in fluorescence. However, ICT-type fluorophores exhibit solvatochromism and their optical properties can be affected by the surrounding medium.24,25 The effects of solvatochromism originate from quenching excited states to form non-radiative decay caused by various factors, including twisted intramolecular charge transfer (TICT)26 and intermolecular hydrogen bonding.27,28 This feature increases the sensitivity of these dyes to the polarity of the microenvironment. Therefore, ICT-type molecules, including a related phenomenon TICT molecule, are widely used for LD polarity and viscosity sensing due to the tunability of the emission properties triggered by conformational changes in the excited state. In general, ICT/TICT-based probes exhibit low fluorescence and bathochromic-shifted emission in polar environments, while an increased fluorescence intensity and blue-shifted emission in non-polar lipid environments are observed (Fig. 2). Furthermore, the high viscosity in LDs would inhibit the nonradiative twisted of the TICT molecule, resulting in enhanced emission.18 In addition, the larger Stokes shift of the ICT/TICT probe can also minimize the impact of incident light to achieve a high imaging signal-to-background ratio for sensitive detection of LDs.2.2. Aggregation-induced emission mechanism
Conventional fluorophores for LD imaging are typically used at low concentrations due to the aggregation quenching (ACQ) effect in aqueous solutions, a common problem in the field. In contrast, AIE fluorogens,29 possess molecular rotators/vibrators and a twisted conformation. They are not emissive or weakly emissive in the dissolved state. In an aggregated state, they exhibit high fluorescence, which can be attributed to the restricted intramolecular motion, thus boosting radiative decay (Fig. 3).30 The intrinsic properties of AIE luminogens (AIEgens) confer a remarkable ability to tolerate high concentrations in solution, overcoming the inherent limitations of ACQ fluorogens.31 Most AIEgens have high lipophilicity, leading to their accumulation within LDs, forming highly luminescent nanoaggregates in a noncompatible environment. Therefore, most AIE-based LD fluorescent probes can specifically accumulate in LDs to generate bright fluorescence because the nonpolar and viscous environments of LDs restrict free intramolecular rotation.2.3. Excited-state intramolecular proton transfer mechanism
ESIPT, a unique luminescence mechanism, involves a distinctive fluorophore with proton donor (hydroxyl or amino groups) and acceptor (carbonyl oxygen or azo nitrogen) groups.32,33 Upon light excitation, the excited state molecule's proton transfers from the donor to the acceptor to form an intramolecular hydrogen bond, leading to significant spectral differences between the normal excited state and the photo-tautomer (Fig. 4).34 ESIPT-based probes enable LD imaging through proton transfer induced by changes in lipid polarity. Upon entering the LD, the probe exhibits a sensitive environmental response to observe a discernible change in spectra, allowing effective lipids identification for the detection of relevant diseases.3. Lipid droplet fluorescent probes for fatty liver imaging
Fluorescent probes are instrumental in diagnosing FLD through LD imaging, offering a non-invasive, real-time method for monitoring the progression of the condition. Benefit from these advantages of the fluorescent probe, the development of different types of probes for fatty liver imaging by lipid droplets detecting. Based on the excitation mode, these probes can be categorized as one-, two- or three-photon probes. Alternatively, in accordance with the emission wavelength, they can be classified into the ultraviolet to visible-light (UV-Vis, 400–650 nm) region, the first near-infrared window (NIR-I, 650–900 nm) region, and the second near-infrared window (NIR-II, 900–1700 nm) region.35 The findings presented in Table 1 summarize the most recent advancements in fluorescent probes for detecting LDs in the pathogenesis of FLD.| Probes | λ abs/λem (nm) | Excitation model/wavelength | Biomarker | clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P/log P/log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
Application range | Ref. |
|---|---|---|---|---|---|---|
| LD-TTP | 402/528 (THF) | One-photon/405 nm | Polarity | 6.698/(N/A) | Fatty liver tissues | 36 |
| LD-HWZ | 490/635 (THF) | One-photon/(N/A) | Polarity | (N/A)/0.78(O/W) | Fatty liver tissues | 13 |
| TPA-TCF4 | 535/635 (1,4-dioxane) | One-photon/561 nm | Polarity | 2.05(O/W) | Fatty liver mice | 37 |
| L3 | 470/610 (DMSO) | One-photon/442 nm | Polarity | N/A | HepG2 cell | 38 |
| LIP-Ser | 471/634 (1,4-dioxane) | One-photon/488 nm | Polarity | N/A | Fatty liver mice | 39 |
| TST | 484/644 (1,4-dioxane) | One-photon/(N/A) | Polarity | 7.635/(N/A) | Fatty liver tissues | 40 |
| PPTH | 450/633 (DMSO) | One-photon/488 nm | Polarity | N/A | Fatty liver tissues | 41 |
| DBC30 | 398/460 (n-hexane) | One-photon/430 nm | Polarity | N/A | Fatty liver tissues | 42 |
| ZP-1 | 426/506 (1,4-dioxane) | One-photon/(N/A) | Polarity | N/A | Fatty liver mice | 43 |
| L1 | 505/596 (THF) | One-photon/(N/A) | Polarity | 0.33 | Fatty liver tissues | 44 |
| B2 | 420/572 (THF) | One-photon/(N/A) | Polarity | N/A | Fatty liver tissues | 45 |
| LD-HW | 520/608 (1,4-dioxane) | One-photon/500 nm | Polarity | N/A | Fatty liver mice | 46 |
| LD-1 | 543/661 (1,4-dioxane) | One-photon/540 nm | Polarity/viscosity | N/A | Fatty liver mice | 47 |
| TSB | 346/597 (THF) | One-photon/(N/A) | Polarity | (N/A)/1.35 | Fatty liver tissues | 48 |
| ANI | 503/591 (THF) | One-photon/(N/A) | Polarity | N/A | Fatty liver tissues | 49 |
| LDs-DM | 450/543 (1,4-dioxane) | One-photon/488 nm | Polarity | N/A | Fatty liver tissues | 50 |
| LipidGreen2 | 456/534 (PBS) | One-photon/470 nm | N/A | N/A | Zebrafish | 51 |
| G1 | 450/565 (1,4-dioxane) | One-photon/(N/A) | Polarity | (N/A)/1.36 | Fatty liver organ | 52 |
| ISO-LD3 | 459/591 (1,4-dioxane) | One-photon/(N/A) | Polarity | N/A | Fatty liver tissues | 53 |
| Flp-13 | 414/568 (ethyl oleate) | One-photon/405 nm | Polarity | N/A | Fatty liver tissues | 54 |
| HOP-NMe | 355/530 (THF) | One-photon/405 nm | Polarity | N/A | Fatty liver organ | 55 |
| DPDO-C | 600/800 (DMSO) | One-photon/561 nm | Polarity | 12.475/(N/A) | Fatty liver tissues | 56 |
| SSR-LDs | 590/663 (THF) | One-photon/600 nm | Polarity | N/A | Fatty liver tissues | 57 |
| CCB | (N/A)/675 (glycerol) | One-photon/490 nm | Polarity | N/A | Fatty liver tissues | 58 |
| TITM | 469/667 (THF) | One-photon/488 nm | Polarity | (N/A)/1.47(O/W) | Fatty liver tissues | 59 |
| TNBD | 547(DMSO)/657 (DMSO) | One-photon/488 nm | Polarity | N/A | Fatty liver tissues | 60 |
| ZH-2 | (N/A)/685 (toluene) | One-photon/633 nm | Viscosity | N/A | Fatty liver organ | 61 |
| DCIQ | 565/675 (toluene) | One-photon/633 nm | Polarity | N/A | Fatty liver tissues | 62 |
| PV-1 | 680/705 (glycerol) | One-photon/660 nm | Viscosity | N/A | Fatty liver mice | 63 |
| MBDP-Py+ | MBDP-Py:562/613 (30% acetonitrile) | One-photon/540–580 nm | ONOO− | 6.04 (MBDP-Py)/(N/A) | Fatty liver organ | 64 |
| LD-Lyso | 330/440 (N/A) | One-photon/405 nm | pH | N/A | Fatty liver tissues | 65 |
| BDP-NIR-Py+ | BDP-NIR-Py:637/661 (50% acetonitrile) | One-photon/(N/A) | ONOO− | 7.21 (BDP-NIR-Py)/(N/A) | Fatty liver mice | 66 |
| TPAT-BSeNT | 865/975 (n-hexane) | One-photon/808 nm | N/A | N/A | Fatty liver mice | 67 |
| LD1 | 485/596 (1,4-dioxane) | Two-photon/800 nm | Polarity | N/A | Fatty liver tissues | 68 |
| LD2 | 531/697 (toluene) | Two-photon/880 nn | Polarity | N/A | Fatty liver tissues | 69 |
| lip-YB | 537/594 (1,4-dioxane) | Two-photon/950 nm | Polarity | N/A | Fatty liver tissues | 70 |
| ABCXF | 448/579 (THF) | Two-photon/850 nm | Polarity/Viscosity | N/A | Fatty liver tissues | 71 |
| 4 | 490/621 (toluene) | Two-photon/880 nm | Polarity | (N/A)/5.3 | Fatty liver tissues | 72 |
| N-Cy | 460/522 (acetone) | Two-photon/840 nm | N/A | N/A | Fatty liver tissues | 73 |
| NAP-CF3 | 420/510 (OA) | Three-photon/1300 nm | Polarity | 6.5/(N/A) | Fatty liver tissues | 74 |
| TPAPhCN | (N/A)/685 (THF) | Three-photon/1200 nm | N/A | N/A | Fatty liver tissues | 75 |
| DMPCN | 450/645 (N/A) | Three-photon/1300 nm | N/A | N/A | Fatty liver tissues | 76 |
3.1. Ultraviolet to the second near-infrared window lipid droplet fluorescent probes
Conventional small-molecule dyes with bright luminescence and tunable wavelength variations are highly regarded in many fields, including biochemistry and photochemistry.77 Based on different frameworks, several UV-Vis LD probes were first developed and have shown great promise in the diagnosis of fatty liver due to their excellent optical properties and easily adjustable structure.Triphenylamine, a classical fluorophore employed in constructing probes for imaging LDs, has been demonstrated to possess effective targeting capabilities due to the low polarity and lipophilicity of the triphenylamine unit.78 Generally, most triphenylamine-based probes exhibit a strong solvatochromism effect, which can be exploited for imaging LDs through the ICT mechanism. In 2021, Dong and colleagues designed a polarity-sensitive probe, LD-TTP, for imaging LDs,36 the probe consists of a triphenylamine donor unit and a pyridine acceptor group, forming a push–pull conformation (Fig. 5). LD-TTP exhibited a redshift of the maximum emission bands from 528 to 548 nm with an increase in solvent polarity, which is attributed to solvatochromism evident due to the larger excited-state geometry relaxation of the torsional configuration. The changes in the unique optical properties demonstrated that LD-TTP can allow qualitative detection of changes in the polarity of the microenvironment. Inspired by the polarity-sensitive characteristics, confocal imaging of LD-TTP was performed by staining HeLa and A549 cells. Following excitation with a 405 nm laser, the LDs showed a bright short-wavelength green fluorescence. Green fluorescence-specific staining of LDs was ascribed to the triphenylamine-based fluorophore in a locally excited state in a nonpolar environment. The phenomenon of stained LDs was validated using commercially available LD-specific Nile Red. Fatty liver, a prevalent metabolic disease, is frequently accompanied by dysfunctional lipid metabolism. Following incubation with LD-TTP, a significant fluorescence signal was observed in fatty liver tissue, whereas no fluorescence was observed in normal liver tissue. Using LD-TTP, visualizing LD-polarity changes to evaluate FLD has been successfully achieved.
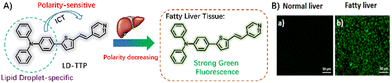 | ||
| Fig. 5 (A) The proposed response mechanism of the probe LD-TTP for imaging lipid droplets, (B) and it uses fluorescence imaging to differentiate between normal and fatty liver tissue. Reprinted with permission from ref. 36. Copyright 2021 American Chemical Society. | ||
A long-wavelength derivative, LD-HWZ,13 comprising a dicyanoisophorone acceptor unit, was constructed to enhance the imaging signal-to-noise ratio (SNR) by modifying the triphenylamine-based dye. In various solvents, LD-HWZ had a maximum absorption peak of approximately 500 nm. Furthermore, the probe exhibited remarkable fluorescence in the presence of lipophilic oleic acid due to the high lipophilicity of LD-HWZ. Using cell imaging measurements, LD-HWZ's distinct red fluorescence image in LDs overlapped with the green fluorescence of commercial BODIPY 493/503, indicating that LD-HWZ can target LDs and is active in low-polarity environments. Based on the specific labelling effect in the LDs of LD-HWZ, fatty liver tissues were imaged using the probe to image fatty liver tissues. Compared to normal liver tissues, strong red fluorescence was observed in fatty liver tissues, which was attributed to increased LDs in the non-alcoholic fatty liver process. These experiments demonstrated that LD-HWZ could be a beneficial tool for a more detailed fatty liver study through the detection of LD changes. Using a receptor substitution strategy, they successively designed probes TPA-TCF437 and L338 to label LD for FLD diagnosis (Fig. 6).
 | ||
| Fig. 6 Triphenylamine-based LD probes (LD-HWZ, TPA-TCF4, L3) and schematic diagram of the imaging of LDs in the fatty liver model. Reprinted with permission from ref. 13. Copyright 2023 The Royal Society of Chemistry. | ||
Yang et al. further enlarged the conjugated system to develop a long-wavelength triphenylamine-based probe (LIP-Ser).39 As the polarity of the solvent decreased, LIP-Ser showed an increase in fluorescence emission at 634 nm, indicating that LIP-Ser can detect changes in polarity. The confocal imaging experiments performed within the cells demonstrated that LIP-Ser selectively stains LDs, as corroborated by colocalization reagent BODIPY 493/503. Non-alcoholic fatty liver disease (NAFLD) is associated with disordered lipid metabolism and accumulation in hepatocytes. Ferroptosis, a form of regulated cell death, involves lipid peroxidation in NAFLD progression. Therefore, the role of ferroptosis in NAFLD was elucidated using the polarity-sensitive LD-targeting probe LIP-Ser. Using in vivo imaging, LIP-Ser demonstrated that NAFLD promotes ferroptosis through real-time LD imaging in high-fat diet (HFD)-treated mice. By introducing dual triphenylamine donors, Wang and colleagues designed a D–π–A–D configuration probe,40 TST, which could visualize the changes in LD polarity in a fatty liver model (Fig. 7).
 | ||
| Fig. 7 Triphenylamine-based LD probes LIP-Ser (A) and TST (B) and their application in the imaging of fatty liver and organs of high-fat diet mice. Reprinted with permission from ref. 39 and 40. Copyright 2023 and 2024 Elsevier B.V. | ||
AIEgens are increasingly being used for biological applications because of their unique optical properties compared to ACQ. Generally, AIEgens comprise flexibly twisted-configuration and rotated units. The triphenylamine group represents an excellent candidate for designing AIE fluorophores because of its flexible and rotating non-planar propeller configuration and strong electron-donating ability.21 Inspired by the excellent performance of AIE dyes, Yu et al. designed a lipid droplet-targetable probe, PPTH, with AIE character for detecting polarity.41 It was substantiated that a ratiometric fluorescence of PPTH was observed along with the change of solvent polarity. Normal and fatty liver tissues were differentiated successfully based on the PPTH polarity response by detecting LD changes (Fig. 8).
 | ||
| Fig. 8 (A) The proposed mechanism of the probe PPTH for sensing polarity in lipid droplets, (B) PPTH (red channel) was used to co-stain mouse liver tissue with LDs Tracker Blue (blue channel) to detect normal and fatty liver. Reprinted with permission from ref. 41. Copyright 2024 Elsevier B. V. | ||
Coumarins, a lipophilicity family of 1-benzopyran-2-one, are composed of the pyrone and benzene units that exhibit fluorescence in the Vis-NIR range.79 The excellent lipophilicity and optical properties are derived from a D–A system of charge-separated excited structures, which have received significant attention in constructing LD probes. In 2023, Yoshihara's group reported a series of coumarin 30-based probes (DBC30s) with blue fluorescence for imaging LDs,42 DBC30s were synthesized by replacing the diethylamino group with a dibutylamino group and substituting on the benzimidazole moiety with various groups. Upon incubating the DBC30 derivatives with oleic acid-pretreated cells, LDs emitted bright emissive dots upon excitation at 400 nm. Conversely, little fluorescence was observed upon incubation with coumarin 30, indicating that the cellular uptake efficiency was significantly enhanced due to the higher lipophilicity of the dibutylamino group of the DBC30 derivatives. Since DBC30 has excellent selectivity towards LDs, dual-color imaging was performed successfully by co-administration with the vascular endothelial probe, FITC-lectin, to visualize the abnormal accumulation of lipids in the fatty liver model (Fig. 9).
 | ||
| Fig. 9 (A) Structure of the coumarin-based LD-targeting probes DBC30s and (B) DBC30 (blue channel) used to co-stain of fatty liver tissue with BODIPY 493/503 and BDIPY 493/503 or FITC-lectin (green channel). Reprinted with permission from ref. 42. Copyright 2023 Elsevier B. V. | ||
By modifying the acceptor, Ma and colleagues reported an LD-specific probe, ZP-1, based on N,N-diethyl-coumarin, connecting an electron-withdrawing trifluoromethylphenyl group bridged by vinyl malononitrile.43 ZP-1 displayed a main absorption peak around 430 nm and a red shift from 426 to 431 nm, attributed to the enhancement of the ICT effect by intensifying of the D–A system. More importantly, the lipophilic ZP-1 showed a bright short-wavelength fluorescence in low-polarity environments without any interference from viscosity and common biologically active substances. ZP-1 entered the cell and accumulated in LD through hydrophobic interactions. ZP-1 showed remarkable anti-photobleaching capacity compared to the commercial LD dyes, BODIPY and HCS. Subsequently, NALFD was investigated by using ZP-1 to monitor lipid homeostasis in vitro and in vivo. Following ZP-1 administration, bright fluorescence was observed in liver tissues and pathological liver organs with NALFD. ZP-1 has revealed the lipid homeostasis mechanisms in NALFD and can guide NALFD diagnosis. In contrast, to bridging electron acceptors, Ding et al. modified a strong electron-donating unit, N-modified aniline, based on the similar coumarin framework to design a series of D–A–π–D fluorophores L1–L3.44 The three compounds differed significantly in their photophysical and bioactive properties, due to the different substituent group present in the aniline side chain. L1 and L3 emerged as dual-state emissions, whereas L2 was an ACQ molecule. L1 exhibited high specificity for LD staining, whereas L2 and L3 exhibited diffuse cytoplasmic distribution. This was attributed to the unsuitable oil–water partition coefficients of the hydroxyl and ester groups, unfavorable for LD targeting. L1 demonstrated high clarity and signal fidelity when used as an imaging agent to differentiate fatty liver from normal liver by visualizing dynamic LD changes in liver slices (Fig. 10).
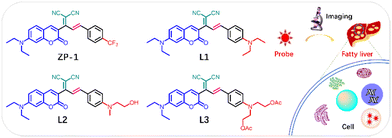 | ||
| Fig. 10 Structure of malononitrile acceptor modified LD probes (ZP-1, L1-3) and schematic diagram of the imaging of LDs in the fatty liver disease. | ||
Based on the advantages of AIE, Gu and colleagues prepared a sequence of LDs targeting probes (B1–B6) based on a lipophilic coumarin (Fig. 11).45 The probe was equipped with a diphenylamine donor and various acceptor moieties to regulate the π-electron density for influencing the photophysical properties, and the coumarin backbone and diphenylamine moiety locked the lipophilicity for targeting LD. The probes B1–B6 showed a clear solvatochromism effect in solvents of different polarities and AIE properties in a mixture of ethanol and phosphate-buffered saline (PBS), except for B5. Benefiting from the lipophilic nature of the diphenylamine and coumarin units, probes B1–B6 could selectively stain LDs and emit bright-spot fluorescence in the cytoplasm. Using probe B2 as an agent to diagnose fatty liver, a strong fluorescence signal was dramatically enhanced in LDs in fatty liver tissue rather than normal liver tissue. This was caused by the excessive accumulation of LDs in the pathological liver tissue.
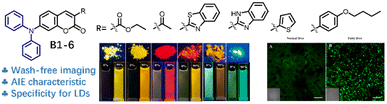 | ||
| Fig. 11 Chemical structures of LD probes (B1-6) and their solid/solution fluorescence performance and application in fatty liver imaging. Reprinted with permission from ref. 45. Copyright 2023 Elsevier Ltd. | ||
BODIPY fluorophores are suitable for LD imaging because of their high hydrophobicity and quantum yield, as demonstrated by the successful use of BODIPY 493/503 as a commercial colocalization reagent.20 However, the symmetrical structure of BODIPY 493/503 and a low ICT effect are disadvantageous for the polarity response. The short wavelength of BODIPY 493/503 is unfavorable for in vivo imaging. Hence, a range of BODIPY derivatives with varying wavelengths was developed for use in LD imaging, exhibiting excellent imaging performance. To further enhance the affinity of the probe to LDs, Wang et al. selected a triphenylamine as an electron donor to conjugate the difluoroboron segment,46 thereby constructing a D–π–A configuration LD probe, LD-HW. The LD-HW probe showed a long-wavelength emission peak in water and a gradual wavelength hypochromatic shift with changing solvent polarity due to the positive solvatochromism effect resulting from the typical ICT effect. Moreover, LD-HW achieved high LD-specific staining due to the appropriate hydrophobicity of the triphenylamine and difluoroboron moieties, which enhanced LD targeting ability. To assess NAFLD, alterations in LD were tracked by combining the polar-sensitive and LD-specific abilities of LD-HW. After LD-HW administration, a 10-fold increase in fluorescence intensity was observed in the HFD tissues compared to the normal liver under 500 nm excitation, demonstrating the considerable potential for non-invasive FLD diagnosis through LD imaging. Based on the BODIPY framework, He et al. used bovine serum albumin (BSA) to assemble LD-1 to form a biocompatible and long-wavelength fluorescent probe, LD-1@BSA,47 which selectively imaged hepatocytes to differentiate NAFLD by monitoring LD accumulation under 500 nm excitation. Then, using triphenylamine to conjugate the difluoroboron segment strategy, a D–π–A probe TSB was designed for identifying LDs to differentiate fatty liver from normal liver (Fig. 12).48
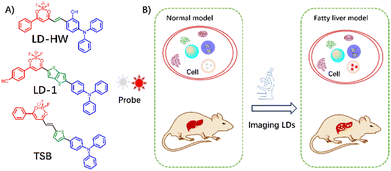 | ||
| Fig. 12 (A) Structures of LD probes (LD-HW, LD-1, TSB) and (B) their schematic representation for imaging fatty liver. | ||
In addition to the common fluorophores, numerous other fluorophores were synthesized and employed in imaging LDs through different detection mechanisms in the FLD model. Niu's group developed a naphthalene-based probe,49 ANI, that exhibited distinct emission peaks from toluene to dimethyl sulfoxide (DMSO). This was attributed to the typical solvatochromic effect observed for D–A-based probes. In addition, the probe demonstrated enhanced emission towards polarity compared with viscosity. The ANI probe can illuminate emission in a low-polarity environment using the TICT mechanism, thereby enabling LD imaging. As shown in the hepatic tissue images, bright green fluorescence appeared in fatty liver tissues of HFD-treated mice under 488 nm excitation, attributable to the specific differentiation of LD accumulated in disease tissues. Similar results were also observed in fatty liver tissues of human samples. Using the naphthalene backbone, Li et al. reported a typical D–π–A fluorescent probe, LDs-DM, with 535 nm emission in oil.50 Moreover, the solvatochromic response nature and ability of LDs-DM to specifically target LDs facilitated the differentiation of fatty liver from normal human liver tissue by visualizing the abnormal lipid droplet accumulation. Other probes have demonstrated remarkable efficacy in quantifying LD alterations. For instance, Bae's group successfully developed a green fluorescent LDs probe, LipidGreen2,51 for staining fatty liver. Wang's52 group designed THE red-emission fluorescent probe, G1, for assessing FLD by tracing LDs. Gu et al. designed a series of AIE probes, ISO-LDs, for the specific identification of LDs under visible light excitation in a NAFLD model (Fig. 13).53
 | ||
| Fig. 13 Chemical structures of LD fluorescent probes (ANI, LDs-DM, LipidGreen2, G1, ISO-LD1-3) for the diagnosis of fatty liver disease. | ||
All the above UV-Vis LD probes were constructed based on the ICT/AIE detection mechanism, utilizing different fluorophores. ESIPT, a unique luminous mechanism, can also be employed in the design of LD probes. In 2020, Liu's group employed a classical ESIPT-based fluorescent dye,54 3-hydroxyflavone, to design a series of LD probes by equipping them with various donor groups (Fig. 14). The microenvironment-sensitive characteristics of the ESIPT dyes enabled a sensitive response to LD lipid media. LDs can be selectively targeted by modifying liposoluble side chains, thus, Flps have been considered promising probes for imaging LDs. Flps exhibited a high dependence on solvent polarity, exhibiting weak fluorescence in water and strong yellow fluorescence in liposoluble solvents. Furthermore, colocalization experiments proved they had superior LD targeting capabilities due to their long carbon chains, which increased their lipophilicity. Finally, Flps were successfully applied to label LDs in fatty liver and other diseased tissues, resulting in strong yellow fluorescence.
 | ||
| Fig. 14 Chemical structures of Flps LD probes and their used in laser scanning confocal microscopy of various mouse tissue slices (including WAT, BAT, fatty liver, skeletal muscle tissues). Reprinted with permission from ref. 54. Copyright 2020 American Chemical Society. | ||
Yu et al. developed a series of novel ESIPT-based chromophores (HOPs) by introducing a hydroxyphenyl group into the purine framework.55 The purine-based dyes displayed characteristic absorption peaks at approximately 350 nm. In a manner analogous to that observed with classical ESIPT-based dyes, HOP-Ph and HOP-NMe exhibited green fluorescence in non-polar solvents, while exhibiting only weak fluorescence in polar solvents. This indicates that the stronger ESIPT effect was controlled to light up the fluorescence in the different solvents, suitable for responding to the lipophilic environment within the LDs. HOP probes can selectively stain LDs and emit light fluorescence to label their distribution when combined with the lipophilic properties of HOPs. More importantly, HOP revealed the lipid-rich phenomenon of LDs in fatty liver, and significant signals were observed in the fatty liver models (Fig. 15).
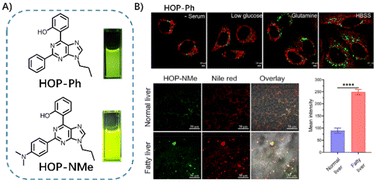 | ||
| Fig. 15 (A) Structure of LD probes HOP-Ph and HOP-NMe, (B) and their use in co-staining cells with Mitochondria Tracker Red or imaging normal/fatty liver with Nile Red. Reprinted with permission from ref. 55. Copyright 2023 Elsevier B.V. | ||
The reported UV-Vis probes have gained a significant advancement in the field of FLD diagnosis through LD imaging. NIR-I probes with longer wavelengths, can penetrate deeper into tissues while minimizing autofluorescence and photodamage.15,80 They offer a distinct advantage over UV-Vis probes for the precise in vivo assessment of fatty liver. This transition expands the potential application spectrum and addresses some limitations of shorter-wavelength UV-Vis probes, facilitating more accurate and non-invasive LD imaging for diagnosing fatty liver.
LDs and mitochondria play a vital role in the process of fat metabolism. Zhou's group designed a typical D–π–A probe, DPDO-C, introducing 3,4-ethylene dioxythiophene to connect the triphenylamine electron donor group and the indole salt electron acceptor,56 which could selectively identify mitochondria and LDs in high or low polarity environments via dual emission (Fig. 16). In non-polar solvents, the DPDO-C probe exhibited a strong absorption at 370 nm and a weak absorption at 600 nm. However, the opposite trend was observed in polar solvents. Furthermore, an obvious green fluorescence at 500 nm and a red fluorescence at 800 nm were observed with excitation in 1,4-dioxane and DMSO at 405 nm and 561 nm, respectively. The DPDO-C fluorescence response towards different solvents was attributed to the probe's local excitation state and efficient ICT state. In addition, a dual-color fluorescence was observed in the LDs and mitochondria due to the differing polarities of these organelles. Based on these excellent features, the probes successfully distinguished between normal and fatty liver tissues by altering the dual-channel fluorescence due to the differential number of LDs.
 | ||
| Fig. 16 Structure of the dual-targeting probe DPDO-C and its confocal fluorescence imaging of fatty liver tissue. Reprinted with permission from ref. 56. Copyright 2022 American Chemical Society. | ||
Lin et al. constructed a push–pull conjugation system probe, SSR-LDs, by difluoroboronated β-diketone bridging of a dimethylaminophenyl moiety (electron donor) and a coumarin moiety (electron acceptor),57 which produced an obvious ICT effect and was highly sensitive to the solvent's polarity, triggering changes in the fluorescence wavelength and intensity. SSR-LDs exhibited strong short-wavelength emission in low-polarity solvents and weak long-wavelength emission in high-polarity solvents due to differences in excited-state energy dissipation. The superior polarity response performance of SSR-LDs enables non-invasive monitoring of fatty liver by tracking the number and distribution of LDs. The fatty liver model showed strong fluorescence unlike the normal liver. The framework was also employed to modify a carbazole derivative (electron donor) to design a polar-sensitive probe, CCB.58 CCB was highly sensitive to polarity, enabling the quantitative detection of LDs through the fluorescence lifetime, which can be used to monitor NAFLD (Fig. 17).
 | ||
| Fig. 17 (A) Structure of Bodipy-based LD probes (SSR-LDs and CCB), (B) Fluorescence and fluorescence lifetime imaging with SSR-LDs or CCB for fatty liver detection. Reprinted with permission from ref. 57 and 58. Copyright 2022 The Royal Society of Chemistry and Elsevier B.V. | ||
Chen's group constructed an imidazole-modified AIE probe, TITM (Fig. 18),59 which exhibited a remarkable emission at 580 nm (toluene) and red-shifted emission with a weak intensity as the solvent polarity increased. Similar to other types of triphenylamine-based dyes, TITM displays excellent polarity sensitivity and lipid droplet targeting ability, rendering it a suitable candidate for intracellular lipid tracking to assess the corresponding disease. Following TITM staining, fatty liver tissue can be differentiated from normal liver tissue by visualizing the distribution and accumulation of disordered LDs and exploring LD-related diseases.
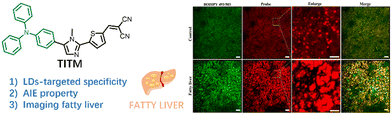 | ||
| Fig. 18 Structure of the TITM probe and its (red channel) use in co-staining liver tissue from mice fed a normal diet and a high-fat diet with BODIPY 493/503 (green channel). Reprinted with permission from ref. 59. Copyright 2022 Elsevier Ltd. | ||
In general, probes for imaging of LDs with long wavelengths are characterized by the presence of large conjugated structures, necessitating the use of complex synthesis methods. Wang et al. prepared a novel triphenylamine-based probe, TNBD, via one-step synthesis for turn-on LD imaging (Fig. 19).60 TNBD exhibited a maximum absorption wavelength at approximately 550 nm, accompanied by weak fluorescence in a DMSO/water mixed solution due to the ACQ effect. Nevertheless, the pronounced red fluorescence at 657 nm increased in intensity when the TNBD was dispersed in an oleic acid solution. This phenomenon indicated that TNBD exhibited good polarity sensitivity due to its lipophilicity. Furthermore, changes in LDs induced by oleic acid in HeLa cells were monitored using TNBD-specifically labeled LDs. Next, TNBD was co-stained with fatty liver tissues to image LDs in fatty liver disease, and obvious fluorescence signals of TNBD were observed in LD clusters of fatty liver tissues, while not in healthy liver tissues. The confocal laser scanning microscopy to image liver tissue substantiated that TNBD has significant potential for fatty liver diagnosis.
 | ||
| Fig. 19 Chemical structure of probe TNBD and its (red channel) co-staining images of LDs with BODIPY 493/503 (green channel) in the liver tissue from mice with fatty liver and normal mice. Reprinted with permission from ref. 60. Copyright 2021 The Royal Society of Chemistry. | ||
In addition, to a further attempt to promote our understanding of the changes in LDs and their dynamic relationship with fatty liver disease, concurrently, other NIR-I LD probes have been reported due to the advantages of the NIR-I fluorescence imaging system. For instance, Wei's,61 Feng's62 designed the NIR-I fluorescence probes ZH-2 and DCIQ. Tang's group designed a viscosity-responsive NIR-I probe, PV-1, for the diagnosis and prognostic assessment of NAFLD using fluorescence and photoacoustic dual-mode imaging (Fig. 20).63
 | ||
| Fig. 20 Chemical structures of LD fluorescent probes (ZH-2, DCIQ, PV-1) for the diagnosis of fatty liver disease. | ||
Except for polarity and viscosity, other factors are closely linked to fatty liver or involved in FLD development, including peroxynitrite (ONOO−) and pH. Therefore, other activable NIR-I probes were constructed to image LDs in the fatty liver disease process. Zhang et al. developed three ONOO−-induced elimination probes to monitor the levels of LDs.64 Of these probes, MBDP-Py+ exhibited high sensitivity to ONOO−, enabling the release of MBDP-Py to target LDs for tracking their dynamic changes. Following the pretreatment of cells with MBDP-Py+, bright fluorescence was initially activated by intracellular reactive oxygen species and subsequently migrated to the LDs, verified using a commercial localization agent, BODIPY 493/503. HFD-fed NAFLD mice image was explored using MBDP-Py+, and the fluorescence signals of MBDP-Py+ increased progressively in the livers of HFD-fed mice under 540–580 nm excitation channel. Higher fluorescence intensity was observed in the model mice with prolonged HFD feeding, which positively correlated with increased oxidative stress and the number of LDs in NAFLD mice. In 2024, Hu et al. reported a pH-triggered hydrophilicity-adjustable probe, LD-Lyso, that could activate 740 nm emission to simultaneously label LDs and lysosomes for NAFLD diagnosis.65 Then, they developed a near-infrared fluorescent probe BDP-NIR-Py+ by molecular engineering strategy modification of the MBDP-Py+,66 which could release near-infrared BDP-NIR-Py to track LD after fast and sensitive response to ONOO− for NAFLD and type 2 diabetes research (Fig. 21).
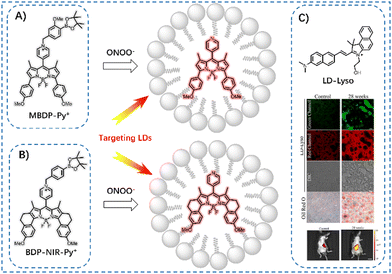 | ||
| Fig. 21 LD probes (A) MBDP-Py+, (B) BDP-NIR-Py+, and (C) LD-Lys for sensing ONOO− and pH in fatty liver models, respectively. Reprinted with permission from ref. 65. Copyright 2024 Elsevier B.V. | ||
UV-Vis/NIR-I small molecule fluorophores have tuned optical properties, functions, and excellent biocompatibility, and several LD-specific probes have been developed based on these dyes. Due to the limited penetration depth capability of UV-Vis/NIR-I emission, it would suffer from strong interference from background autofluorescence and tissue scattering in biological tissues, which would limit LD tracing in living tissues.81 Compared to the visible/NIR region, imaging in the NIR-II region has demonstrated the predominance of low-scattering and background autofluorescence for achieving high spatial resolution imaging of deep tissues,82,83 which is of great advantage for monitoring and visualizing real-time information in vivo. In 2020, Wu and colleagues reported NIR-I/NIR-II emitting donor–acceptor-donor dyes, TPAT-BNT and TPAT-BSeNT,67 which displayed bright fluorescence with emission above 900 nm. Moreover, TPAT-BNT and TPAT-BSeNT exhibited polarity-sensitive properties, and the fluorophores were sensitive to the mimicking LDs microenvironment, resulting in a 60-fold fluorescence enhancement. These features successfully facilitated fluorescence imaging of LDs in living cells and mouse models of fatty liver disease using NIR-I/NIR-II fluorescence imaging (Fig. 22).
 | ||
| Fig. 22 (A) The proposed mechanism of the NIR-II probes TPAT-BNT/BSeNT for fatty liver imaging, (B) fluorescence imaging of normal and oleic acid-treated HeLa cells, (C) fluorescence imaging and H&E staining of living mice/tissue. Reprinted with permission from ref. 67. Copyright 2022 Wiley-VCH. | ||
For a long time, LDs were considered simple neutral lipid reservoirs. However, these probes with different wavelengths for LD imaging offer distinct information of LD in the FLD process, which further dissect the relationship of FLD and LDs. UV-Vis probes with high quantum efficiency enable high-contrast imaging of lipid droplets to explore the mechanisms of fatty liver disease in cellular models. NIR probes, especially NIR-II probes, are widely used for in vivo fatty liver imaging due to the deep tissue penetration capability of photons. Ultimately, these probes provide a powerful tool for advancing our understanding of lipid homeostasis and its relevance in the FLD process. However, these types of LD probes face the problem of targeted competition from the lipid-soluble environment.
3.2. Two/Three-photon lipid droplet fluorescent probes
Based on the advantages of fluorescence imaging, various LD probes enable LD visualization with high spatial selectivity for assessing fatty liver disease. However, most LD probes reported using one-photon microscopy imaging with short excitation wavelengths, which presents significant limitations. The limitations include the following: the short excitation wavelength limits the penetration depth for tracking changes in LDs in deep tissues; photobleaching/phototoxicity makes these probes unsuitable for prolonged imaging; autofluorescence from living tissues interferes with the imaging signal.84 In contrast, two-photon (TP)/three-photon fluorescence microscopy could translate the short-wavelength excitation to NIR-I and II windows, allowing the two/three photons of lower energy excitation to achieve probe imaging, effectively reducing photobleaching, phototoxicity, auto-fluorescence interference and increasing penetration depth.85,86 Therefore, Kim et al. constructed a small-molecule probe (LD1) using a benzofuran ring as a conjugation bridge to connect a pyrrolidine-fused tetrahydroquinoxaline electron donor and a dicyanobenzene electron acceptor.68 LD1 emitted a bright fluorescence in non-polar solvents while a weak fluorescence in polar solvents because the dipole–dipole interaction with the solvents led to the different degree of charge separation. Notably, LD1 had a peak absorption at 495 nm and obvious two-photon action cross-sections, which also increased with decreasing solvent polarity. Furthermore, LD1 was highly selective for staining LDs, likely because of its high lipophilicity, which facilitates cell membrane penetration to label LDs. Encouraged by LD1's high LD targeting and sensitive response ability, as well as its excellent two-photon properties, LD1 was used for in vivo two-photon microscopy (TPM) imaging of LDs in fatty liver mice models. They then designed another probe, LD2, by replacing the acceptor with benzothiadiazole, to track LDs,69 and combined it with green fluorescent protein-label macrophages to reveal the correlation between macrophages and LDs and the progression of fatty liver using TPM imaging (Fig. 23). | ||
| Fig. 23 Structures of the two-photon probes LD1–2 (A) and two-photon microscopy fluorescence images of tissue from normal or methionine/choline-deficient mice and Cx3cr1-GFP mice (B). Reprinted with permission from ref. 68 and 69. Copyright 2022 and 2024 American Chemical Society. | ||
To improve the imaging quality, Zhou et al. reported a two-photon probe, lip-YB, with high brightness to visualize the multi-dynamic behavior of LDs.70 lip-YB was equipped with a coumarin donor moiety, an organoboron acceptor moiety, and a lipophilic cyclohexane modification segment to form a strong ICT effect molecule. Coumarin and cyclohexane endowed the probe with the potential to target LDs because of its markedly enhanced lipophilicity. In contrast, the potential of coumarin for two-photon imaging has been confirmed. As expected, lip-YB exhibited a sensitive polarity response upon polarity change from non-polar to polar solvents, accompanied by a red shift in absorption from 530 to 568 nm. Meanwhile, the fluorescence intensity increased with decreasing solvent polarity. A 2113-fold enhancement in fluorescence was observed in oleic acid compared to water. Moreover, lip-YB could light up the maximum emission under 970 nm excitation, demonstrating its superior two-photon nature. And the confocal images showed satisfactory LDs localization, which was further verified by directly labelling the LDs in a fatty liver model for disease diagnosis (Fig. 24).
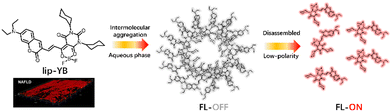 | ||
| Fig. 24 The proposed mechanism of LD probe lip-YB for imaging fatty liver and its image of fatty liver. Reprinted with permission from ref. 70. Copyright 2022 American Chemical Society. | ||
The reported two-photon LD's probes were also exposed to the ACQ effect. Tang and colleagues developed a novel π-conjugation AIE probe, ABCXF,71 to address this issue through a straightforward nucleophilic reaction. The probe exhibited a bright solid-state red emission, attributed to the nonaromatic rotor (CF3), which endowed it with its AIE nature. The AIE property of ABCXF was validated in a tetrahydrofuran/water mixture, where increased emission at 585 nm was observed with increasing water fraction. It is noteworthy that ABCXF exhibited blue-shifted and enhanced fluorescence at 525 nm in the mimic the environment of LDs, and its maximum two-photon action cross-section reached 180 GM at 850 nm. Subsequently, imaging of cells with ABCXF indicated that it could emit bright fluorescence in LDs, which endowed the probe with the ability to assess fatty liver disease by visualizing changes in LDs. ABCXF demonstrated the ability to achieve high-contrast two-photon imaging of the FLD tissue when excited at a wavelength of 850 nm and exhibited superior photobleaching resistance compared to commercial LD probes (Fig. 25).
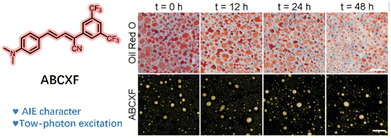 | ||
| Fig. 25 Structure of the two-photon probe ABCXF and its bright-field and fluorescence images of pig liver tissue pretreated with oleic acid at different times. Reprinted with permission from ref. 71. Copyright 2021 The Royal Society of Chemistry. | ||
Small molecular weight, limited conjugation length and high lipophilicity of the fluorophore are helpful for LD imaging. Kim et al. installed electron-rich portions of benzofuran to benzothiadiazole to construct the D–A probe 4 for imaging LD.72 The probe exhibited high selectivity and sensitivity for LD, which is helpful in diagnosing fatty liver disease by imaging the LD accumulation. Yu et al. designed a two-amphipathic small-molecule probe, N–Cy, using an indol cation to modify the lipophilic fluorophore nitrobenzoxadiazole.73 Then, an interface-targeting strategy was proposed to improve LD targeting by hydrophobic and electrostatic interactions, which could achieve high-fidelity and background-free LD imaging. Finally, using a two-photon imaging system, N–Cy not only visually monitored changes in the size, number and morphology of LDs, but also clearly showed their abnormalities in liver tissue during the process of fatty liver disease (Fig. 26).
 | ||
| Fig. 26 (A) Two-photon microscopy images of liver tissue from mice pretreated with oleic acid or tunicamycin with probe 4, (B) and fluorescence images of fatty liver tissue with probe N–Cy. Reprinted with permission from ref. 72 and 73. Copyright 2020 and 2018 American Chemical Society. | ||
Unlike two-photon microscopy, three-photon microscopy relies on non-linear excitation light in the NIR-II (900–1700 nm) region, which can penetrate deeper tissues to provide high spatio-temporal resolution and signal-to-noise ratio imaging in vivo.87 Therefore, three-photon fluorescence microscopy technology is an ideal approach for tracking and monitoring changes in LDs in vivo. As an electron-accepting group, the cyano group can modify the electron-donating group to form an ICT electronic configuration fluorophore, which can improve the three-photon absorbance and regulate the polarity sensitivity of the fluorophore. Qian et al. incorporated electron-rich portions of naphthalene into acrylonitrile to construct the D–A probe, NAP-CF3, for imaging LDs.74 As expected, the D–A conjugated fluorophore exhibited enhanced emission in nonpolar solvents compared to polar solvents. The hydrophobicity of NAP-CF3 contributed to its targeting ability and the probe labelled LDs with high specificity. NAP-CF3 showed satisfactory three-photon performance due to the pronounced ICT effect, achieving maximum three-photon absorption at 1300 nm. NAP-CF3 was successfully used to evaluate the developmental progress of fatty liver using three-photon fluorescence imaging to achieve high depth and resolution imaging (Fig. 27).
 | ||
| Fig. 27 (A) Structure and spectrum of the three-photon probe NAP-CF3 and (B) its images of pig liver tissue pretreated with high-fat at different times. Reprinted with permission from ref. 74. Copyright 2022 World Scientific. | ||
Liu et al. selected triphenylamine and dicyanovinyl groups to construct a D–A configuration probe, TPAPhCN, with emission in the NIR region.75 The rotor structure of triphenylamine endowed its AIE activity, and an increase in fluorescence intensity was monitored from fW of 70–99%. Then, TPAPhCN was encapsulated to form TPAPhCN dots using a nanoprecipitation method, which specifically stained the LDs, as evidenced by the co-staining of BODIPY 493/503. More importantly, TPAPhCN dots exhibited an apparent three-photon absorption cross section at 1200 nm and a maximum two-photon absorption cross section at 900 nm, as measured by femtosecond laser excitation. Compared with two-photon imaging, a greater imaging depth was observed with the three-photon excitation. Therefore, the TPAPhCN dots were employed to investigate fatty liver disease by tracking LDs. Obvious fluorescent dots accumulated in the LDs, and imaging with a lower background was achieved with three-photon excitation than with two-photon excitation (Fig. 28).
 | ||
| Fig. 28 The schematic illustration of the preparation of TPAPhCN dots and TPAPhCN dots were used for imaging fatty liver tissue. Reprinted with permission from ref. 75. Copyright 2021 Wiley-VCH. | ||
In 2022, Hua et al. designed the π-conjugated D–A configuration molecule, DPCN, by linking malononitrile to an amine-based electronic donor, N,N′-diphenyl-dihydrophenazine (DHP).76 Methyl groups were further modified at the ortho-positions of the DHP ring to tune the molecular conformations to prepare SMPCN and DMPCN. All compounds exhibited comparable deep-red emissions at approximately 640 nm in cyclohexane and demonstrated significant solvatochromic effects due to the apparent ICT effect. As a result of the ortho-methyl effects, SMPCN and DMPCN showed a larger Stokes shift than DPCN. They were encapsulated in mPEG-PLGA to guarantee optimal biocompatibility to form DPCN, SMPCN, and DMPCN NPs. Among them, DMPCN NPs exhibited obvious three-photon signals, benefiting from the large π conjugation of the molecule. The high lipophilicity of the DMPCN NPs effectively facilitated precipitation in the cell membrane for LD-specific labelling, resulting in high fluorescence brightness for imaging LDs. Finally, the DMPCN NPs were successfully used for in vivo labelling of LDs in a fatty liver disease model and exhibited a much higher signal-to-background ratio, excellent depth under three-photon fluorescence microscopy (Fig. 29).
 | ||
| Fig. 29 (A) Chemical structures of LD probes and the schematic illustration of the preparation of DMPCN NPs, (B) DMPCN NPs used for imaging fatty liver tissue and Gaussian fitting profile along the white line across the LDs at an imaging depth of 10 μm. Reprinted with permission from ref. 76. Copyright 2022 American Chemical Society. | ||
Multiphoton microscopy, including two-/three-photon, exploits the non-linear excitation of photons at infrared wavelengths, allowing deep tissue penetration with minimal autofluorescence background, affording high-contrast imaging of lipid droplets, providing insights into the mechanisms underlying fatty liver development and potential therapeutic interventions. However, most probes are limited to imaging at the tissue level and achieving multiphoton imaging of fatty liver at the in vivo level is still worth exploring.
4. Summary and outlook
4.1. Achievements in optimizing the properties of lipid droplet fluorescent probes
FLD is a pathological condition closely related to changes in LDs and has a significant impact on the quality of life of affected individuals. Fluorescence imaging is a promising non-invasive imaging method with the advantages of simplicity and high temporal-spatial controllability. Therefore, in recent years, developing LD fluorescent probes have contributed to FLD diagnosis by LD imaging. As previously discussed, these probes with different excitation models and emission wavelengths provide high-resolution imaging signals, enabling a deeper understanding of the relationship between LDs and FLD. Notably, there have been some achievements in optimizing the properties of the LD probes developed, including (1) expansion of the range of available emission wavelengths to encompass wavelengths from Vis to NIR-II; (2) improved photophysical properties, such as superior brightness, stability, and Stokes shift; (3) improved selectivity and specificity; and (4) capability of sensitively capturing changes in LD including polarity, viscosity, and others.4.2. Limitations and future developments of fluorescent probes for imaging lipid droplets
Despite considerable efforts to enhance the characteristics of LD fluorescent probes, several pivotal issues persist as obstacles to clinical implementation.Author contributions
Long He: investigation, writing and editing; Hang Li: investigation, writing; Yao Tang: investigation, writing; Tian-Bing Ren: funding acquisition, reviewing and supervision and Lin Yuan: supervision, reviewing and editing.Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Science Foundation of China (22374039), the Natural Science Foundation of Hunan Province (2023JJ20004), and the Fundamental Research Funds for the Central Universities.References
- X. Qi, S. Zheng, M. Ma, N. Lian, H. Wang, L. Chen, A. Song, C. Lu, S. Zheng and H. Jin, Front. Pharmacol., 2022, 13, 912825 CrossRef CAS.
- Y.-C. Zhao, G.-J. Zhao, Z. Chen, Z.-G. She, J. Cai and H. Li, Hypertension, 2020, 75, 275–284 CrossRef CAS.
- N. L. Gluchowski, M. Becuwe, T. C. Walther and R. V. Farese, Nat. Rev. Gastroenterol. Hepatol., 2017, 14, 343–355 CrossRef CAS PubMed.
- P. Dalhaimer, Cells, 2019, 8, 974 CrossRef CAS.
- D. H. Ipsen, J. Lykkesfeldt and P. Tveden-Nyborg, Cell. Mol. Life Sci., 2018, 75, 3313–3327 CrossRef CAS.
- F. Seebacher, A. Zeigerer, N. Kory and N. Krahmer, Semin. Cell Dev. Biol., 2020, 108, 72–81 CrossRef CAS.
- S. Zhang, B. Ji, J. Li, W. Ji, C. Yang and L. Yang, Cell. Signalling, 2023, 112, 110905 CrossRef CAS.
- A. Chorlay and A. R. Thiam, J. Cell Biol., 2020, 219, e201907099 CrossRef CAS.
- J. A. Olzmann and P. Carvalho, Nat. Rev. Mol. Cell Biol., 2019, 20, 137–155 CrossRef CAS.
- Z. Huo, X. Cao, D. Sun, W. Xu, B. Yang and S. Xu, ACS Sens., 2023, 8, 1939–1949 CrossRef CAS.
- X. Zhang, Y. Wang and P. Liu, Protein Cell, 2016, 8, 4–13 CrossRef.
- N. Krahmer, R. V. Farese and T. C. Walther, EMBO Mol. Med., 2013, 5, 973–983 CrossRef.
- J. Yang, Z. Wang, Y. Deng, C. Zhang, X. Shen, J. He, L. Hu and H. Wang, Org. Biomol. Chem., 2023, 21, 8767–8771 RSC.
- L. Guo, M. Tian, Z. Zhang, Q. Lu, Z. Liu, G. Niu and X. Yu, J. Am. Chem. Soc., 2021, 143, 3169–3179 CrossRef CAS PubMed.
- J. Huang, L. He, J. Xu, J. Wang and L. Yuan, Synlett, 2024, 21–28 CrossRef CAS.
- F. Yang, P. Lu, T.-B. Ren, X.-B. Zhang and L. Yuan, Smart Mol., 2023, 1, e20220002 CrossRef.
- P.-Z. Liang, Z. Li, X.-X. Zhang, F.-Y. Yang, S.-L. Liu, T.-B. Ren, L. Yuan and X.-B. Zhang, Chem. Biomed. Imaging, 2024, 2, 185–193 CrossRef CAS.
- Y. Zhao, W. Shi, X. Li and H. Ma, Chem. Commun., 2022, 58, 1495–1509 RSC.
- T. K. Fam, A. S. Klymchenko and M. Collot, Materials, 2018, 11, 1768 CrossRef PubMed.
- H. Tian, A. C. Sedgwick, H.-H. Han, S. Sen, G.-R. Chen, Y. Zang, J. L. Sessler, T. D. James, J. Li and X.-P. He, Coord. Chem. Rev., 2021, 427, 213577 CrossRef CAS.
- L. Wang, X. Chen, X. Ran, H. Tang and D. Cao, Dyes Pigm., 2022, 203, 110332 CrossRef CAS.
- S. Sasaki, G. P. C. Drummen and G.-I. Konishi, J. Mater. Chem. C, 2016, 4, 2731–2743 RSC.
- F. Bureš, RSC Adv., 2014, 4, 58826–58851 RSC.
- S. Singha, D. Kim, B. Roy, S. Sambasivan, H. Moon, A. S. Rao, J. Y. Kim, T. Joo, J. W. Park, Y. M. Rhee, T. Wang, K. H. Kim, Y. H. Shin, J. Jung and K. H. Ahn, Chem. Sci., 2015, 6, 4335–4342 RSC.
- D. Zúñiga-Núñez, R. A. Zamora, P. Barrias, C. Tirapegui, H. Poblete, G. Cárdenas-Jirón, E. I. Alarcon and A. Aspée, Phys. Chem. Chem. Phys., 2018, 20, 27621–27629 RSC.
- C. Wang, W. Chi, Q. Qiao, D. Tan, Z. Xu and X. Liu, Chem. Soc. Rev., 2021, 50, 12656–12678 RSC.
- C. A. Hoelzel, H. Hu, C. H. Wolstenholme, B. A. Karim, K. T. Munson, K. H. Jung, H. Zhang, Y. Liu, H. P. Yennawar, J. B. Asbury, X. Li and X. Zhang, Angew. Chem., Int. Ed., 2020, 59, 4785–4792 CrossRef CAS PubMed.
- T.-B. Ren, W. Xu, Q.-L. Zhang, X.-X. Zhang, S.-Y. Wen, H.-B. Yi, L. Yuan and X.-B. Zhang, Angew. Chem., Int. Ed., 2018, 57, 7473–7477 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- X. Cai and B. Liu, Angew. Chem., Int. Ed., 2020, 59, 9868–9886 CrossRef CAS.
- D. Wang and B. Z. Tang, Acc. Chem. Res., 2019, 52, 2559–2570 CrossRef CAS PubMed.
- A. P. Demchenko, K.-C. Tang and P.-T. Chou, Chem. Soc. Rev., 2013, 42, 1379–1408 RSC.
- V. S. Padalkar and S. Seki, Chem. Soc. Rev., 2016, 45, 169–202 RSC.
- A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Chem. Soc. Rev., 2018, 47, 8842–8880 RSC.
- Z. Mao, H. Rha, J. Kim, X. You, F. Zhang, W. Tao and J. S. Kim, Adv. Sci., 2023, 10, 2301177 CrossRef CAS.
- L. Fan, X. Wang, Q. Zan, L. Fan, F. Li, Y. Yang, C. Zhang, S. Shuang and C. Dong, Anal. Chem., 2021, 93, 8019–8026 CrossRef CAS PubMed.
- J. Yang, Y. Wang, Z. Wang, J. Wang, C. Zhang, X. Gu, L. Hu and H. Wang, Anal. Chim. Acta, 2024, 1312, 342747 CrossRef CAS PubMed.
- Z. Wang, Y. Deng, J. Ge, F. Ding, H. Du, X. Shen, C. Zhang, X. Gu, L. Hu and H. Wang, Tetrahedron Lett., 2024, 141, 155070 CrossRef CAS.
- W. Wang, L. Chai, X. Chen, Z. Li, L. Feng, W. Hu, H. Li and G. Yang, Biosens. Bioelectron., 2023, 231, 115289 CrossRef CAS.
- J. Ge, Z. Wang, Y. Deng, F. Ding, J. Wang, X. Shen, C. Zhang, C. Wang, L. Hu and H. Wang, Spectrochim. Acta, Part A, 2024, 318, 124479 CrossRef CAS.
- X. Tian, D. Wu, W. Wei, G. Dai, Z. Li, B. Wang and M. Yu, Chin. Chem. Lett., 2024, 35, 108912 CrossRef CAS.
- K. Purevsuren, Y. Shibuta, S. Shiozaki, M. Tsunoda, K. Mizukami, S. Tobita and T. Yoshihara, J. Photochem. Photobiol., A, 2023, 438, 114562 CrossRef CAS.
- T. Liu, J. Chen, Y. Dai, Z. Wang, P. Chen, Y. Zhou, H. Wang, Y. Li, Z. Mo, C. Yang, X. Zhang, B. Sun, J. Yin, L. Li, G. Li and J. Ma, Sens. Actuators, B, 2024, 399, 134825 CrossRef CAS.
- H. Wang, C. Zhang, L. Hu, F. Tang, Y. Wang, F. Ding, J. Lu and A. Ding, Chem. – Asian J., 2023, 18, e202201291 CrossRef CAS PubMed.
- H. Wang, C. Zhang, X. Shen, Z. Wang, J. Yang, S. Shen, L. Hu, J. Pan and X. Gu, Dyes Pigm., 2023, 212, 111137 CrossRef CAS.
- L. Hu, J. Pan, C. Zhang, K. Yu, S. Shen, Y. Wang, X. Shen, X. Gu, J. Han and H. Wang, Biosens. Bioelectron., 2022, 216, 114618 CrossRef CAS PubMed.
- H.-M. Wang, Y.-C. Li, L.-L. Sun, M.-Y. Tang, J. Liu, J. Cai, L. Dong, J. Li, Y. Zang, H.-H. Han and X.-P. He, Chin. Chem. Lett., 2024, 35, 109603 CrossRef CAS.
- Y. Deng, Z. Wang, J. Wang, S. Zhang, J. Li, A. Sun, X. Zhang, L. Hu and H. Wang, New J. Chem., 2024, 48, 10427–10431 RSC.
- C.-J. Wu, X.-Y. Li, T. Zhu, M. Zhao, Z. Song, S. Li, G.-G. Shan and G. Niu, Anal. Chem., 2022, 94, 3881–3887 CrossRef CAS PubMed.
- Z. Zhan, W. Zhuang, Q. Lei, S. Li, W. Mao, M. Chen and W. Li, Chem. Commun., 2022, 58, 4020–4023 RSC.
- H.-S. Chun, J. H. Jeon, H. S. Pagire, J. H. Lee, H.-C. Chung, M. J. Park, J.-H. So, J.-H. Ryu, C.-H. Kim, J. H. Ahn and M. A. Bae, Mol. BioSyst., 2013, 9, 630–633 RSC.
- C. Wang, L. Hu, J. Yang, J. Wang, Y. Wang, X. Gu and H. Wang, Microchem. J., 2024, 201, 110591 CrossRef CAS.
- H. Wang, L. Hu, J. Yang, C. Zhang, Z. Wang, X. Shen, X. Chen, J. He, J. Pan and X. Gu, Spectrochim. Acta, Part A, 2024, 306, 123588 CrossRef CAS.
- G. Jiang, Y. Jin, M. Li, H. Wang, M. Xiong, W. Zeng, H. Yuan, C. Liu, Z. Ren and C. Liu, Anal. Chem., 2020, 92, 10342–10349 CrossRef CAS.
- J. Zhou, K. Li, L. Shi, H. Zhang, H. Wang, Y. Shan, S. Chen and X.-Q. Yu, Chin. Chem. Lett., 2023, 34, 107689 CrossRef CAS.
- S. Wang, M. Zhou, L. Chen, M. Ren, Y. Bu, J. Wang, Z.-P. Yu, X. Zhu, J. Zhang, L. Wang and H. Zhou, ACS Appl. Bio Mater., 2022, 5, 3554–3562 CrossRef CAS PubMed.
- Y. Tang, S. Song, J. Peng, Q. Zhang and W. Lin, J. Mater. Chem. B, 2022, 10, 6974–6982 RSC.
- C. Lai, Y. Zhao, X. Zou, Y. Liang and W. Lin, Sens. Actuators, B, 2022, 369, 132267 CrossRef CAS.
- C. Li, W. Zhuang, Y. Wang, S. Li, J. Chen, L. Zhou, Y. Liao, M. Chen and J. You, Dyes Pigm., 2022, 204, 110439 CrossRef CAS.
- S. Li, W. Zhuang, J. Chen, G. Li, C. Li, L. Chen, Y. Liao, M. Chen and Y. Wang, J. Mater. Chem. B, 2021, 9, 4050–4055 RSC.
- Y. Zhang, H. Zhao, J. Tang, S. Nan, L. Lu, P. Zhang and C. Wei, Bioorg. Chem., 2023, 140, 106800 CrossRef CAS PubMed.
- J. Hong, Y. Liu, X. Tan and G. Feng, Biosens. Bioelectron., 2023, 240, 115646 CrossRef CAS PubMed.
- Y. Zhou, P. Li, X. Wang, C. Wu, N. Fan, X. Liu, L. Wu, W. Zhang, W. Zhang, Z. Liu and B. Tang, Chem. Sci., 2020, 11, 12149–12156 RSC.
- N. Wang, X. Lu, J. Wang, H. Wang, B. Zhang, W. Zhao and J. Zhang, Anal. Chem., 2023, 95, 5967–5975 CrossRef CAS.
- S. Wu, X. Li, M. Zhou, Y. Cui, W. Wu, J. Ping, X. Guo and Q. Hu, Biosens. Bioelectron., 2024, 251, 116084 CrossRef CAS PubMed.
- N. Wang, X. Lu, J. Wang, R. Han, X. Ma, B. Zhang, W. Zhao and J. Zhang, Sens. Actuators, B, 2024, 412, 135806 CrossRef CAS.
- X. Zhou, K. Zhang, C. Yang, Y. Pei, L. Zhao, X. Kang, Z. Li, F. Li, Y. Qin and L. Wu, Adv. Funct. Mater., 2022, 32, 2109929 CrossRef CAS.
- H. W. Lee, I.-J. Lee, S.-J. Lee, Y. R. Kim and H. M. Kim, ACS Sens., 2022, 7, 1027–1035 CrossRef PubMed.
- E. S. Kim, J.-M. Lee, J.-Y. Kwak, H. W. Lee, I.-J. Lee and H. M. Kim, Anal. Chem., 2024, 96, 8467–8473 CrossRef CAS PubMed.
- H. Huang, Y. Bu, Z.-P. Yu, M. Rong, R. Li, Z. Wang, J. Zhang, X. Zhu, L. Wang and H. Zhou, Anal. Chem., 2022, 94, 13396–13403 CrossRef CAS PubMed.
- H. Park, S. Li, G. Niu, H. Zhang, Z. Song, Q. Lu, J. Zhang, C. Ma, R. T. K. Kwok, J. W. Y. Lam, K. S. Wong, X. Yu, Q. Xiong and B. Z. Tang, Mater. Chem. Front., 2021, 5, 1853–1862 RSC.
- M. K. Cho, M. J. Seo, V. Juvekar, J. H. Jo, W. Kim, K. S. Choi and H. M. Kim, Anal. Chem., 2020, 92, 11223–11231 CrossRef CAS PubMed.
- L. Guo, M. Tian, R. Feng, G. Zhang, R. Zhang, X. Li, Z. Liu, X. He, J. Z. Sun and X. Yu, ACS Appl. Mater. Interfaces, 2018, 10, 10706–10717 CrossRef CAS PubMed.
- M. He, H. Park, G. Niu, Q. Xia, H. Zhang, B. Z. Tang and J. Qian, J. Innov. Opt. Heal. Sci., 2023, 16, 2250033 CrossRef CAS.
- S. Wang, X. Li, S. Y. Chong, X. Wang, H. Chen, C. Chen, L. G. Ng, J.-W. Wang and B. Liu, Adv. Mater., 2021, 33, 2007490 CrossRef CAS PubMed.
- S. Li, M. He, X. Jin, W. Geng, C. Li, X. Li, Z. Zhang, J. Qian and J. Hua, Chem. Mater., 2022, 34, 5999–6008 CrossRef CAS.
- K. Umezawa, A. Matsui, Y. Nakamura, D. Citterio and K. Suzuki, Chem. – Eur. J., 2009, 15, 1096–1106 CrossRef CAS.
- S. Pei, H. Li, J. Li, Y. Liu, G. Zhang, L. Shi, W. Liang, C. Zhang, S. Shuang and C. Dong, ACS Biomater. Sci. Eng., 2023, 9, 3590–3596 CrossRef CAS PubMed.
- D. Cao, Z. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Lin, Chem. Rev., 2019, 119, 10403–10519 CrossRef CAS.
- Y. V. Suseela, N. Narayanaswamy, S. Pratihar and T. Govindaraju, Chem. Soc. Rev., 2018, 47, 1098–1131 RSC.
- B. Li, L. Lu, M. Zhao, Z. Lei and F. Zhang, Angew. Chem., Int. Ed., 2018, 57, 7483–7487 CrossRef CAS.
- L. He, L. H. He, S. Xu, T. B. Ren, X. X. Zhang, Z. J. Qin, X. B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2022, 61, e202211409 CrossRef CAS PubMed.
- T. B. Ren, Z. Y. Wang, Z. Xiang, P. Lu, H. H. Lai, L. Yuan, X. B. Zhang and W. Tan, Angew. Chem., Int. Ed., 2021, 60, 800–805 CrossRef CAS PubMed.
- L. Wu, J. Liu, P. Li, B. Tang and T. D. James, Chem. Soc. Rev., 2021, 50, 702–734 RSC.
- Z. Zheng, H. Zhang, H. Cao, J. Gong, M. He, X. Gou, T. Yang, P. Wei, J. Qian, W. Xi and B. Z. Tang, ACS Nano, 2022, 16, 6444–6454 CrossRef CAS PubMed.
- H. Li, Q. Yao, J. Fan, J. Du, J. Wang and X. Peng, Biosens. Bioelectron., 2017, 94, 536–543 CrossRef CAS PubMed.
- Z. Xu, Z. Zhang, X. Deng, J. Li, Y. Jiang, W.-C. Law, C. Yang, W. Zhang, X. Chen, K. Wang, D. Wang and G. Xu, ACS Nano, 2022, 16, 6712–6724 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |