Sulfur-doped carbon nanofibers as stable and high performance anode materials for sodium-ion batteries†
Mengwei
Lu
,
Ying
Huang
 *,
Xianping
Du
and
Xitong
Sheng
*,
Xianping
Du
and
Xitong
Sheng
The MOE Key Laboratory of Material Physics and Chemistry under Extrodinary Conditions, School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an 710072, PR China. E-mail: yingh@nwpu.edu.cn
First published on 31st May 2024
Abstract
Carbon nanofibers are deemed ideal sodium-ion battery (SIB) anode materials due to their unique structure, but their low capacity has limited further development. Heteroatom modification is one of the common methods to improve the electrochemical properties of carbon materials. Therefore, we prepared sulfur-doped carbon nanofiber (CNF-nS) composites by heat-treating sublimed sulfur and carbon nanofiber precursors under an argon atmosphere. When it serves as the anode of a sodium ion half-cell, the reversible discharge specific capacity of CNF-2S is 368.2 mA h g−1, which is better than the 203.4 mA h g−1 of the carbon nanofiber (CNF). The CNF-2S anode also has good high-rate cycling performance, and its discharge specific capacity is 122 mA h g−1 at 10 A g−1 after 1000 cycles. Furthermore, the CNF-2S anode and the Na3V2(PO4)3 (NVP) cathode were paired and assembled into a full cell, which displayed a satisfactory capacity of 182.6 mA h g−1 after 200 cycles at 0.1 A g−1. In short, the CNF-2S anode exhibits good sodium storage performance, because sulfur doping increases the layer spacing of the CNF, promotes the transfer of sodium ions, and enhances the electronic conductivity.
1. Introduction
Despite the constantly expanding new energy market and the growing requirement for sustainable energy, the global shortage of lithium resources, extremely uneven distribution, rapid consumption, and low safety issues of lithium-ion batteries (LIBs) greatly restrict the further promotion and application of LIBs.1–3 Therefore, it is imperative to find electrochemical energy storage systems that are highly safe, low-cost, long-lasting, and environmentally friendly, and can appropriately replace or supplement lithium-ion batteries.4–11 Of the multitude of promising energy storage systems in the days to come, sodium-ion batteries (SIBs) have the superiorities of low price, abundant reserves and high safety compared with LIBs.12–15 As one of the critical factors that affect the properties of SIBs, the development of anode materials is relatively slow compared with cathode materials, which leads to the commercial application of SIBs being limited. Therefore, the research focus in recent years has been to synthesize good performance anode materials suitable for SIBs.16–19The carbon material has the benefits of low price, high stability and abundant reserves, and is one of the most potential SIB anode materials.20–22 However, the low theoretical capacity limits the further development of carbon materials in SIBs. Doping with heteroatoms is effective to enhance the electronic structural performance and surface chemical properties of carbon-based materials, and is often used for the modification of carbon materials.23–28 Currently, the commonly used heteroatoms include B, S, N, P, and so on. There have been reports that B doping can make carbon materials absorb sodium more easily and improve their initial coulombic efficiency.29 Tang et al.30 successfully synthesized B, N co-doped carbon materials with NaBH4 as the B doping source. Its initial coulombic efficiency can reach up to 96.1%, and the reversible charge–discharge specific capacity of the obtained composite material is 308 mA h g−1 at a current density of 0.05 A g−1. Because the atomic radius of sulfur is greater than that of carbon, sulfur doping is a common method to expand the layer spacing between carbon materials. Aristote et al.31 obtained sulfur-doped hard carbon (S-Cmph) with good properties by pyrolysis of camphor tree and sulfur powder at different temperatures. After S doping, the crystal plane spacing of the material increases to 0.398 nm, and the charge–discharge specific capacity of S-Cmph-700 can reach 145.6 mA h g−1 at a current density of 2 A g−1 after 500 cycles. Compared to other heteroatoms, N atoms are the most commonly doped elements because of their chemical properties similar to carbon atoms. For instance, Fu et al.32 synthesized nitrogen-doped active porous carbon fiber with polypyrrole as the raw material and investigated its sodium storage performance. Qin et al.33 successfully obtained N, P double-doped carbon sheets with corn stalk skin as the raw material through a hydrothermal reaction. The material presents a good capacity of 105 mA h g−1 at a current density of 1 A g−1 after 2000 cycles, suggesting a good rate capability and outstanding cycle stability.
In recent years, a great deal of scholars have devoted much effort to preparing various carbon materials with extended interlayer spacing.34–38 Liu et al.34 successfully synthesized N-rich graphene ranging from 0.45 nm to 0.51 nm by adjusting different temperatures, achieving excellent long cycle stability and ultra-high rate performance. Therefore, by increasing the interlayer spacing of carbon materials, the sodium ion diffusion distance in the intercalation process can be shortened, and the sodium ion storage capacity of the N-rich graphene can be improved, thus improving their electrochemical properties. In addition, the construction of novel structures is often used to improve the properties of carbon anode materials, such as hollow structures,39–41 porous structures42–45 and fiber structures,46–48 which can effectively improve the sodium storage properties. Among them, the carbon fiber structure is considered a good candidate anode material for SIBs on account of its many surface defects and large specific surface area. At present, research on improving the sodium storage performance of carbon nanofibers by heteroatom doping has been reported.49–53 Nevertheless, it is yet scarce and challenging to synthesize anode materials for SIBs with better electrochemical properties by simple methods.
In this article, we have prepared sulfur-doped carbon nanofiber (CNF-nS) composites by a simple preparation method. The CNF-nS film is a self-supporting and flexible anode material, which can be used to directly assemble button cells without a binder and conductive carbon black. As the anode for SIBs, CNF-2S displays a favorable long cycle performance (122 mA h g−1 after 1000 cycles at 10 A g−1). When matched with the Na3V2(PO4)3 (NVP) cathode, the CNF-2S anode also exhibits a favorable specific capacity in full cells.
2. Experimental section
2.1. Material synthesis
CNF-nS thin films were prepared by the electrospinning method. First, 1 g polyacrylonitrile (PAN) was put in 10 mL N,N-dimethylamide (DMF) and agitated at room temperature until it was completely dissolved. Then the above solution was poured into a plastic injector for electrostatic spinning. During spinning, the positive high pressure and the negative high pressure were 8.45 kV and 1.29 kV respectively, and the injection rate was 0.06 mm min−1. Next, the obtained fiber films were heated at 280 °C for 2 hours using a muffle furnace. Finally, the fiber film and sublimated sulfur were placed in the same porcelain boat according to a certain mass ratio (mfiber film![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) msublimated sulfur = 1
msublimated sulfur = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and heat treated at 800 °C in a tube furnace for 2 hours under an argon atmosphere. According to the different proportions of the fiber film and sulfur powder, the obtained materials were named CNF-0.5S, CNF-1S, CNF-2S and CNF-3S. In addition, CNF was synthesized by the same method except that no sulfur powder was mixed.
3) and heat treated at 800 °C in a tube furnace for 2 hours under an argon atmosphere. According to the different proportions of the fiber film and sulfur powder, the obtained materials were named CNF-0.5S, CNF-1S, CNF-2S and CNF-3S. In addition, CNF was synthesized by the same method except that no sulfur powder was mixed.
2.2. Materials characterization
In order to understand the crystal structure and composition of the fiber membrane, it was characterized by XRD (Kratos XRD-7000) and Raman spectroscopy (WITec Alpha 300R). The elemental composition and content of the CNF-nS were measured by X-ray photoelectron spectroscopy (Kratos AXIS Ultra DLD). The morphologies of the CNF and CNF-nS were studied using an FEI Verios G4 scanning electron microscope (SEM). High-resolution transmission electron microscopy (HRTEM) and energy dispersive spectroscopy (EDS) were performed using an FEI Talos F200X transmission electron microscope.2.3. Electrochemical test
All the electrochemical property studies were performed on CR2016 button batteries. CNF and CNF-nS self-supporting fiber membranes can be directly used without the need for electrode preparation processes. For the production of the NVP cathode, NVP, Super-P and polyvinylidene fluoride (PVDF) were put into N-methyl-2-pyrrolidone (NMP) according to the ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, stirred into a uniform slurry and coated on Al foil. The coated film was dried overnight at 80 °C in a vacuum drying oven to obtain NVP electrode plates. The electrolyte is 1 M NaClO4 in a 1
10, stirred into a uniform slurry and coated on Al foil. The coated film was dried overnight at 80 °C in a vacuum drying oven to obtain NVP electrode plates. The electrolyte is 1 M NaClO4 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio with diethyl carbonate (DEC) and ethylene carbonate (EC), plus 5% fluoroethylene carbonate (FEC) additive. The separator was glassy fiber. When the CNF, CNF-nS and NVP are used as electrodes alone, sodium metal is used as the working electrode. In the full cells, the NVP electrode and CNF-2S electrode are used as the cathode and anode respectively, and the weight ratio is 3
1 volume ratio with diethyl carbonate (DEC) and ethylene carbonate (EC), plus 5% fluoroethylene carbonate (FEC) additive. The separator was glassy fiber. When the CNF, CNF-nS and NVP are used as electrodes alone, sodium metal is used as the working electrode. In the full cells, the NVP electrode and CNF-2S electrode are used as the cathode and anode respectively, and the weight ratio is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. All coin batteries were put together in an argon-filled glovebox. Cycle performance and the galvanostatic charge and discharge tests were conducted on a LAND BT2000 battery measurement system. Electrochemical impedance spectra (EIS) and cyclic voltammetry (CV) curves were recorded using a Gamry workstation.
1. All coin batteries were put together in an argon-filled glovebox. Cycle performance and the galvanostatic charge and discharge tests were conducted on a LAND BT2000 battery measurement system. Electrochemical impedance spectra (EIS) and cyclic voltammetry (CV) curves were recorded using a Gamry workstation.
3. Results and discussion
Fig. 1 exhibits the preparation procedure of CNF-nS. The sulfur-doped carbon nanofiber membrane was prepared by one-step vulcanization and carbonization of the PAN fiber membrane obtained by electrospinning. After high-temperature calcination, the PAN fibers undergo oxidation, cross-linking and other reactions, and the internal structure of the fibers is transformed into a carbon–carbon structure.54 Meanwhile, the sublimated sulfur becomes small molecules embedded in carbon materials, and finally CNF-nS is obtained.53 It is important to note that the N element is derived from PAN.The microstructure and surface morphology of the materials were characterized through SEM and TEM. From the SEM pictures (Fig. 2(a and b) and S1†) of CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S, it can be observed that the average diameter of the fibers is about 400 nm. The nanofibers in the samples are cross-linked to form a three-dimensional net structure, which makes the carbon fibers have a certain degree of flexibility.52 In the meantime, there are large gaps between the carbon fibers, which are advantageous to promoting the diffusion of sodium ions and reaction kinetics. Fig. 2c and S2† show high resolution transmission electron microscopy images of the CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S, respectively. It can be seen that CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S are not long-range ordered crystal structures and the carbon layer has a spacing of 0.391, 0.401, 0.408, 0.413 and 0.420 nm, respectively. This shows that sulfur doping improves the interlayer spacing of carbon nanofibers and makes them have wider sodium ion storage or transportation channels.51,53 The EDS test results of CNF-2S are shown in Fig. 2d. The elemental mapping exhibits the distribution of C, N and S on the sample surface, confirming the successful synthesis of CNF-2S.
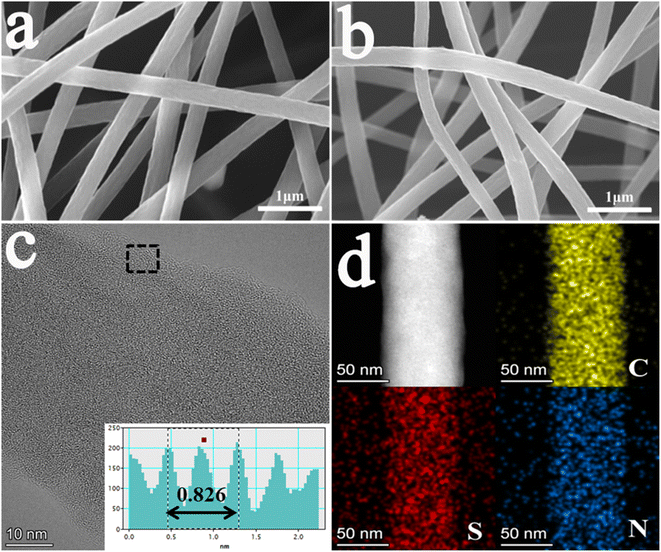 | ||
| Fig. 2 SEM pictures of (a) CNF and (b) CNF-2S; (c) TEM pictures of CNF-2S; (d) EDS mapping of CNF-2S. | ||
The structure and elementary composition of the samples were characterized through XRD, Raman and XPS. From the XRD pattern of the materials (Fig. 3a), it can be seen that the crystal structure of the CNF does not vary with the doping of sulfur. In the figure, the diffraction peak is the (002) crystal plane representing amorphous carbon. At the same time, it can be seen that the 2θ value of the crystal plane (002) shifts towards a low angle with the doping of sulfur, indicating that sulfur doping extends the interplanar crystal spacing of the materials, which will be helpful to the intercalation and de-intercalation of sodium ions in the samples to improve the electrochemical performance.31,49,50 The peak positions of the CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S are 23.2°, 22.6°, 22.2°, 21.9 °and 21.5°, respectively. The corresponding lattice spacing is 0.3911, 0.4008, 0.4077, 0.4130 and 0.4203 nm, which is consistent with the HRTEM test results.
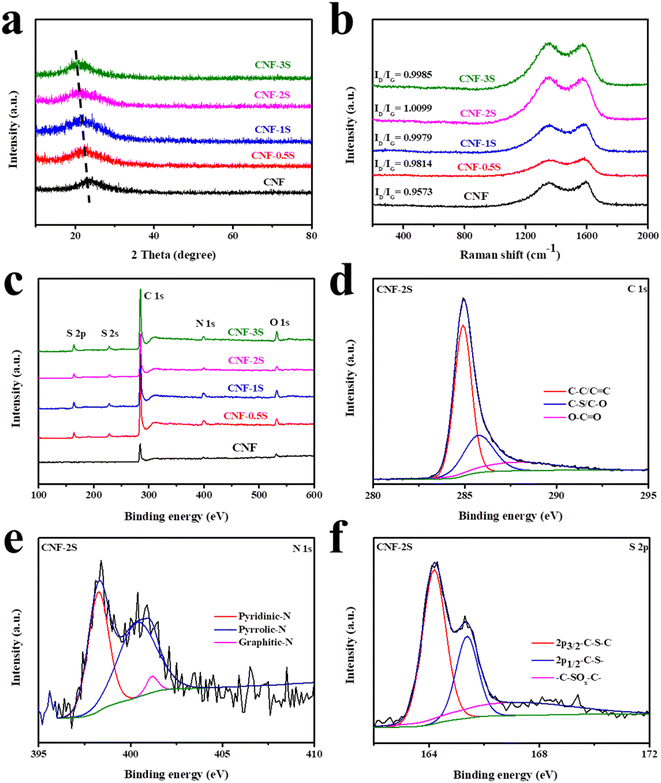 | ||
| Fig. 3 (a) XRD spectra, (b) Raman spectra, and (c) XPS full spectra of the CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S. (d) C 1s, (e) N 1s, and (f) S 2p spectra of CNF-2S. | ||
The curve shown in Fig. 3b was obtained by characterizing the sample using Raman spectroscopy. It can be seen that the carbon atom crystals' characteristic peaks are around 1300 cm−1 and 1580 cm−1, respectively. Among them, the peaks near 1580 cm−1 are G peaks due to sp2 hybridized carbon atoms' in-plane vibration, while the peak near 1300 cm−1, also known as the D peak, stands for the disordered vibration peak due to the lattice vibration of carbon atom crystal defect deviation from the Brillouin zone.55–57 By calculating the ratio of ID and IG, the graphitization degree and the defects of the samples can be analyzed. With the increase of sublimed sulfur content, the ID/IG value also increases. When the sublimed sulfur content is twice as much as the CNF, the ID/IG value of the material reaches the maximum value, indicating that there are more defects in the sample. As displayed in Fig. 3c, it can be seen that in the CNF there is a C 1s peak at around 285.8 eV, a N 1s peak at about 398.4 eV, and a O 1s peak located at 532.2 eV, while there is a S 2p peak around 164.8 eV and a S 2s peak around 227.4 eV after sulfur doping, indicating that the sulfur element is successfully doped into the CNF.58,59 Among them, Table S1† shows the specific content of each element in the samples. The content of the sulfur element in the CNF-0.5S, CNF-1S, CNF-2S and CNF-3S is 8.9%, 10.43%, 12.71% and 13.09%. In Fig. 3d–f, the results of peak fitting processing on the narrow spectrum of CNF-2S are shown. The C 1s peak can be fitted as three characteristic peaks representing C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–S/C–O and O–C
C, C–S/C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which are around 284.9 eV, 285.6 eV and 288 eV, respectively (Fig. 3d). In Fig. 3e, the N 1s peak is split into three peaks around 398.3 eV, 400.5 eV, and 401.2 eV, which represent pyridinic-N, pyrrolic-N, and graphitic-N, respectively. For the narrow spectrum of the S element, the main method is to fit the S 2p peak, and the result is revealed in Fig. 3f. It can be seen that S 2p is also fitted as three characteristic peaks, which belong to 2p3/2–C–S–C, 2p1/2–C–S– and –C–SOx–C–, and are located around 164.2 eV, 165.5 eV and 168 eV, respectively.52 Among them, the detection of the –C–S–C covalent bond once again confirmed the successful doping of the S element into the CNF, indicating that the previous EDS test result was accurate.
O, which are around 284.9 eV, 285.6 eV and 288 eV, respectively (Fig. 3d). In Fig. 3e, the N 1s peak is split into three peaks around 398.3 eV, 400.5 eV, and 401.2 eV, which represent pyridinic-N, pyrrolic-N, and graphitic-N, respectively. For the narrow spectrum of the S element, the main method is to fit the S 2p peak, and the result is revealed in Fig. 3f. It can be seen that S 2p is also fitted as three characteristic peaks, which belong to 2p3/2–C–S–C, 2p1/2–C–S– and –C–SOx–C–, and are located around 164.2 eV, 165.5 eV and 168 eV, respectively.52 Among them, the detection of the –C–S–C covalent bond once again confirmed the successful doping of the S element into the CNF, indicating that the previous EDS test result was accurate.
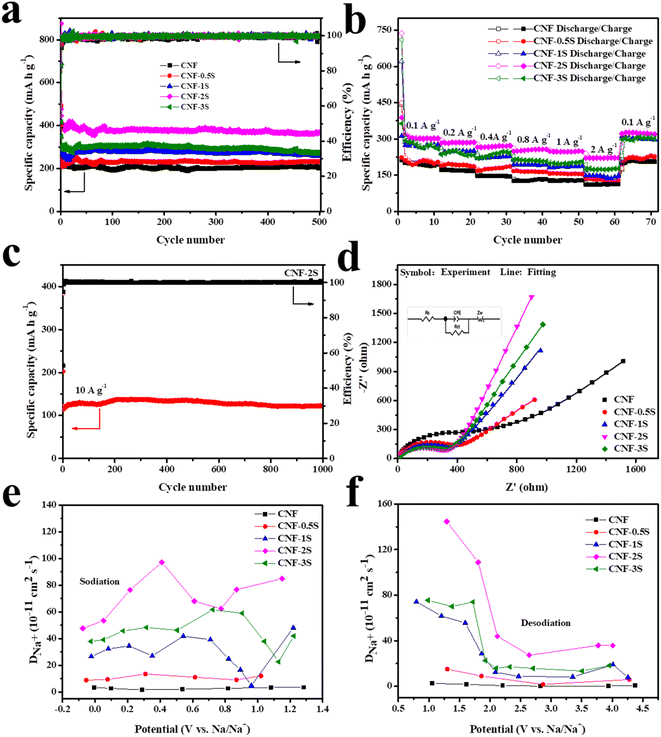
The electrochemical performance of the samples was tested by assembling CR2016 half-cells with a voltage range of 0.01–3 V (vs. Na/Na+). The CV curves of the CNF and CNF-2S in the first three cycles at a scanning speed of 0.1 mV s−1 are shown in Fig. 4a and b. As can be seen from the figure, two irreversible cathode peaks are located at 0.5 V and 1.2 V during the first discharge, which is because of the formation of the solid electrolyte interface (SEI) film. The redox peak pairs in the range of 0.01–0.2 V represent the intercalation and extraction process of Na+ between the carbon nanofiber layers. In addition, a pair of redox peaks of the CNF appeared at about 1 V, which represents the reaction among the functional groups on the surface of carbon fibers and sodium pieces. From Fig. 4b and S3,† it can be found that after S doping, the charge curve exhibits an oxidation peak at around 2.0 V, which represents the reaction between sodium ions and S atoms.49 After the first cycle, the CV curves almost coincide, which shows that the material has favorable electrochemical stability and reversibility. In Fig. 4c and d and S3,† the first three charge/discharge curves of the CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S at a current density of 0.1 A g−1 are revealed. The initial discharge/charge specific capacities of the CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S were 479.1/213.7, 493.6/242.3, 691.3/348.1, 874.1/476.3 and 679/346.7 mA h g−1, and the relevant coulombic efficiencies are 48.36%, 49.08%, 50.35%, 54.49% and 51.06%, respectively. The lower coulombic efficiency may be due to the formation of the SEI membrane and side reaction with the electrolyte. It is worth noting that the charge curves of CNF-nS exhibit a voltage plateau around 2 V, which conforms to the CV test results. In conclusion, sulfur doping can enhance the sodium storage capacity of the CNF.
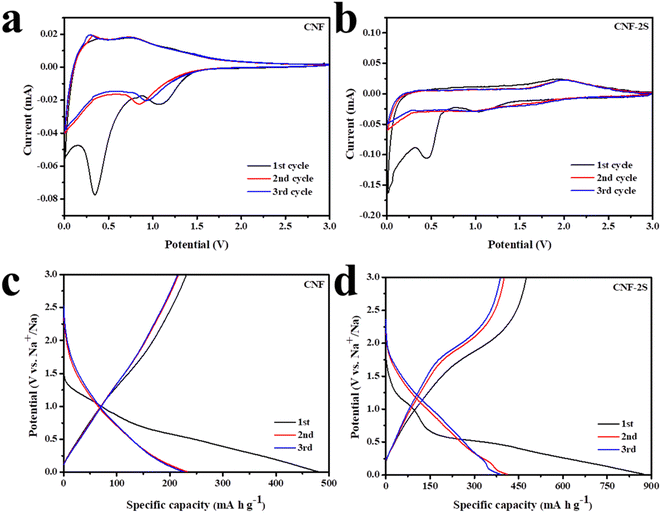 | ||
| Fig. 4 CV of (a) CNF and (b) CNF-2S with the scan speed of 0.1 mV s−1; the charge/discharge curves of the first three cycles of (c) CNF and (d) CNF-2S at 0.1 A g−1. | ||
The cycling performance of the CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S at 0.1 A g−1 is shown in Fig. 5a. It can be seen that the discharge specific capacities of the CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S after 500 cycles are 203.4, 232.6, 258.9, 368.2 and 273 mA h g−1, respectively. The rate capability of the CNF, CNF-0.5S, CNF-1S, CNF-2S, and CNF-3S is revealed in Fig. 5b. At the current densities of 0.1, 0.2, 0.4, 0.8, 1, and 2 A g−1, the reversible capacity of the CNF is 195.9, 168.5, 145.5, 133.3, 128.5, and 112.2 mA h g−1, which are worse than that of CNF-0.5S (202.1, 186.4, 184.8, 163.9, 155, and 130 mA h g−1), CNF-1S (276.6, 249.8, 228.7, 192.7, 187.5, and 145.1 mA h g−1), CNF-2S (302.6, 286.5, 272.6, 257.5, 249, and 222.7 mA h g−1) and CNF-3S (262.4, 246.6, 243.9, 207.4, 202.8, and 177.2 mA h g−1). It is worth noting that when the current density returns to 0.1 A g−1, the charge/discharge specific capacities of all electrodes are greater than the initial value, which may be due to the activation of the carbon materials.50,52 Because of the relatively favorable electrochemical properties of CNF-2S, its cycling performance at 10 A g−1 was tested (Fig. 5c). It was found that the discharge specific capacity of the CNF-2S electrode was 122 mA h g−1 after 1000 cycles. Compared with sulfur-doped carbon materials reported in the literature (Table S2†), CNF-2S has better electrochemical properties. Fig. 5d displays electrochemical impedance spectroscopy (EIS) spectra of the materials. The charge transfer impedance (Rct) of the electrode is represented by the semicircle in the mid-frequency region, while the slash in the low-frequency region is related to the diffusion process of Na+ in the electrode materials. The Rct values of the CNF, CNF-0.5S, CNF-1S, CNF-2S and CNF-3S are 588.6, 384.3, 339.9, 306.8 and 317.1 Ω, respectively. The Rct value of the CNF is the largest, showing that S doping promotes charge transfer and improves electrode conductivity. Meanwhile, the curves obtained through equivalent circuit fitting coincide with the test results, which demonstrate the rationality of the equivalent circuit.
In addition, GITT technology was used to investigate the diffusion behavior of sodium ions in materials. The sodium ion apparent diffusion coefficient (DNa+) can be obtained from the GITT curve (Fig. S4†) and the following eqn (1):
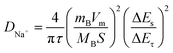 | (1) |
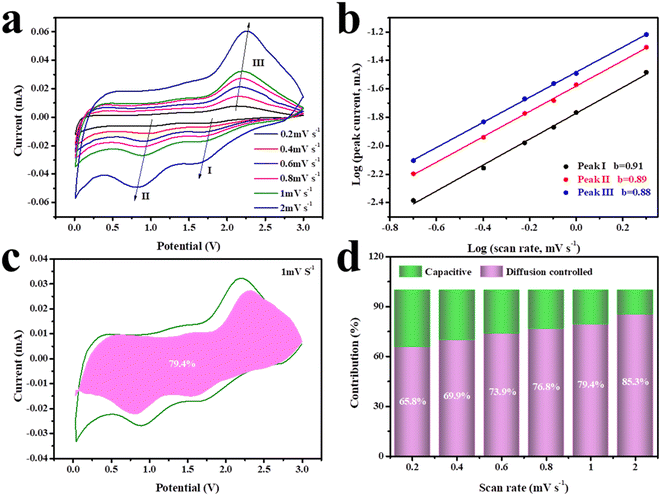
For the purpose of analyzing the sodium ion reaction kinetics of the CNF-2S electrode, its CV curves were recorded at various scan speeds ranging from 0.2 to 2 mV s−1, and the results are shown in Fig. 6. It can be seen that the shape of these CV curves is basically the same at different sweep speeds (Fig. 6a), which shows that the CNF-2S electrode has good rate properties. The connection between the peak current (i) of the oxidation/reduction peak of the electrode in the CV curves and the scanning speed (v) adheres to the following eqn (2):
log(i) = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v) + log(a) log(v) + log(a) | (2) |
| i(v) = k1v + k2v1/2 | (3) |
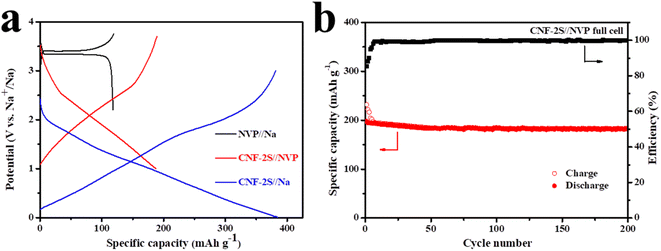
CNF-2S was used as the anode and NVP as the cathode to assemble a full battery, and the electrochemical properties were tested in the voltage range of 1–3.7 V to further understand the practical suitability of the CNF-2S anode for Na storage. Fig. 7a exhibits the charge and discharge curves of the NVP cathode, the CNF-2S anode, and the CNF-2S//NVP full cell at the 50th cycle at a current density of 0.1 A g−1. It can be seen that the charge and discharge voltage platform of the full cell (about 2.4 V) is basically the same as the voltage difference between the NVP cathode (about 3.4 V) and CNF-2S anode (about 1 V). At a current density of 0.1 A g−1, the charge/discharge specific capacity of the full battery is 182.6/182.4 mA h g−1 after 200 cycles, as displayed in Fig. 7b. These results further indicate that CNF-2S has good sodium storage properties and can be regarded as an anode material for sodium ion half/full cells.
4. Conclusion
In brief, we successfully prepared CNF-nS thin films by carbonizing and vulcanizing carbon nanofiber precursors synthesized by electrospinning. The results of XPS and TEM show that the sulfur element is successfully doped into carbon nanofibers, and the doping amount of sulfur in CNF-2S films is the largest. Compared with CNF, CNF-nS has more defects and greater interlayer spacing, which will be more advantageous to the sodium ion diffusion and electron transport, so the electrochemical properties of CNF-nS are superior to those of the CNF. The CNF-2S electrode reveals a discharge specific capacity of 368.2 mA h g−1 at a current density of 0.1 A g−1 after 500 cycles, and even at a current density of 10 A g−1, a reversible capacity of 122 mA h g−1 was also obtained after 1000 cycles. When combined with the NVP cathode to assemble a full cell, CNF-2S delivers a charge specific capacity of 182.6 mA h g−1 after 200 cycles at a current density of 0.1 A g−1. In a word, CNF-2S has a good prospect as an anode material for SIBs on account of its simple synthesis process and good sodium storage properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the Analytical & Testing Center of Northwestern Polytechnical University for equipment support.References
- X. Song, X. Li, H. Shan, J. Wang, W. Li, K. Xu, K. Zhang, H. M. K. Sari, L. Lei, W. Xiao, J. Qin, C. Xie and X. Sun, Adv. Funct. Mater., 2024, 34, 2303211 CrossRef CAS.
- H. Qian, H. Ren, Y. Zhang, X. He, W. Li, J. Wang, J. Hu, H. Yang, H. M. K. Sari, Y. Chen and X. Li, Electrochem. Energy Rev., 2022, 5, 2 CrossRef CAS.
- M. Guo, W. Tang, Y. Hong, B. Wei, J. Hu, F. Yu and C. Fan, Sci. China Mater., 2023, 66, 2621 CrossRef CAS.
- L. Wang, H. Tian, X. Yao, Y. Cai, Z. Gao and Z. Su, Chemelectrochem, 2024, 11, 414 Search PubMed.
- L. Song, Y. Wang, Y. Kuang, Y. Xia and Z. Xiao, CIESC J., 2022, 73, 4814 Search PubMed.
- J. Chen, G. Adit, L. Li, Y. Zhang, D. H. C. Chua and P. S. Lee, Energy Environ. Mater., 2023, 6, 12633 CrossRef.
- T. Yu, G. Li, Y. Duan, Y. Wu, T. Zhang, X. Zhao, M. Luo and Y. Liu, J. Alloys Compd., 2023, 958, 170486 CrossRef CAS.
- P. Molaiyan, G. S. Dos Reis, D. Karuppiah, C. M. Subramaniyam, F. Garcia-Alvarado and U. Lassi, Batteries, 2023, 9, 116 CrossRef CAS.
- A. Fereydooni, C. Yue and Y. Chao, Small, 2023, 230, 7275 Search PubMed.
- Y. Xi, X. Wang, H. Wang, M. Wang, G. Wang, J. Peng, N. Hou, X. Huang, Y. Cao, Z. Yang, D. Liu, X. Pu, G. Cao, R. Duan, W. Li, J. Wang, K. Zhang, K. Xu, J. Zhang and X. Li, Adv. Funct. Mater., 2024, 34, 2309701 CrossRef CAS.
- H. Raza, S. Bai, J. Cheng, S. Majumder, H. Zhu, Q. Liu, G. Zheng, X. Li and G. Chen, Electrochem. Energy Rev., 2023, 6, 29 CrossRef CAS.
- X. Bai, N. Wu, G. Yu and T. Li, Inorganics, 2023, 11, 289 CrossRef CAS.
- Y. She, X. Li, Y. Zheng, D. Chen, X. Rui, X. Lin and Y. Qin, Energy Environ. Mater., 2023, 7, 12538 CrossRef.
- Z. Y. Gu, X. T. Wang, Y. L. Heng, K. Y. Zhang, H. J. Liang, J. L. Yang, E. H. Ang, P. F. Wang, Y. You, F. Du and X. L. Wu, Sci. Bull., 2023, 68, 2302 CrossRef CAS PubMed.
- J. M. Cao, K. Y. Zhang, J. L. Yang, Z. Y. Gu and X. L. Wu, Chin. Chem. Lett., 2024, 35, 109304 CrossRef CAS.
- A. N. Singh, M. Islam, A. Meena, M. Faizan, D. Han, C. Bathula, A. Hajibabaei, R. Anand and K. W. Nam, Adv. Funct. Mater., 2023, 33, 2304617 CrossRef CAS.
- X. Yin, S. Sarkar, S. Shi, Q. A. Huang, H. Zhao, L. Yan, Y. Zhao and J. Zhang, Adv. Funct. Mater., 2020, 30, 2070071 CrossRef.
- E. Gabriel, C. Ma, K. Graff, A. Conrado, D. Hou and H. Xiong, Escience, 2023, 3, 100139 CrossRef.
- C. Peng, X. Xu, F. Li, L. Xi, J. Zeng, X. Song, X. Wan, J. Zhao and J. Liu, Small Struct., 2023, 4, 150 Search PubMed.
- Y. Xu, Y. Zhu, Y. Liu and C. Wang, Adv. Energy Mater., 2013, 3, 128 CrossRef CAS.
- M. S. Balogun, Y. Luo, W. Qiu, P. Liu and Y. Tong, Carbon, 2016, 98, 162 CrossRef CAS.
- S. Zhao, Z. Guo, J. Yang, C. Wang, B. Sun and G. Wang, Small, 2021, 17, 7431 Search PubMed.
- G. Wang, M. Shao, H. Ding, Y. Qi, J. Lian, S. Li, J. Qiu, H. Li and F. Huo, Angew. Chem., Int. Ed., 2019, 58, 13584 CrossRef CAS PubMed.
- J. M. Xie, R. Zhuang, Y. X. Du, Y. W. Pei, D. M. Tan and F. Xu, New Carbon Mater., 2023, 38, 305 CrossRef CAS.
- W. Chen, M. Wan, Q. Liu, X. Xiong, F. Yu and Y. Huang, Small Methods, 2019, 3, 1800323 CrossRef CAS.
- L. Zou, Y. Lai, H. Hu, M. Wang, K. Zhang, P. Zhang, J. Fang and J. Li, Chem. - Eur. J., 2017, 23, 14261 CrossRef CAS PubMed.
- Q. Li, Y. N. Zhang, S. Feng, D. Liu, G. Wang, Q. Tan, S. Jiang and J. Yuan, Int. J. Energy Res., 2020, 45, 7082 CrossRef.
- K. Y. Zhang, Y. Q. Fu, H. H. Liu, J. L. Yang, M. Y. Su, Y. Wang and X. L. Wu, Phys. Scr., 2023, 98, 125977 CrossRef.
- O. Mykhailiv, K. Brzezinski, B. Sulikowski, Z. Olejniczak, M. Gras, G. Lota, A. Molina-Ontoria, M. Jakubczyk, L. Echegoyen and M. E. Plonska-Brzezinska, Chem. - Eur. J., 2017, 23, 7132 CrossRef CAS PubMed.
- Y. Tang, X. Wang, J. Chen, D. Wang and Z. Mao, Chem. Eng. J., 2022, 427, 131951 CrossRef CAS.
- N. T. Aristote, C. Liu, X. Deng, H. Liu, J. Gao, W. Deng, H. Hou and X. Ji, J. Electroanal. Chem., 2022, 923, 116769 CrossRef CAS.
- L. Fu, K. Tang, K. Song, P. A. van Aken, Y. Yu and J. Maier, Nanoscale, 2014, 6, 1384 RSC.
- D. Qin, Z. Liu, Y. Zhao, G. Xu, F. Zhang and X. Zhang, Carbon, 2018, 130, 664 CrossRef CAS.
- J. Liu, Y. Zhang, L. Zhang, F. Xie, A. Vasileff and S. Z. Qiao, Adv. Mater., 2019, 31, 1901261 CrossRef PubMed.
- Z. Li, Y. Cao, G. Li, L. Chen, W. Xu, M. Zhou, B. He, W. Wang and Z. Hou, Electrochim. Acta, 2021, 366, 137466 CrossRef CAS.
- X. C. Xie, H. L. Shuai, X. Wu, K. J. Huang, L. N. Wang, R. M. Wang and Y. Chen, J. Alloys Compd., 2020, 847, 156288 CrossRef CAS.
- J. Sun, Y. Sun, J. A. S. Oh, Q. Gu, W. Zheng, M. Goh, K. Zeng, Y. Cheng and L. Lu, J. Energy Chem., 2021, 62, 497 CrossRef CAS.
- B. Yuan, L. Zeng, X. Sun, Y. Yu and Q. Wang, Nano Res., 2018, 11, 2256 CrossRef CAS.
- D. S. Bin, Y. Li, Y. G. Sun, S. Y. Duan, Y. Lu, J. Ma, A. M. Cao, Y. S. Hu and L. J. Wan, Adv. Energy Mater., 2018, 8, 1800855 CrossRef.
- L. Zhang, H. Dong, X. He, L. Li, L. Li, X. Wu and S. Chou, Chem. J. Chin. Univ., 2023, 44, 20220620 Search PubMed.
- W. Hong, Y. Zhang, L. Yang, Y. Tian, P. Ge, J. Hu, W. Wei, G. Zou, H. Hou and X. Ji, Nano Energy, 2019, 65, 104038 CrossRef CAS.
- H. Hou, C. E. Banks, M. Jing, Y. Zhang and X. Ji, Adv. Mater., 2015, 27, 7861 CrossRef CAS PubMed.
- Z. Xu, Y. Huang, C. Chen, L. Ding, Y. Zhu, Z. Zhang and Z. Guang, Ceram. Int., 2020, 46, 4532 CrossRef CAS.
- J. Hao, L. Xu, J. Bai, X. Wang, Q. Guo, Y. Wang, Y. Yang and J. Zhao, Ionics, 2021, 27, 667 CrossRef CAS.
- Y. Chen, X. Guo, A. Liu, H. Zhu and T. Ma, Sustainable Energy Fuels, 2021, 5, 3017 RSC.
- M. Wang, D. Li, G. Li, Y. Li, D. S. Butenko, G. Milinevsky, J. Li and W. Han, Appl. Surf. Sci., 2022, 605, 154633 CrossRef CAS.
- W. Chen, X. Liu, J. Wu, Q. Wang, Y. Zhang, S. Yan, P. Hou and S. Luo, J. Electroanal. Chem., 2023, 929, 117095 CrossRef CAS.
- Y. Luo, Y. Xu, X. Li, K. Zhang, Q. Pang and A. Qin, Nanomaterials, 2023, 13, 881 CrossRef CAS PubMed.
- B. Long, R. Zhao, J. Zhang, L. Wang, X. Chen, Y. Du, G. Yuan, Z. Dong and X. Li, J. Mater. Sci., 2022, 57, 17711 CrossRef CAS.
- D. Chen, Z. Huang, S. Sun, H. Zhang, W. Wang, G. Yu and J. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 44369 CrossRef CAS PubMed.
- M. Yu, Z. Yin, G. Yan, Z. Wang, H. Guo, G. Li, Y. Liu, L. Li and J. Wang, J. Power Sources, 2020, 449, 227514 CrossRef CAS.
- Q. Yu, T. Dong, R. Qiu and H. Wang, Mater. Res. Bull., 2021, 138, 111211 CrossRef CAS.
- X. Sun, C. Wang, Y. Gong, L. Gu, Q. Chen and Y. Yu, Small, 2018, 14, 2218 Search PubMed.
- O. Pech and S. Maensiri, J. Alloys Compd., 2019, 781, 541 CrossRef CAS.
- T. Yin, Z. Zhang, L. Xu, C. Li and D. Han, Chemistryopen, 2024, 10, 178 Search PubMed.
- G. Shen, B. Li, Y. Xu, X. Chen, S. Katiyar, L. Zhu, L. Xie, Q. Han, X. Qiu, X. Wu and X. Cao, J. Colloid Interface Sci., 2024, 653, 1588 CrossRef CAS PubMed.
- Y. Qiao, M. Ma, Y. Liu, S. Li, Z. Lu, H. Yue, H. Dong, Z. Cao, Y. Yin and S. Yang, J. Mater. Chem. A, 2016, 4, 15565 RSC.
- D. Xu, H. Wang, R. Qiu, Q. Wang, Z. Mao, Y. Jiang, R. Wang, B. He, Y. Gong, D. Li and X. Hu, Energy Storage Mater., 2020, 28, 91 CrossRef.
- H. Zhai, H. Jiang, Y. Qian, X. Cai, H. Liu, Y. Qiu, M. Jin, F. Xiu, X. Liu and L. Lai, Mater. Chem. Phys., 2020, 240, 122139 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se00478g |
| This journal is © The Royal Society of Chemistry 2024 |