Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dinuclear platinum(II) complexes emitting through TADF: new ligand design to minimise aggregation and the S1–T1 energy gap†
Piotr
Pander‡
 *ab,
Yana M.
Dikova‡
*ab,
Yana M.
Dikova‡
 c,
Emma V.
Puttock
c,
Emma V.
Puttock
 c and
J. A. Gareth
Williams
c and
J. A. Gareth
Williams
 *c
*c
aFaculty of Chemistry, Silesian University of Technology, M. Strzody 9, 44-100 Gliwice, Poland. E-mail: piotr.pander@polsl.pl
bCentre for Organic and Nanohybrid Electronics, Silesian University of Technology, Konarskiego 22B, 44-100 Gliwice, Poland
cDepartment of Chemistry, Durham University, South Road, Durham, DH1 3LE, UK
First published on 18th September 2024
Abstract
Dinuclear platinum(II) complexes of a new, ditopic, bis-tridentate NCN–NCN-coordinating ligand, appended with four mesityl groups, are reported. The high radiative rate constants and correspondingly efficient luminescence of the complexes involves thermally activated delayed fluorescence (TADF), thanks to a near-zero energy gap between the S1 and T1 states. The mesityl groups also serve to hinder the aggregation that was detrimental to electroluminescence efficiency in previous studies, allowing a ∼4-fold increase in OLED efficiency to be achieved (i.e. from 2.3% previously to 10% in this work). Oxidation of one of the Pt(II) complexes led to a dinuclear Pt(IV) complex of unprecedented structure.
Introduction
Organoplatinum(II) complexes are widely used as the luminophore or sensitizer in diverse applications, often complementing their iridium(III) counterparts.1–3 Important uses include bioimaging,4–9 photodynamic therapy,10 photocatalysis,11–14 and organic light-emitting diodes (OLEDs).15–18 The luminescence displayed by such complexes is normally considered phosphorescence, whereby the high spin–orbit coupling associated with the heavy metal relaxes the spin selection rule, accelerating the rate of the otherwise forbidden T1 → S0 process.2,19 However, recent findings by some of the present authors have revealed that another mechanism may be at work in at least some such complexes, involving thermally activated delayed fluorescence (TADF).20–22 Dinuclear Pt(II) complexes of ditopic ligands featuring a pyrimidine bridge were found to have a small energy gap ΔEST between the lowest singlet S1 and triplet T1 excited states.21 It leads to a shortening of the decay lifetime by thermal activation of T1 to S1 and subsequent emission through the allowed S1 → S0 transition (i.e., following the TADF model).23,24 A similar phenomenon has since been observed by others in a mononuclear Pt(II) complex,25 and the future discovery of many further examples seems likely. While preparing this manuscript, another work reporting both mono- and dinuclear Pt(II) complexes showing TADF has been published, reporting high OLED efficiency and low efficiency roll-off.26 This further underscores the potential advantages that TADF complexes of this metal offer.In the present work, we took our original ligand design and appended it with four 2,4,6-trimethylphenyl (mesityl) groups, with a view to improving the solubility of the resulting complex and reducing aggregation. We also show how this seemingly otherwise insignificant structural change leads to a reduction in ΔEST, which in turn enhances the TADF contribution to emission and substantially improves performance.
Results and discussion
Synthesis
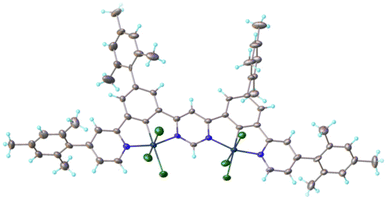
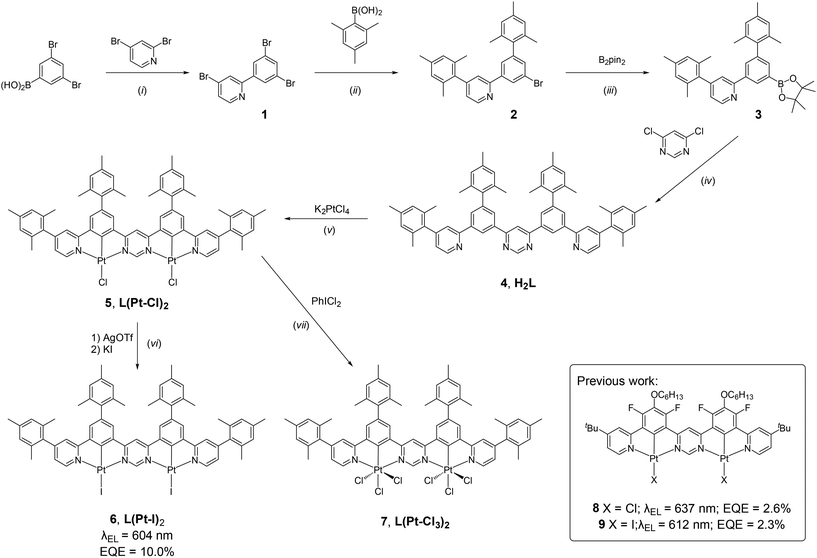
The new ditopic NCN–NCN proligand H2L (compound 4 in Scheme 1) was prepared by a sequence of Pd-catalysed cross-coupling reactions from readily obtainable starting materials; full experimental details and the characterisation of new compounds are given in the ESI.† Compound H2L was platinated using K2PtCl4 in acetic acid to give the dinuclear complex L(Pt–Cl)2 (5), from which the iodo derivative L(Pt–I)2 (6) was prepared by metathesis of the monodentate ligand upon treatment with Ag(SO3CF3) followed by KI. We were unable to obtain crystals of 5 or 6 of sufficient quality for an X-ray diffraction study, but the oxidation of 5 with PhICl2 led cleanly to L(Pt–Cl3)2 (7), a dinuclear Pt(IV) complex of unprecedented structure that was amenable to crystallography. Although the thrust of the current work is the Pt(II) systems, there is growing interest in the less widely explored +4 oxidation state,27,28 and 7 represents an interesting structure in that context for future elaboration by replacement of the chloride ligands.29 Here, it offers insight into the likely structures of 5 and 6. The structure (Fig. 1) shows the two Pt centres in very similar pseudo-octahedral environments, each bound to an NCN unit with three chlorides completing the coordination sphere. The torsion angles between the mesityl and phenyl rings are in the range 69 ± 3° and, between the pyridyl and mesityl rings, 69 ± 5°. These angles are a little less than that of 80° in the related mesityl-appended NCHN proligand,30 suggesting a slightly greater degree of conjugation across the rings upon complexation, attenuating the steric preference for orthogonality.DFT and TD-DFT calculations
The excited states in 5 and 6 were probed by DFT/TD-DFT at the B3LYP/def2-SVP level of theory using ORCA 5.0.3,31,32 and by quasi-degenerate perturbation theory (QDPT).33,34 The optimised T1 geometries (Fig. 2) show torsions between the plane of the NCN–NCN unit and the pendent mesityl rings that are similar to those observed experimentally in the Pt(IV) complex (atomic coordinates for T1 and S0 are given as separate ESI†). The mesityl groups thus form a congested shield around the complex, which is expected to inhibit intermolecular interactions between NCN–NCN planes.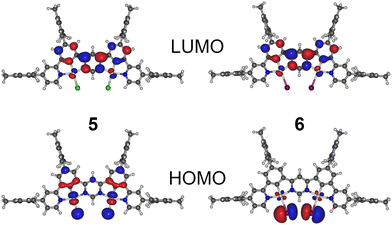 | ||
| Fig. 2 HOMO and LUMO of complexes 5 and 6 calculated using the B3LYP/def2-SVP//B3LYP/def2-TZVP level of theory. | ||
The frontier molecular orbital isosurfaces of 5 and 6 (Fig. 2) resemble those of 8 and 9,21,22 with the LUMO distributed over the pyrimidine linker and the neighbouring benzene rings, but also including a small admixture of d orbitals from both Pt(II) centres. The HOMO comprises d orbitals of both the Pt centres and p orbitals of their respective monodentate halide ligands. For 6, there are no significant contributions from other parts of the molecule, such that the degree of HOMO–LUMO overlap is small. In 5, the HOMO includes additional contributions from π orbitals of the organic ligand, which serves to increase the HOMO–LUMO overlap. In both complexes, the S1 and T1 are associated mainly with the HOMO → LUMO transition, and thus may be classed as predominantly MXLCT or metal-halogen-to-ligand charge-transfer in character. Complex 6 appears to display a stronger MXLCT character of the excited state, while 5 shows a rather MXLCT + LC (LC – ligand-centred) character due to a clear contribution of the NCN–NCN ligand to HOMO.
When spin–orbit coupling (SOC) is included in the calculations, excited states of predominantly triplet or singlet nature are identified (Fig. S4.1, Tables S4.1 and S4.2†). The lowest triplet state splits into three closely separated levels, the separation between the first and third, ΔE1–3, being the zero-field splitting (ZFS). Values of 2 meV (13 cm−1) and 15 meV (119 cm−1) are calculated for 5 and 6 respectively. In 5, state 7 is the first excited state with predominant singlet character (68%; states 4–6 are associated with T2); thus ΔEST = ΔE1–7, which is calculated to be 291 meV (2345 cm−1). For 6, the first predominantly singlet excited state (81.4%) is state 4, and ΔEST = ΔE1–4 = 19 meV (149 cm−1). The trend to smaller ΔEST upon changing X from Cl to I is consistent with that observed previously for 8 and 9.
Solution-state photophysics
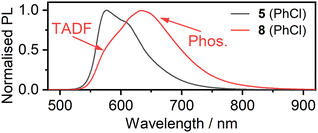
The solvent of choice for photophysical measurements is toluene (PhMe) (based on 9 having previously shown the highest radiative rate in this solvent22). Here, we studied 6 in toluene, but 5 has poorer solubility and intermolecular interactions suppress its emission leading to a lower ΦPL. We believe that the larger diameter of the X = I as compared to X = Cl hinders close π⋯π and Pt⋯Pt intermolecular interactions, leading to the better overall solubility of 6. This is in line with our previous work where the X = I auxiliary ligand was found to disrupt aggregation of the closely related mono-Pt(II) complexes of the Pt(NCN)-X type.35 The full characterisation of 5 was therefore performed in chlorobenzene (PhCl), where such interactions were essentially absent.§,¶ There is substantial overlap between the photoluminescence (PL) spectrum and the lowest-energy absorption band in both complexes (Fig. 3) – a clear indication that the PL originates not from the lowest-energy T1 state but rather from a higher state, and a strong clue for the involvement of TADF.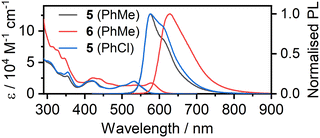 | ||
| Fig. 3 Absorption and photoluminescence spectra of 5 and 6 in dilute (c = 10−5 M) toluene (PhMe) and (for 5) chlorobenzene (PhCl) solutions.§,¶. | ||
The PL spectrum of 6 is very similar to that of the analogous complex 9 (λPLmax = 627 and 628 nm respectively),22 but the emission spectrum of 5 (λPLmax = 576 nm) is drastically different from that of 8 (λPLmax = 617 nm), Fig. 4. The difference between the PL of 5 and 8 in chlorobenzene can be attributed to a significantly smaller ΔEST in the former with TADF dominating the spectrum at RT as opposed to 8 with phosphorescence being the dominant component. As the PL onsets are similar in either case, the S1 energy is likely comparable in both complexes, but the structural differences between the two luminophores lead to a higher T1 energy in 5 (Table S5.2†).
The PL spectra of the complexes in solution are sensitive to temperature (Fig. S5.1 and S5.2†), in line with the TADF mechanism. For 5 in PhCl, the interplay between phosphorescence and TADF at intermediate temperatures is evident, as in 8. For 6, however, the PL spectrum blue shifts very slightly at lower temperatures, indicating a visible influence of suppressed molecular motion. Even at 160 K, there is no evidence of phosphorescence (in stark contrast to the behaviour of 9 for example), suggesting that ΔEST in 6 is very small indeed, with TADF consequently predominating even at low temperatures.
Complexes 5 and 6 display unusually large radiative decay rate constants kr for platinum(II) complexes: τ = 0.34 μs, ΦPL = 0.11, kr = 3.3 × 105 s−1 for 5 in PhMe [τ = 2.1 μs, ΦPL = 0.45, kr = 2.1 × 105 s−1 in PhCl]; τ = 0.40 μs, ΦPL = 0.23, kr = 5.7 × 105 s−1 for 6 in PhMe.|| The kr values are significantly higher than for 8 and 9 (Table S5.1†), suggesting more efficient TADF. We use the method of Strickler and Berg36 to estimate the singlet-state radiative rate constants kSr and f(S1 → S0) oscillator strengths, from the lowest energy absorption bands.37,38 For 5 in PhCl, kSr = 5.3 × 107 s−1 and f(S1 → S0) = 0.12; corresponding values for 6 in PhMe are 3.3 × 107 s−1 and 0.09. The values are two orders of magnitude higher than the experimental values, showing (as expected) that the emission is not fluorescence, but rather pointing to the likely involvement of TADF.
Solid-state photophysics
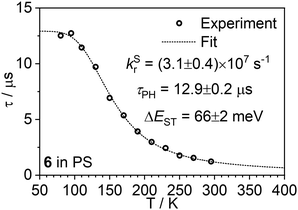
More conclusive evidence of a TADF mechanism comes from the temperature dependence of the complexes’ PL spectra in a dilute polystyrene (PS) film, over the range 295–80 K (Fig. 5). The spectra display a clear change, shifting to the red as the temperature decreases, from which it is apparent that TADF dominates at 295 K, while phosphorescence from the lower-energy T1 state dominates at 80 K. The evolution of the spectra correlates with a change in the radiative lifetime (Fig. 6, Fig. S5.4 and S5.5†). Fitting of the lifetime data of 6 to an established Boltzmann-based expression (eqn (S2)†) gives a natural fluorescence lifetime τ0 ≈ 32 ns, phosphorescence lifetime τPH ≈ 13 μs, and ΔEST ≈ 66 meV, not dissimilar to the value calculated by TD-DFT. The kSr figures for both complexes (∼107 s−1) are in good agreement with the estimates obtained using the Strickler–Berg method. It is striking that the radiative lifetime at 295 K is an order of magnitude shorter than at 80 K thanks to the involvement of TADF, underscoring how TADF can benefit Pt(II) complexes despite the heavy atom effect that might otherwise be expected to funnel emission through phosphorescence.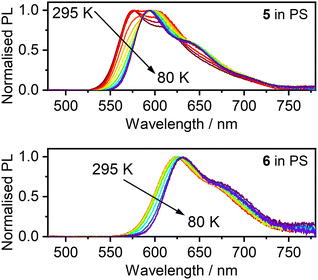 | ||
| Fig. 5 PL spectra of 5 and 6 in polystyrene dispersion (c = 0.2% w/w) over the temperature range indicated. | ||
OLED devices
Owing to the good PL properties of 6 and its low susceptibility to aggregative quenching (in contrast to 5), it was tested as an emitter in a solution-processed organic light-emitting diode (OLED). Three pairs of device architectures (Tables S6.1 and S6.3†) were trialled to optimise the efficiency: Devs 1, 3, and 5 at 3% w/w loading of the complex in the host, and Devs 2, 4, and 6 at 5%, to probe the effect of concentration. The pair of devices 1 and 2 use a simple structure with a relatively thick TCTA:PO-T2T host emissive layer.39 Devices 3 and 4 feature a mCP:PO-T2T host. Devices 5 and 6 employ a mCP:PBD host with a poly(vinyl-carbazole) hole-transporting and electron-blocking layer (as previously used with 9 and hence serving as a reference22). The OLED data are presented in Fig. 7, Fig. S6.1–S6.8 and Table S6.1.†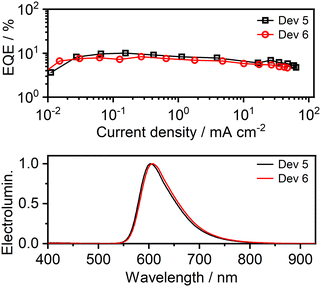 | ||
| Fig. 7 Characteristics of OLEDs 5 and 6: (top) External quantum efficiency (EQE); (bottom) EL spectra. | ||
Comparison of Devices 5 and 6 with those reported previously using 9 shows that the use of the new complex leads to a ∼4-fold higher EQE (up to 10%) than complex 9, and a ∼2–4-fold higher luminance (up to 8700 cd m−2). Although several factors may have contributed to this result, the main one appears to be suppression of emitter aggregation in the solid state by the mesityl substituents. This effect can be identified from a comparison of the respective EL spectra (Fig. 8). Despite identical emitter loading and OLED structure, Device 6 displays a visibly narrower EL spectrum, lacking the long wavelength tail >700 nm that the previously reported Device 7 shows (using complex 9). That tail is due to dimeric or oligomeric species formed through aggregation; it is detrimental both to the overall OLED EQE and to colour purity.** Thus, the encumbered molecular design is successful in promoting efficiency in OLEDs using dinuclear Pt(II) TADF emitters.
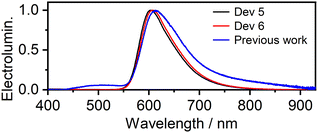 | ||
| Fig. 8 Comparison of the EL spectra of OLEDs 5 and 6 with the EL spectrum of OLED 7 (using complex 9) from previous work.22 | ||
Conclusions
In conclusion, the design of ligand 4 – featuring a ditopic NCN–NCN core decorated with four mesityl units – successfully limits aggregation of complex 6, inhibiting the detrimental effect on EL in an OLED. Complex 6 is highly soluble in toluene and other solvents and displays a lower susceptibility to aggregation in the solid state than the previously reported complex 9. The hexyl chains and t-butyl substituents in 9 are insufficient to impede intermolecular interactions, whereas the mesityl groups of 6 do so. The solution-processed OLEDs reach an EQE of 10% with a maximum luminance of 5400 cd m−2 (Dev 5), or EQE of 9.2% with a maximum luminance of 8700 cd m−2 (Dev 3). These figures exceed by ∼4-fold the values reported for the structurally similar complex 9, and are the highest values reported for a TADF-based Pt(II) emitter. Moreover, the use of ligand 4 also leads to a significant reduction of the ΔEST (e.g., to 0.07 eV in 5 in PS, compared to 0.20 eV reported for complex 8). This is achieved by increasing the T1 energy in complex 5.The high solubility attained using ligand 4 could also be exploited to oxidise the dinuclear Pt(II) 5 complex to the corresponding dinuclear Pt(IV) complex 7. Analogous attempts to prepare such materials from related, less substituted NCN–NCN ligands have led to intractable mixtures, probably due to poor solubility.
In summary, this study has shown how modifying the design of rigid, ditopic, bis-tridentate ligands can simultaneously lead to improvements in several properties of the corresponding Pt2 complexes, including enhanced solubility, lower propensity to aggregation, and a reduction in ΔEST that serves to accelerate radiative decay through TADF. We expect TADF to be implicated in the emission of many other 3rd row phosphors and that these findings will help inform the future design of such molecules.
Author contributions
P. P. – Conceptualization, funding acquisition, project administration, formal analysis, investigation (synthesis, photophysics, OLED devices, computations), visualization, writing – original draft, writing – review & editing; Y. M. D. – investigation (synthesis), writing – review & editing; E. V. P. – conceptualization, investigation (synthesis), writing – review & editing; J. A. G. W. – conceptualization, funding acquisition, resources, project administration, supervision, writing – original draft, writing – review & editing.Data availability
Our supporting research data is available from the Durham Research Online DATAsets Archive (DRO-DATA) open data repository. DOI: https://doi.org/10.15128/r2tt44pm914.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Dr Dmitry Yufit for determining the structure of compound 7 through X-ray diffraction. P. P. thanks the National Science Centre, Poland for funding under grant no. 2022/47/D/ST4/01496, and the Rector's pro-quality grant, Silesian University of Technology, Poland, grant no: 04/040/RGJ24/0279. E. V. P. and J. A. G. W. acknowledge funding from EPRSC (grant ref. EP/S012788/1). Y. M. D. thanks Durham University Chemistry for partial support of a PhD studentship. P. P. thanks Dr F. B. Dias and Prof. A. P. Monkman for access to facilities. This work made use of the Hamilton High Performance Computing Service of Durham University.References
- P. L. dos Santos, P. Stachelek, Y. Takeda and P. Pander, Recent advances in highly-efficient near infrared OLED emitters, Mater. Chem. Front., 2024, 8, 1731–1766 RSC.
- K. Li, G. S. M. Tong, Q. Wan, G. Cheng, W. Tong, W.-H. Ang, W.-L. Kwong and C. M. Che, Highly phosphorescent platinum(II) emitters: photophysics, materials and biological applications, Chem. Sci., 2016, 7, 1653–1673 RSC.
- B. Ma, P. I. Djurovich, S. Garon, B. Alleyne and M. E. Thompson, Platinum Binuclear Complexes as Phosphorescent Dopants for Monochromatic and White Organic Light-Emitting Diodes, Adv. Funct. Mater., 2006, 16, 2438–2446 CrossRef CAS.
- W. A. Tarran, G. R. Freeman, L. Murphy, A. M. Benham, R. Kataky and J. A. G. Williams, Platinum(II) Complexes of N ∧ C ∧ N-Coordinating 1,3-Bis(2-pyridyl)benzene Ligands: Thiolate Coligands Lead to Strong Red Luminescence from Charge-Transfer States, Inorg. Chem., 2014, 53, 5738–5749 CrossRef CAS PubMed.
- M. Mauro, A. Aliprandi, D. Septiadi, N. S. Kehr and L. De Cola, When self-assembly meets biology: luminescent platinum complexes for imaging applications, Chem. Soc. Rev., 2014, 43, 4144–4166 RSC.
- C.-K. Koo, K.-L. Wong, C. W.-Y. Man, Y.-W. Lam, L. K.-Y. So, H.-L. Tam, S.-W. Tsao, K.-W. Cheah, K.-C. Lau, Y.-Y. Yang, J.-C. Chen and M. H.-W. Lam, A Bioaccumulative Cyclometalated Platinum(II) Complex with Two-Photon-Induced Emission for Live Cell Imaging, Inorg. Chem., 2009, 48, 872–878 CrossRef CAS PubMed.
- P. Wu, E. L. Wong, D. Ma, G. S. Tong, K. Ng and C. Che, Cyclometalated Platinum(II) Complexes as Highly Sensitive Luminescent Switch–On Probes for Practical Application in Protein Staining and Cell Imaging, Chem. – Eur. J., 2009, 15, 3652–3656 CrossRef CAS PubMed.
- K. Mitra, C. E. Lyons and M. C. T. Hartman, A Platinum(II) Complex of Heptamethine Cyanine for Photoenhanced Cytotoxicity and Cellular Imaging in Near–IR Light, Angew. Chem., Int. Ed., 2018, 57, 10263–10267 CrossRef CAS.
- J. Wu, B. Xu, Y. Xu, L. Yue, J. Chen, G. Xie and J. Zhao, Reblooming of the cis -Bis(2-phenylpyridine) Platinum(II) Complex: Synthesis Updating, Aggregation-Induced Emission, Electroluminescence, and Cell Imaging, Inorg. Chem., 2023, 62, 19142–19152 CrossRef CAS PubMed.
- A. Upadhyay, A. Nepalia, A. Bera, D. K. Saini and A. R. Chakravarty, A Platinum(II) Boron–dipyrromethene Complex for Cellular Imaging and Mitochondria–targeted Photodynamic Therapy in Red Light, Chem. – Asian J., 2023, 18, 1–11 Search PubMed.
- P. K. Chow, C. Ma, W. P. To, G. S. M. Tong, S. L. Lai, S. C. F. Kui, W. M. Kwok and C. M. Che, Strongly phosphorescent palladium(II) complexes of tetradentate ligands with mixed oxygen, carbon, and nitrogen donor atoms: Photophysics, photochemistry, and applications, Angew. Chem., Int. Ed., 2013, 52, 11775–11779 CrossRef CAS PubMed.
- M. Yoshida, K. Saito, H. Matsukawa, S. Yanagida, M. Ebina, Y. Maegawa, S. Inagaki, A. Kobayashi and M. Kato, Immobilization of luminescent Platinum(II) complexes on periodic mesoporous organosilica and their water reduction photocatalysis, J. Photochem. Photobiol., A, 2018, 358, 334–344 CrossRef CAS.
- D. Gómez de Segura, A. Corral-Zorzano, E. Alcolea, M. T. Moreno and E. Lalinde, Phenylbenzothiazole-Based Platinum(II) and Diplatinum(II) and (III) Complexes with Pyrazolate Groups: Optical Properties and Photocatalysis, Inorg. Chem., 2024, 63, 1589–1606 CrossRef PubMed.
- P. Domingo-Legarda, A. Casado-Sánchez, L. Marzo, J. Alemán and S. Cabrera, Photocatalytic Water-Soluble Cationic Platinum(II) Complexes Bearing Quinolinate and Phosphine Ligands, Inorg. Chem., 2020, 59, 13845–13857 CrossRef CAS PubMed.
- Y.-C. Wei, S. F. Wang, Y. Hu, L.-S. Liao, D.-G. Chen, K.-H. Chang, C.-W. Wang, S.-H. Liu, W.-H. Chan, J.-L. Liao, W.-Y. Hung, T.-H. Wang, P.-T. Chen, H.-F. Hsu, Y. Chi and P.-T. Chou, Overcoming the energy gap law in near-infrared OLEDs by exciton–vibration decoupling, Nat. Photonics, 2020, 14, 570–577 CrossRef CAS.
- S.-F. Wang, B.-K. Su, X.-Q. Wang, Y.-C. Wei, K.-H. Kuo, C.-H. Wang, S.-H. Liu, L.-S. Liao, W.-Y. Hung, L.-W. Fu, W.-T. Chuang, M. Qin, X. Lu, C. You, Y. Chi and P.-T. Chou, Polyatomic molecules with emission quantum yields 20% enable efficient organic light-emitting diodes in the NIR(II) window, Nat. Photonics, 2022, 16, 843–850 CrossRef CAS.
- G. Cheng, P.-K. Chow, S. C. F. Kui, C.-C. Kwok and C.-M. Che, High–Efficiency Polymer Light–Emitting Devices with Robust Phosphorescent Platinum(II) Emitters Containing Tetradentate Dianionic O ∧ N ∧ C ∧ N Ligands, Adv. Mater., 2013, 25, 6765–6770 CrossRef CAS PubMed.
- Y. Yuan, J. L. Liao, S. F. Ni, A. K. Y. Jen, C. S. Lee and Y. Chi, Boosting Efficiency of Near-Infrared Organic Light-Emitting Diodes with Os(II)-Based Pyrazinyl Azolate Emitters, Adv. Funct. Mater., 2020, 30, 1–8 Search PubMed.
- H. Yersin, Highly Efficient OLEDs with Phosphorescent Materials, Wiley, 2008 Search PubMed.
- P. Pander, R. Daniels, A. V. Zaytsev, A. Horn, A. Sil, T. J. Penfold, J. A. G. Williams, V. N. Kozhevnikov and F. B. Dias, Exceptionally fast radiative decay of a dinuclear platinum complex through thermally activated delayed fluorescence, Chem. Sci., 2021, 12, 6172–6180 RSC.
- P. Pander, A. V. Zaytsev, A. Sil, J. A. G. Williams, P.-H. Lanoe, V. N. Kozhevnikov and F. B. Dias, The role of dinuclearity in promoting thermally activated delayed fluorescence (TADF) in cyclometallated, N^C^N-coordinated platinum(II) complexes, J. Mater. Chem. C, 2021, 9, 10276–10287 RSC.
- P. Pander, A. V. Zaytsev, A. Sil, J. A. G. Williams, V. N. Kozhevnikov and F. B. Dias, Enhancement of thermally activated delayed fluorescence properties by substitution of ancillary halogen in a multiple resonance-like diplatinum(II) complex, J. Mater. Chem. C, 2022, 10, 4851–4860 RSC.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang and W. Huang, Thermally Activated Delayed Fluorescence Materials Towards the Breakthrough of Organoelectronics, Adv. Mater., 2014, 26, 7931–7958 CrossRef CAS.
- A. Russegger, S. M. Fischer, A. C. Debruyne, H. Wiltsche, A. D. Boese, R. I. Dmitriev and S. M. Borisov, Tunable Self-Referenced Molecular Thermometers via Manipulation of Dual Emission in Platinum(II) Pyridinedipyrrolide Complexes, ACS Appl. Mater. Interfaces, 2024, 16, 11930–11943 CrossRef CAS.
- J.-G. Yang, N. Li, J. Li, X.-F. Song, M.-D. Li, J. Zhang and K. Li, A thermally activated delayed fluorescent platinum(II) complex for red organic light emitting diodes with high efficiencies and small roll-off, J. Mater. Chem. A, 2024, 12, 18977–18985 RSC.
- F. Juliá, D. Bautista, J. M. Fernández-Hernández and P. González-Herrero, Homoleptic tris-cyclometalated platinum(IV) complexes: a new class of long-lived, highly efficient 3 LC emitters, Chem. Sci., 2014, 5, 1875–1880 RSC.
- R. J. Ortiz, J. D. Braun, J. A. G. Williams and D. E. Herbert, Brightly Luminescent Platinum Complexes of N ∧ C – ∧ N Ligands Forming Six-Membered Chelate Rings: Offsetting Deleterious Ring Size Effects Using Site-Selective Benzannulation, Inorg. Chem., 2021, 60, 16881–16894 CrossRef CAS.
- Y. M. Dikova, D. S. Yufit and J. A. G. Williams, Platinum(IV) Complexes with Tridentate, NNC -Coordinating Ligands: Synthesis, Structures, and Luminescence, Inorg. Chem., 2023, 62, 1306–1322 CrossRef CAS.
- S. J. Farley, D. L. Rochester, A. L. Thompson, J. A. K. Howard and J. A. G. Williams, Controlling Emission Energy, Self-Quenching, and Excimer Formation in Highly Luminescent N∧C∧N-Coordinated Platinum(II) Complexes, Inorg. Chem., 2005, 44, 9690–9703 CrossRef CAS.
- F. Neese, The ORCA program system, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- F. Neese, Software update: The ORCA program system—Version 5.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1606 Search PubMed.
- M. Roemelt, D. Maganas, S. DeBeer and F. Neese, A combined DFT and restricted open-shell configuration interaction method including spin-orbit coupling: Application to transition metal L-edge X-ray absorption spectroscopy, J. Chem. Phys., 2013, 138, 204101 CrossRef.
- B. de Souza, G. Farias, F. Neese and R. Izsák, Predicting Phosphorescence Rates of Light Organic Molecules Using Time-Dependent Density Functional Theory and the Path Integral Approach to Dynamics, J. Chem. Theory Comput., 2019, 15, 1896–1904 CrossRef CAS PubMed.
- R. J. Salthouse, P. Pander, D. S. Yufit, F. B. Dias and J. A. G. Williams, Near-infrared electroluminescence beyond 940 nm in Pt(N^C^N)X complexes: influencing aggregation with the ancillary ligand X, Chem. Sci., 2022, 13, 13600–13610 RSC.
- S. J. Strickler and R. A. Berg, Relationship between Absorption Intensity and Fluorescence Lifetime of Molecules, J. Chem. Phys., 1962, 37, 814–822 CrossRef CAS.
- M. Urban, P. H. Marek-Urban, K. Durka, S. Luliński, P. Pander and A. P. Monkman, TADF Invariant of Host Polarity and Ultralong Fluorescence Lifetimes in a Donor–Acceptor Emitter Featuring a Hybrid Sulfone–Triarylboron Acceptor, Angew. Chem., Int. Ed., 2023, 62, e202217530 CrossRef CAS.
- P. Pander, A. V. Zaytsev, L. G. Franca, F. B. Dias and V. N. Kozhevnikov, Unusual Excimer/Dimer Behavior of a Highly Soluble C,N Platinum(II) Complex with a Spiro-Fluorene Motif, Inorg. Chem., 2023, 62, 18465–18473 CrossRef CAS.
- P. Pander, A. V. Zaytsev, A. Sil, G. V. Baryshnikov, F. Siddique, J. A. G. Williams, F. B. Dias and V. N. Kozhevnikov, Thermally activated delayed fluorescence in a deep red dinuclear iridium(III) complex: a hidden mechanism for short luminescence lifetimes, Chem. Sci., 2023, 14, 13934–13943 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic details and characterisation of new materials; X-ray diffraction and crystal data; further information on the equipment and methods for theory, photophysical characterisation, and OLED devices. CCDC 2365020. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi02069c |
| ‡ These authors contributed equally to the experimental work. |
| § A slight difference between the PL spectra of 5 in toluene and chlorobenzene can be ascribed to a variation in the ΔEST, in line with the previous report of complex 8.21 Here, 5 displays a larger ΔEST in the higher polarity chlorobenzene than in toluene, leading to a visible phosphorescence contribution in chlorobenzene, manifest on the low-energy side of the spectrum. |
| ¶ Complex 7 does not show any detectable emission, as expected based on earlier studies.28,29 The absorption spectrum of 7 is included in the ESI for completeness (Fig. S5.3†). |
| || kr is estimated from the lifetime and quantum yield, assuming unit population of the emissive state, where kr = Φ/τ. The quoted τ and Φ values were measured in deoxygenated solution at 295 K. |
| ** Some deleterious interactions nevertheless remain, as the 3%-loaded devices are generally more efficient than the 5% devices, a trend also widely observed with, for example, Ir(III) emitters. |
| This journal is © the Partner Organisations 2024 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: