Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-driven formation of siloxanes via dehydrocoupling between hydrosilanes and silanols†
Martyna
Markwitz
 a,
Kacper
Łyczek
a,
Kacper
Łyczek
 a,
Qingqing
Bu
a,
Qingqing
Bu
 b and
Krzysztof
Kuciński
b and
Krzysztof
Kuciński
 *a
*a
aFaculty of Chemistry, Adam Mickiewicz University, Poznan, Uniwersytetu Poznanskiego St. 8, 61-614 Poznan, Poland. E-mail: kucinski.k@amu.edu.pl
bSchool of Chemistry and Chemical Engineering/State Key Laboratory Incubation Base for Green Processing of Chemical Engineering, Shihezi University, North Fourth Road, 832003 Shihezi, Xinjiang, People's Republic of China
First published on 26th June 2024
Abstract
In this study, we introduce a method for directly synthesizing various siloxanes from hydrosilanes and silanols under ambient conditions. This process relies on the use of Stryker's reagent ([(PPh3)CuH]6) as the first copper catalyst, enabling this specific cross-dehydrogenative coupling. This method stands out for its exceptional chemoselectivity, making it a superb alternative to established catalytic systems. It operates under mild conditions yet maintains high process efficiency. Throughout the investigation, we demonstrated the potential for modifying the resulting hydrosiloxanes through alcoholysis to produce silyl ethers and via hydrosilylation to yield functionalized siloxanes.
Introduction
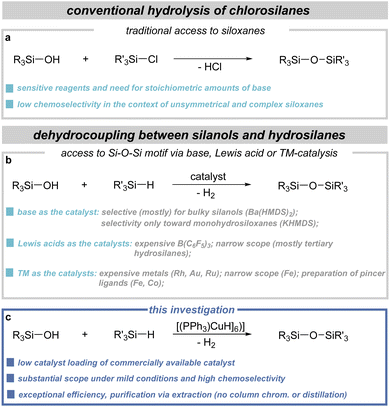
Siloxanes represent a category of compounds defined by a sequence of alternating silicon and oxygen atoms. The mentioned Si–O–Si motif is widely encountered in nature, although the synthetically derived combinations revolutionized industry.1–6 This was driven by the versatility of siloxanes, which are used, among other things, as sealants, adhesives, coatings, plastics, cosmetics, etc.7–13 In synthetic chemistry, siloxanes and silyl ethers also serve as very important reagents and building blocks.14–22Regardless of their structure, chain length, or shape, the main method leading to the formation of Si–O–Si sequences is associated with the process of chlorosilane hydrolysis (Fig. 1a).2 Despite many significant advantages, mainly related to the wide availability of substrates, these methods are accompanied by numerous limitations. This becomes particularly critical in instances where the synthesis of unsymmetrical compounds or complex structures with particular functional groups is envisaged. This, in turn, led scientists to begin searching for alternative synthetic methods replacing chlorosilanes, and using silanols in combination with an appropriate silylating agent.2,23,24 Consequently, a series of methods based on coupling reactions were developed, where instead of a corrosive byproduct such as HCl, dihydrogen (dehydrogenative coupling),25–32 alkene (dealkenative coupling),33,34 or alkyne35 (dealkynative coupling) is obtained. Additionally, the possibility of functionalizing existing siloxanes, for example through hydrosilylation of tetramethyldisiloxane, has also been demonstrated.36–39 Among the aforementioned methodologies, the approach based on the dehydrocoupling reaction between silanols and hydrosilanes appears to be the most extensively explored, particularly concerning the potential utilization of the released dihydrogen (Fig. 1b). Among the strategies for selective SiO–H/Si–H dehydrocoupling, approaches involving the utilization of strong bases,27,28,31 Lewis acids,40–47 4d/5d metal catalysts,25,48 and increasingly prevalent 3d metal complexes have emerged.26,30,32 Each type of catalyst has its own advantages and disadvantages. One of the main limitations of base-catalyzed protocols is the challenge of achieving desired chemoselectivity. When using Lewis acids, we are restricted to the costly tris(pentafluorophenyl)borane and primarily the use of tertiary silanes. Catalysts based on noble metals are very expensive, so the focus is primarily on utilizing 3d metal complexes. Of notable interest is the employment of cobalt complexes featuring NNN-type30 and PNP-type32 pincer ligands. However, the preparation of the ligands themselves can be considered a drawback. Copper compounds have shown effectiveness in synthesizing silyl ethers,49–54 yet they have not been applied to the production of siloxanes. Within this context, pivotal methodologies, as relevant to this study, have been pioneered by Schubert and Lorenz, leveraging Stryker's reagent ([(PPh3)CuH]6) to obtain diverse alkoxysilanes and silanols.54,55 Moreover, there exists a sole example where copper compounds have been applied during the synthesis of siloxanes.29 However, in this scenario, reactions must be performed with copper(II) triflate at 80 °C, where mechanistic studies show that silyl triflate molecules, formed in situ, are the true catalytic species.
Considering the precedent research by Schubert and Lorenz,54 as well as our expertise in forging of Si–O bonds, we embarked on an investigation into the application of commercially available (triphenylphosphine)copper hydride hexamer, known as Stryker's reagent,56–59 within the field of siloxane synthesis (Fig. 1c). It is worth adding here that, until now, this compound and its variations with different phosphine ligands have been used as very useful (pre)catalysts in a range of synthetic processes.60–64 Our initial hypothesis posited that this easily accessible copper cluster would serve as an exceptionally useful catalyst for the dehydrocoupling between silanols and hydrosilanes, thus circumventing the necessity for intricate pincer ligands commonly utilized in prior instances involving 3d metal complexes. Thus, this approach would yield a catalytic system based on copper, which is much more abundant in nature than cobalt. This endeavor resulted in the development of a highly efficient methodology for siloxane synthesis. Moreover, we substantiated the practical viability of our approach through scaled-up experimentation and exemplification of further product modification via subsequent alcoholysis and hydrosilylation reactions.
Results and discussion
In Table 1, we compared the initial findings from our investigation into the dehydrogenative coupling reaction involving phenylsilane (1a) and tert-butyldimethylsilanol (2a). These comparisons were important for optimizing the entire process. This is particularly significant in terms of chemoselectivity. The use of primary silane may lead, depending on the reagent ratio, to the substitution of one, two, or even three silanol molecules. Theoretically, this may yield three distinct products: disiloxane, trisiloxane, and a branched variant. Hence, it is crucial to establish reaction conditions that ensure selectivity and facilitate straightforward isolation of the desired products. It should be noted that due to the sensitivity of all reagents (substrates and catalyst), they were stored in a glovebox and each reaction was set up there. Subsequently, the sealed vials were removed from the glovebox and mixed for a predetermined duration. We initiated our research by examining how the catalyst affects the progression of the dehydrocoupling reaction. Firstly, two tests were carried out (entries 1 and 2). In both tests, all reagents were mixed for 20 hours without the catalyst present. One test maintained the reaction in a closed vial (entry 1), while the second vial after being set up in the glovebox was opened briefly and exposed to air, then sealed again and mixed for the specified duration (entry 2). Both tests affirmed the necessity of a copper catalyst for the reaction to proceed, prompting us to continue our research with its inclusion. Across all experiments (entries 3–12), it became evident that a catalyst amount as low as 0.125 mol% is sufficient. Any further reduction in catalyst amount began to impact the conversion rate of the substrates. Additionally, these experiments revealed the versatility of conducting the reaction in either an argon atmosphere or in air. The presence of air only shortens the duration of the process compared to carrying out the entire reaction in an argon atmosphere (entries 5 and 6). This observation aligns with the pioneering findings of Lorenz and Schubert, who similarly noted the expedited reaction rate in the presence of air.54| Entry | Variation of conditions | Catalyst loading (mol%) | Molar ratio | Conversion of 1a or 2ai [%]; {isolated yield}; | Selectivityj [%] |
|---|---|---|---|---|---|
[1a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [2a] [2a] |
[3a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [4a] [4a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [5a] [5a] |
||||
a All reaction conditions: 1a (1 eq.), 2a (1 eq.), toluene (0.25 mL), rt, under argon atmosphere, 20 h.
b All reaction conditions: 1a (1 eq.), 2a (1 eq.), toluene, rt, under air atmosphere, 20 h.
c Under argon atmosphere, 20 h.
d Under air atmosphere, 30 min.
e Under air atmosphere, 1 h.
f Under air atmosphere, 3 h.
g Under air atmosphere, 6 h.
h Under air atmosphere, in toluene, 20 h.
i Conversion of 1a or 2a determined by GC.
j Selectivity of [mono-sub]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [di-sub] [di-sub]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [tri-sub] products determined by GC. [tri-sub] products determined by GC.
|
|||||
| 1 | No catalysta | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | — |
| 2 | No catalystb | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | — |
| 3 | In toluenec | 0.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2a) | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 4 | In toluened | 0.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2a) | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 5 | In toluenec | 0.125 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2a); {3a, 80%} | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 6 | In toluened | 0.125 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2a); {3a, 79%} | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 7 | In toluened | 0.125 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2a); {3a, 82%} | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 8 | In hexanee | 0.125 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2a); {3a, 81%} | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 9 | In toluened | 0.125 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2a); {3a, 87%} | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 10 | In hexanee | 0.125 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2a); {3a, 84%} | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 11 | In acetonitrilef | 0.125 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 (2a); {3a, 86%} | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 12 | In 2-methyltetrahydrofurang | 0.125 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 (2a) | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 13 | In toluened | 0.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
99 (1a); {4a, 92%} | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 14 | In toluenee | 0.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
99 (1a) | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 15 | CuCl2 instead of [(PPh3)CuH]6h | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3 (1a) | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 16 | Cu(OAc)2 instead of [(PPh3)CuH]6h | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2 (1a) | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
Next, by conducting the reaction in toluene with 0.125 mol% of the catalyst and a reagent ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a = 2
2a = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, we achieved efficient and highly selective synthesis, exclusively yielding the disiloxane product 3a (entry 9). Subsequently, we explored the possibility of substituting two molecules of silanol to obtain the corresponding trisiloxane. Once again, toluene proved to be the most effective solvent (entry 13). Interestingly, despite increasing the amount of silanol in the system (entry 14) and simultaneously increasing the catalyst amount, we did not observe the substitution of a third silanol molecule. Subsequent studies, to be discussed later, indirectly suggest that steric effects likely play a crucial role in preventing the substitution of the third molecule of this bulky silanol. Finally, we investigated the impact of simple copper salts on the progression of this reaction (entries 15 and 16). Both copper(II) chloride and copper(II) acetate demonstrated limited activity in the dehydrocoupling process, yielding only traces of the desired products (less than 3%).
1, we achieved efficient and highly selective synthesis, exclusively yielding the disiloxane product 3a (entry 9). Subsequently, we explored the possibility of substituting two molecules of silanol to obtain the corresponding trisiloxane. Once again, toluene proved to be the most effective solvent (entry 13). Interestingly, despite increasing the amount of silanol in the system (entry 14) and simultaneously increasing the catalyst amount, we did not observe the substitution of a third silanol molecule. Subsequent studies, to be discussed later, indirectly suggest that steric effects likely play a crucial role in preventing the substitution of the third molecule of this bulky silanol. Finally, we investigated the impact of simple copper salts on the progression of this reaction (entries 15 and 16). Both copper(II) chloride and copper(II) acetate demonstrated limited activity in the dehydrocoupling process, yielding only traces of the desired products (less than 3%).
With the optimized conditions in hand, we carried out experiments utilizing different hydrosilanes and silanols to showcase the wide-ranging utility of our protocol. Our reaction conditions demonstrated effectiveness across a diverse array of primary and secondary silanes in the process of mono-dehydrogenative coupling (Fig. 2). It has been shown that various silanols, including those containing bulky groups like isopropyl, are reactive under the studied conditions. All of them easily undergo coupling with phenylsilane or p-tolylsilane to give dihydrosiloxanes (3a–3k) in very good yields (76–99%). In the case of trimethylsilanol, a significant excess of 1a was needed (10.0 eq.). Otherwise, we observed the formation of disubstituted product.
 | ||
| Fig. 2 Substrate scope for mono-dehydrogenative coupling reaction between various silanes and silanols. | ||
At this point, a brief comparison can be made between the previously known methods of synthesizing dihydrosiloxanes and the method described in this article. Because the authors of all discussed studies often used various reagents and reaction conditions, often focusing on different aspects, this comparison is very general in nature. However, the obtained results are highly satisfactory. Firstly, comparable numbers of products were achieved as when using Au catalyst,25 and more than with cobalt complexes.30,32 In the protocol where Ba(HMDS)2 was used as a catalyst,27 only bulky silanols were investigated, resulting in the production of dihydrosiloxanes. However, there is insufficient knowledge about the potential synthesis of dihydrosiloxanes from non-hindered silanols in the presence of a barium base. However, when a similar catalyst like KHMDS was used, no product containing the SiH2 motif was observed. Even after altering the substrate ratio, the reaction did not exclusively yield dihydrosiloxanes.31
Following that, we advanced to examine various secondary hydrosilanes (Fig. 2). In this scenario, irrespective of the substituents’ character around the silicon atom – aliphatic or aromatic – the anticipated monohydrosiloxane products (3g–3k) were consistently acquired with good yields, ranging between 80–98%.
This notable outcome sets apart our method from, for instance, the one utilizing KHMDS, which exclusively yields products of double dehydrogenative coupling.31 Finally, we wanted to verify whether suitable tertiary hydrosilanes would undergo a single dehydrogenative coupling reaction leading to unsymmetrical disiloxanes. The initial test using a combination of dimethylphenylsilane and trimethylsilanol as reagents resulted in the desired product 3l with a yield of 90%. This sets apart the copper-catalyzed method from those employing cobalt complexes with PNP-type ligands.32 In the latter, it was impossible to carry out the dehydrogenative coupling of any tertiary hydrosilane. However, attempts to expand the scope to include more sterically hindered silanols were unsuccessful. The main reason undoubtedly lies in steric hindrance. This is evidenced by the possibility of only attaching trimethylsilanol – the smallest of the investigated molecules.
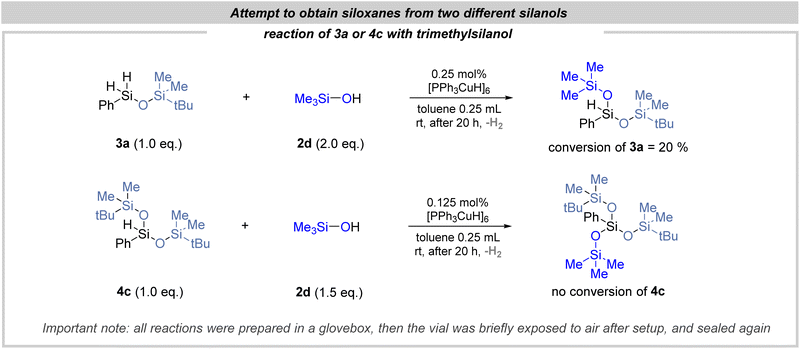
Equipped with prior knowledge of the mono-dehydrogenative coupling reaction, we launched investigations into the substrate's compatibility in the double dehydrogenative coupling reaction (Fig. 3). In the case of various primary hydrosilanes, the situation was quite clear, and a series of monohydrosiloxanes 4a–4i was obtained (with yields up to 99%). Secondary hydrosilanes displayed a distinct pattern: only reactions employing the least sterically hindered trimethylsilanol resulted in the formation of products via double dehydrogenative coupling (products 4j–4l). Conversely, when bulkier silanols were utilized, only products of mono-dehydrogenative coupling were detected. Notably, di-tert-butylsilane stood as a unique case, failing to react with any silanol due to its inherent steric hindrance. Finally, we also investigated whether it was possible to obtain a product of triple dehydrogenative coupling in the case of primary hydrosilanes (Fig. 4). It turned out that in combination with the least sterically hindered trimethylsilanol, branched siloxanes were successfully obtained (Fig. 4). Unfortunately, endeavors to selectively synthesize siloxanes from two distinct silanols yielded no success (Fig. 5). In pursuit of this, previous products 3a and 4c were subjected to reactions with trimethylsilanol. Upon analysis of the post-reaction mixture, the anticipated product originating from 3a was detected; however, the conversion of the starting substrates was notably low (20%). This is particularly intriguing considering the anticipation of a substantially higher conversion in this scenario.
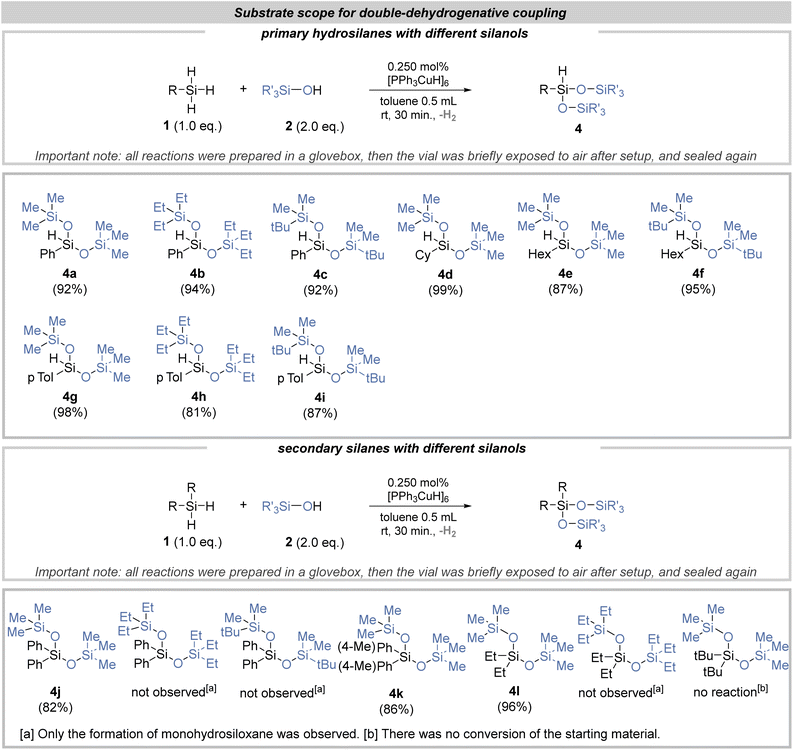 | ||
| Fig. 3 Substrate scope for double-dehydrogenative coupling reaction between various silanes and silanols. | ||
 | ||
| Fig. 4 Substrate scope for triple-dehydrogenative coupling reaction between aliphatic and aromatic hydrosilane and trimethylsilanol. | ||
In the subsequent phase, our aim was to illustrate the potential for functionalizing the hydrosiloxanes we had synthesized (Fig. 6). Initially, drawing on research by Schubert and Lorenz,54 we performed alcoholysis of the previously obtained product 4a using the same catalyst. This process yielded a silyl ether 6a (Fig. 6, part a). Following this, we focused on employing product 4e in a hydrosilylation process with platinum as the catalyst (Fig. 6, part b). Platinum-based catalysts are widely utilized and studied in this field.65–67 The reaction of product 4e with vinylsilane resulted in the formation of product 6b with 85% yield. These reactions collectively highlight the significant application potential of the hydrosiloxanes obtained in our study. Furthermore, we successfully demonstrated the scalability of the original siloxane synthesis reaction by conducting it on an 8-fold larger scale (Fig. 6, part c).
Next, to get some mechanistic insights into copper catalysis, we carried out some of preliminary experiments (Fig. 7). Initially, we conducted tests involving the radical scavenger – TEMPO (Fig. 7, part a).32 We observed no significant changes in the siloxane synthesis process, regardless of the atmosphere (air or argon) or the amount of TEMPO used (1.0 or 2.0 eq.). This suggests that the process may not proceed via a radical mechanism. Subsequently, a mercury drop test was performed to determine the nature of the catalysis, whether homogeneous or heterogeneous (Fig. 7, part b).68–70 Once again, irrespective of the atmosphere, the reaction proceeded in the presence of mercury, indicating homogeneous catalysis.
Additionally, Stryker's reagent, characterized by its brick-red color and utilized in all reactions, exhibited excellent solubility in solvents like benzene or toluene. Analysis of its solution in benzene-d6via1H NMR spectroscopy confirmed the existence of a copper–hydrogen bond (broad signal at 3.51 ppm).71 Then, we employed real-time in situ FT-IR spectroscopy to observe the reaction system. The kinetic profiles derived from the silylation of phenylsilane with tert-butyldimethylsilane at room temperature substantiated a swift vanishing (within less than 5 minutes) of the distinctive peak at 920 cm−1 attributed to the Si–OH functionalities, accompanied by a concomitant increase in the intensity of the Si–O–Si band at 1050 cm−1 (Fig. 7, part c). Moreover, the instrumentation indicated the exothermic nature of the reaction. Then, an attempt was made to verify the catalytic system using NMR spectroscopy. The behaviour of phenylsilane and tert-butyldimethylsilanol in the presence of Stryker's reagent was monitored using both 1H and 29Si NMR (Fig. 7, part d and e). The visible change was observed for the silicon atom of the tert-butyldimethylsilanol molecule. Under normal conditions, the signal from this silicon atom appears at 15.2 ppm. Investigating the same molecule but in the presence of a copper complex showed a shift in the signal to 17.9 ppm. Both of these signals appeared in the spectrum recorded after 15 minutes of the reaction between tert-butyldimethylsilanol and phenylsilane, with an observed signal from the reaction product 4c also at 13.2 ppm (Fig. 7, part d). Furthermore, using 1H NMR, the evolution of hydrogen was demonstrated (signal at 4.48 ppm; Fig. 7, part e), which was also observed visually in the case of each reaction (formation of gas bubbles, particularly vigorous upon opening the vial to the air). Given that the NMR analysis of the phenylsilane/catalyst mixture revealed no discernible changes in the spectrum, in contrast to the silanol/catalyst system, and drawing from prior literature indicating that copper hydrides typically undergo protonolysis in the presence of alcohols or weak acids to produce H2 gas,49,72,73 we propose a plausible mechanism outlined in Fig. 7 (part f). In the initial step, Stryker's reagent goes through protonolysis, which forms a copper–silanolate complex and releases hydrogen. Subsequently, the copper complex undergoes transmetalation, resulting in product formation and the regeneration of copper(I) hydride. It has been indirectly demonstrated that the silanolate form of the complex and its size play a crucial role in driving the reaction forward. Hence, when using the smallest silanol, trimethylsilanol, even trisubstitution reactions were achievable. However, even a minor alteration to triethylsilanol promptly stops the reaction at the disubstitution stage.
Finally, the acceleration of the reaction in the presence of oxygen (air), compared to under argon, is not well understood and requires further experimentation. Schubert54 and Riant74 observed the same effect with copper hydrides, presenting a challenging and interesting problem to solve.
Conclusions
In summary, we have documented the first copper-catalyzed cross-dehydrogenative coupling between hydrosilanes and silanols under remarkably gentle conditions (room temperature), without the need for additional activators or specialized pincer ligands. In this study, commercially available Stryker's reagent proved to be an effective catalyst with just 0.125 mol% loading. This resulted in a catalytic system that is not only straightforward and easily accessible but also significantly more competitive compared to previously used – more expensive gold or cobalt-based systems. Consequently, a range of products including unsymmetrical disiloxanes, symmetrical trisiloxanes, and branched siloxanes were synthesized. This also encompasses hydrosiloxanes containing free Si–H groups, which offer further functionalization potential, as demonstrated through alcoholysis and hydrosilylation processes. Moreover, the employed catalytic system allows for scalability, enhancing the practicality of this approach. Mechanistic investigations highlight the homogeneous nature of the catalysis and the absence of free radicals. Consequently, a proposed mechanism revolves around the formation of a copper–silanolate complex followed by its transmetalation with hydrosilanes.Data availability
The ESI contains general information, synthetic procedures, characterization data and spectra of the obtained products.The authors have cited additional references within the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75–86
75–86
Author contributions
Methodology, K. K.; Synthesis of products – K. K., M. M., K. Ł.; Formal analysis, K. K., M. M., K. Ł.; writing—original draft preparation, K. K. and Q. B.; visualization, K. K. and Q. B.; supervision, K. K.; funding acquisition, K. K. All authors reviewed the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a National Science Centre Grant UMO-2021/43/D/ST4/00132.References
- T. Köhler, A. Gutacker and E. Mejía, Industrial synthesis of reactive silicones: reaction mechanisms and processes, Org. Chem. Front., 2020, 7, 4108–4120 RSC.
- K. Kuciński, H. Stachowiak-Dłużyńska and G. Hreczycho, Catalytic silylation of O–nucleophiles via Si–H or Si–C bond cleavage: A route to silyl ethers, silanols and siloxanes, Coord. Chem. Rev., 2022, 459, 214456 CrossRef.
- M. Selvan T. and T. Mondal, Radiation curable polysiloxane: synthesis to applications, Soft Matter, 2021, 17, 6284–6297 RSC.
- J. Hou, J. Sun and Q. Fang, Recent Advance in Low-Dielectric-Constant Organosilicon Polymers, Chin. J. Chem., 2023, 41, 2371–2381 CrossRef CAS.
- F. Ganachaud and S. Boileau, in Handbook of Ring-Opening Polymerization, John Wiley & Sons, Ltd, 2009, pp. 65–95 Search PubMed.
- M. Woźnica, M. Sobiech and P. Luliński, A Fusion of Molecular Imprinting Technology and Siloxane Chemistry: A Way to Advanced Hybrid Nanomaterials, Nanomaterials, 2023, 13, 248 CrossRef PubMed.
- N. A. Chekina, V. N. Pavlyuchenko, V. F. Danilichev, N. A. Ushakov, S. A. Novikov and S. S. Ivanchev, A new polymeric silicone hydrogel for medical applications: synthesis and properties, Polym. Adv. Technol., 2006, 17, 872–877 CrossRef CAS.
- A. C. Marmo and M. A. Grunlan, Biomedical Silicones: Leveraging Additive Strategies to Propel Modern Utility, ACS Macro Lett., 2023, 12, 172–182 CrossRef CAS PubMed.
- X. Zhang, J. Liu, R. Qu, H. Suo, Z. Xin and Y. Qin, Synthesis and characterization of siloxane functionalized CO2-based polycarbonate, Polymer, 2023, 266, 125623 CrossRef CAS.
- S. Grade, X. Zhang, C.-H. Yang, I. Oduro and C. Wang, Cross-linking lignin and cellulose with polymers using siloxane compounds, Front. Mater., 2024, 11, 1355976 CrossRef.
- L. Lu, F. Dong, X. Chen, T. Guo, J. Qian, X. Xu, Y. Liu, L. Ma, L. Pang, R. Chen, P. Wang and X. Tang, Preparation and properties of an antimicrobial silane-modified polyether sealant, Polym. Bull., 2023, 80, 13129–13141 CrossRef CAS.
- F. de Buyl, V. Hayez, B. Harkness, J. Kimberlain and N. Shephard, in Advances in Structural Adhesive Bonding, ed. D. A. Dillard, Woodhead Publishing, 2nd edn, 2023, pp. 179–219 Search PubMed.
- E. V. Ivanova, E. O. Minyaylo, M. N. Temnikov, L. G. Mukhtorov and Yu. M. Atroshchenko, Silicones in Cosmetics, Polym. Sci., Ser. B, 2023, 65, 578–594 CrossRef CAS.
- A. S. Madsen, H. M. E. Kristensen, G. Lanz and C. A. Olsen, The Effect of Various Zinc Binding Groups on Inhibition of Histone Deacetylases 1–11, ChemMedChem, 2014, 9, 614–626 CrossRef CAS PubMed.
- P. Nareddy, F. Jordan and M. Szostak, Ruthenium(II)-catalyzed ortho-C–H arylation of diverse N-heterocycles with aryl silanes by exploiting solvent-controlled N-coordination, Org. Biomol. Chem., 2017, 15, 4783–4788 RSC.
- P. Nareddy, F. Jordan and M. Szostak, Ruthenium(II)-Catalyzed Direct C–H Arylation of Indoles with Arylsilanes in Water, Org. Lett., 2018, 20, 341–344 CrossRef CAS PubMed.
- J. Zhang, Y. Hou, Y. Ma and M. Szostak, Synthesis of Amides by Mild Palladium-Catalyzed Aminocarbonylation of Arylsilanes with Amines Enabled by Copper(II) Fluoride, J. Org. Chem., 2019, 84, 338–345 CrossRef CAS PubMed.
- I. K. Goncharova, E. A. Ulianova, R. A. Novikov, A. D. Volodin, A. A. Korlyukov and A. V. Arzumanyan, Siloxane-containing derivatives of benzoic acid: chemical transformation of the carboxyl group, New J. Chem., 2022, 46, 18041–18047 RSC.
- T. Sokolnicki, A. Franczyk, R. Kozak and J. Walkowiak, Coupling agents with 2,4,6,8-tetramethylcyclotetrasiloxane core – synthesis and application in styrene–butadiene rubber production, Inorg. Chem. Front., 2023, 10, 5897–5907 RSC.
- A. Shimojima and K. Kuroda, Alkoxy- and Silanol-Functionalized Cage-Type Oligosiloxanes as Molecular Building Blocks to Construct Nanoporous Materials, Molecules, 2020, 25, 524 CrossRef CAS PubMed.
- M. Kikuchi, T. Hayashi, T. Matsuno, K. Kuroda and A. Shimojima, Direct cross-linking of silyl-functionalized cage siloxanes via nonhydrolytic siloxane bond formation for preparing nanoporous materials, Dalton Trans., 2024, 53, 6256–6263 RSC.
- T. Hayashi, M. Kikuchi, N. Murase, T. Matsuno, N. Sugimura, K. Kuroda and A. Shimojima, Hexagonal Prismatic Siloxanes Functionalized with Organosilyl Groups as Building Blocks of Nanoporous Materials, Chem. – Eur. J., 2024, 30, e202304080 CrossRef CAS PubMed.
- K. Kuciński and G. Hreczycho, A Highly Effective Route to Si–O–Si Moieties through O-Silylation of Silanols and Polyhedral Oligomeric Silsesquioxane Silanols with Disilazanes, ChemSusChem, 2019, 12, 1043–1048 CrossRef PubMed.
- B. Yang, P. Guo, X. He and K.-Y. Ye, Cobalt-catalyzed dehydrative approach for the synthesis of unsymmetric disiloxanes and polysiloxanes, Org. Chem. Front., 2024 10.1039/D4QO00706A.
- Y. Satoh, M. Igarashi, K. Sato and S. Shimada, Highly Selective Synthesis of Hydrosiloxanes by Au-Catalyzed Dehydrogenative Cross-Coupling Reaction of Silanols with Hydrosilanes, ACS Catal., 2017, 7, 1836–1840 CrossRef CAS.
- T. Takeshita, K. Sato and Y. Nakajima, Selective hydrosiloxane synthesis via dehydrogenative coupling of silanols with hydrosilanes catalysed by Fe complexes bearing a tetradentate PNNP ligand, Dalton Trans., 2018, 47, 17004–17010 RSC.
- E. L. Coz, S. Kahlal, J.-Y. Saillard, T. Roisnel, V. Dorcet, J.-F. Carpentier and Y. Sarazin, Barium Siloxides and Catalysed Formation of Si–O–Si’ Motifs, Chem. – Eur. J., 2019, 25, 13509–13513 CrossRef PubMed.
- J. Kaźmierczak and G. Hreczycho, Highly effective functionalization of silsesquioxanes mediated by inexpensive earth-abundant metal catalyst – Potassium tert-butoxide, J. Catal., 2019, 378, 90–96 CrossRef.
- J. Kaźmierczak and G. Hreczycho, Copper(II) triflate-mediated synthesis of functionalized silsesquioxanes via dehydrogenative coupling of POSS silanols with hydrosilanes, Dalton Trans., 2019, 48, 6341–6346 RSC.
- S. Pattanaik and C. Gunanathan, Cobalt-Catalyzed Selective Synthesis of Disiloxanes and Hydrodisiloxanes, ACS Catal., 2019, 9, 5552–5561 CrossRef CAS.
- K. Kuciński, H. Stachowiak and G. Hreczycho, Silylation of silanols with hydrosilanes via main-group catalysis: the synthesis of unsymmetrical siloxanes and hydrosiloxanes, Inorg. Chem. Front., 2020, 7, 4190–4196 RSC.
- E. Szafoni, K. Kuciński and G. Hreczycho, Cobalt-catalyzed dehydrogenative cross-coupling reaction: Selective access to dihydrosiloxanes, hydrosiloxanes and functionalized silsesquioxanes, J. Catal., 2023, 423, 1–9 CrossRef CAS.
- Y.-R. Yeon, Y. J. Park, J.-S. Lee, J.-W. Park, S.-G. Kang and C.-H. Jun, Sc(OTf)3–Mediated Silylation of Hydroxy Functional Groups on a Solid Surface: A Catalytic Grafting Method Operating at Room Temperature, Angew. Chem., Int. Ed., 2008, 47, 109–112 CrossRef CAS PubMed.
- B. Marciniec, P. Pawluć, G. Hreczycho, A. Macina and M. Madalska, Silylation of silanols with vinylsilanes catalyzed by a ruthenium complex, Tetrahedron Lett., 2008, 49, 1310–1313 CrossRef CAS.
- K. Kuciński, H. Stachowiak and G. Hreczycho, Silylation of Alcohols, Phenols, and Silanols with Alkynylsilanes–an Efficient Route to Silyl Ethers and Unsymmetrical Siloxanes, Eur. J. Org. Chem., 2020, 4042–4049 CrossRef.
- R. Januszewski, I. Kownacki, H. Maciejewski and B. Marciniec, An efficient catalytic and solvent-free method for the synthesis of mono-organofunctionalized 1,1,3,3-tetramethyldisiloxane derivatives, J. Organomet. Chem., 2017, 846, 263–268 CrossRef CAS.
- R. Januszewski, M. Grzelak, B. Orwat, M. Dutkiewicz and I. Kownacki, Simple catalytic approach to highly regioselective synthesis of monofunctionalized disiloxanes decorated with metalloids, J. Catal., 2020, 390, 103–108 CrossRef CAS.
- J. Szyling, R. Januszewski, K. Jankowska, J. Walkowiak, I. Kownacki and A. Franczyk, Synthesis of bifunctional disiloxanes via subsequent hydrosilylation of alkenes and alkynes, Chem. Commun., 2021, 57, 4504–4507 RSC.
- J. Szyling, J. Walkowiak, A. Czapik and A. Franczyk, Synthesis of unsymmetrically and symmetrically functionalized disiloxanes via subsequent hydrosilylation of C≡C bonds, Sci. Rep., 2023, 13, 10244 CrossRef CAS PubMed.
- S. Shinke, T. Tsuchimoto and Y. Kawakami, Stereochemistry in Lewis acid-catalyzed silylation of alcohols, silanols, and methoxysilanes with optically active methyl(1-naphthyl)phenylsilane, Silicon Chem., 2007, 3, 243–249 CrossRef CAS.
- K. M. Rabanzo-Castillo, M. Hanif, T. Söhnel and E. M. Leitao, Synthesis, characterisation and electronic properties of naphthalene bridged disilanes, Dalton Trans., 2019, 48, 13971–13980 RSC.
- J. Peng, Y. Bai and J. Li, Piers-Rubinsztajn Reaction and the Application in Siloxane/Polysiloxane Chemistry, Lett. Org. Chem., 2019, 16, 525–530 CrossRef CAS.
- K. M. Rabanzo-Castillo, V. B. Kumar, T. Söhnel and E. M. Leitao, Catalytic Synthesis of Oligosiloxanes Mediated by an Air Stable Catalyst, (C6F5)3B(OH2), Front. Chem., 2020, 8, 477 CrossRef CAS PubMed.
- J. Kaźmierczak, D. Lewandowski and G. Hreczycho, B(C6F5)3-Catalyzed Dehydrocoupling of POSS Silanols with Hydrosilanes: A Metal-Free Strategy for Effecting Functionalization of Silsesquioxanes, Inorg. Chem., 2020, 59, 9206–9214 CrossRef PubMed.
- A. M. Szawiola, B. H. Lessard, H. Raboui and T. P. Bender, Use of Piers-Rubinsztajn Chemistry to Access Unique and Challenging Silicon Phthalocyanines, ACS Omega, 2021, 6, 26857–26869 CrossRef CAS PubMed.
- H. Gao, A. Battley and E. M. Leitao, The ultimate Lewis acid catalyst: using tris(pentafluorophenyl) borane to create bespoke siloxane architectures, Chem. Commun., 2022, 58, 7451–7465 RSC.
- S. Rubinsztajn, J. Chojnowski and U. Mizerska, Tris(pentafluorophenyl)borane-catalyzed Hydride Transfer Reactions in Polysiloxane Chemistry—Piers–Rubinsztajn Reaction and Related Processes, Molecules, 2023, 28, 5941 CrossRef CAS PubMed.
- Z. M. Michalska, Reactions of organosilicon hydrides with organosilanols catalysed by homogeneous and silica-supported rhodium (L) complexes, Transition Met. Chem., 1980, 5, 125–129 CrossRef CAS.
- H. Ito, A. Watanabe and S. Masaya, ersatile Dehydrogenative Alcohol Silylation Catalyzed by Cu(I)– Phosphine Complex, Org. Lett., 2005, 7, 1869–1871 CrossRef CAS PubMed.
- Y. Gunji, Y. Yamashita, T. Ikeno and T. Yamada, Convenient and selective preparation of mono-alkoxyphenylsilanes from phenylsilane and alcohols, Chem. Lett., 2006, 35, 714–715 CrossRef CAS.
- G.-B. Liu, H.-Y. Zhao and T. Thiemann, Two New Catalysts for the Dehydrogenative Coupling Reaction of Carboxylic Acids with Silanes—Convenient Methods for an Atom–Economical Preparation of Silyl Esters, Synth. Commun., 2007, 37, 2717–2727 CrossRef CAS.
- J. F. Blandez, A. Primo, A. M. Asiri, M. Alvaro and H. García, Copper nanoparticles supported on doped graphenes as catalyst for the dehydrogenative coupling of silanes and alcohols, Angew. Chem., Int. Ed., 2014, 53, 12581–12586 CrossRef CAS PubMed.
- X. Wang, Y. Bai, X. Zhai, B. Wu and Y. Zhou, Synthesis of poly(silyl ether)s via copper-catalyzed dehydrocoupling polymerization, Chin. Chem. Lett., 2022, 33, 2639–2642 CrossRef CAS.
- C. Lorenz and U. Schubert, An efficient catalyst for the conversion of hydrosilanes to alkoxysilanes, Chem. Ber., 1995, 128, 1267–1269 CrossRef CAS.
- U. Schubert and C. Lorenz, Conversion of Hydrosilanes to Silanols and Silyl Esters Catalyzed by [Ph3PCuH]6, Inorg. Chem., 1997, 36, 1258–1259 CrossRef CAS PubMed.
- W. S. Mahoney, D. M. Brestensky and J. M. Stryker, elective hydride-mediated conjugate reduction of .alpha.,.beta.-unsaturated carbonyl compounds using [(Ph3P)CuH]6, J. Am. Chem. Soc., 1988, 110, 291–293 CrossRef CAS.
- B. H. Lipshutz, W. Chrisman and K. Noson, Hydrosilylation of aldehydes and ketones catalyzed by [Ph3P(CuH)]6, J. Organomet. Chem., 2001, 624, 367–371 CrossRef CAS.
- D. Lee and J. Yun, Direct synthesis of Stryker's reagent from a Cu(II) salt, Tetrahedron Lett., 2005, 46, 2037–2039 CrossRef CAS.
- J. F. Daeuble, J. M. Stryker, P. Chiu and W. H. Ng, in Encyclopedia of Reagents for Organic Synthesis, American Cancer Society, 2014, pp. 1–9 Search PubMed.
- D. H. Appella, Y. Moritani, R. Shintani, E. M. Ferreira and S. L. Buchwald, Asymmetric Conjugate Reduction of α,β-Unsaturated Esters Using a Chiral Phosphine–Copper Catalyst, J. Am. Chem. Soc., 1999, 121, 9473–9474 CrossRef CAS.
- R. Moser, Ž. V. Bošković, C. S. Crowe and B. H. Lipshutz, CuH-Catalyzed Enantioselective 1,2-Reductions of α,β-Unsaturated Ketones, J. Am. Chem. Soc., 2010, 132, 7852–7853 CrossRef CAS PubMed.
- K. Junge, B. Wendt, D. Addis, S. Zhou, S. Das and M. Beller, Copper–Catalyzed Enantioselective Hydrosilylation of Ketones by Using Monodentate Binaphthophosphepine Ligands, Chem. – Eur. J., 2010, 16, 68–73 CrossRef CAS PubMed.
- S. Nishino, M. Miura and K. Hirano, An umpolung-enabled copper-catalysed regioselective hydroamination approach to α-amino acids, Chem. Sci., 2021, 12, 11525–11537 RSC.
- N. M. Harmon, N. R. Gehrke and D. F. Wiemer, Conjugate reduction of vinyl bisphosphonates, Tetrahedron Lett., 2022, 106, 154078 CrossRef CAS PubMed.
- H. Stachowiak, K. Kuciński, F. Kallmeier, R. Kempe and G. Hreczycho, Cobalt–Catalyzed Dehydrogenative C– H Silylation of Alkynylsilanes, Chem. – Eur. J., 2022, 28, e202103629 CrossRef CAS PubMed.
- A. Walczak, H. Stachowiak, G. Kurpik, J. Kaźmierczak, G. Hreczycho and A. R. Stefankiewicz, High catalytic activity and selectivity in hydrosilylation of new Pt(II) metallosupramolecular complexes based on ambidentate ligands, J. Catal., 2019, 373, 139–146 CrossRef CAS.
- H. Stachowiak-Dłużyńska, M. Gruszczyński, M. Kubicki and G. Hreczycho, Pt(II) complexes bearing P,N-donor ligands as catalysts in chemoselective hydrosilylation, hydrogermylation, and hydroboration of terminal alkenes, J. Catal., 2024, 433, 115494 CrossRef.
- T. Jurado-Vázquez, E. Rosaldo, A. Arévalo and J. J. García, Levulinic Acid Hydrogenation with Homogeneous Cu(I) Catalyst, ChemCatChem, 2022, 14, e202200628 CrossRef.
- T. Tewari, R. Kumar and S. H. Chikkali, Iron–Catalyzed Magnesium–Mediated Formal Hydroformylation of Alkynes and Alkenes, ChemCatChem, 2023, 15, e202201394 CrossRef CAS.
- D. Lewandowski and G. Hreczycho, Cobalt pincer-type complexes demonstrating unique selectivity for the hydroboration reaction of olefins under mild conditions, Inorg. Chem. Front., 2023, 10, 3656–3663 RSC.
- E. L. Bennett, P. J. Murphy, S. Imberti and S. F. Parker, Characterization of the Hydrides in Stryker's Reagent: [HCu{P(C6H5)3}]6, Inorg. Chem., 2014, 53, 2963–2967 CrossRef CAS PubMed.
- C. M. Wyss, B. K. Tate, J. Bacsa, T. G. Gray and J. P. Sadighi, Bonding and reactivity of a μ-hydrido dicopper cation, Angew. Chem., Int. Ed., 2013, 52, 12920–12923 CrossRef CAS PubMed.
- R. L. M. Bienenmann, A. J. Schanz, P. L. Ooms, M. Lutz and D. L. J. Broere, A Well–Defined Anionic Dicopper(I) Monohydride Complex that Reacts like a Cluster, Angew. Chem., Int. Ed., 2022, 61, e202202318 CrossRef CAS PubMed.
- N. Mostefaï, S. Sirol, J. Courmarcel and O. Riant, Air-Accelerated Enantioselective Hydrosilylation of Ketones Catalyzed by Copper(I) Fluoride-Diphosphine Complexes: Investigations of the Effects of Temperature and Ligand Structure, Synthesis, 2007, 1265–1271 Search PubMed.
- P. D. Prince, M. J. Bearpark, G. S. McGrady and J. W. Steed, Hypervalent hydridosilicates: synthesis, structure and hydride bridging, Dalton Trans., 2007, 2, 271–282 Search PubMed.
- H. Zhang and D. Zhao, Synthesis of Silicon-Stereogenic Silanols Involving Iridium-Catalyzed Enantioselective C–H Silylation Leading to a New Ligand Scaffold, ACS Catal., 2021, 11, 10748–10753 CrossRef CAS.
- Y. Lin, K.-Z. Jiang, J. Cao, Z.-J. Zheng, Z. Xu, Y.-M. Cui and L.-W. Xu, Iridium–Catalyzed Intramolecular C–H Silylation of Siloxane–Tethered Arene and Hydrosilane: Facile and Catalytic Synthesis of Cyclic Siloxanes, Adv. Synth. Catal., 2017, 359, 2247–2252 CrossRef CAS.
- K. Hirabayashi, J. Ando, J. Kawashima, Y. Nishihara, A. Mori and T. Hiyama, Novel Carbon-Carbon Bond Formation through Mizoroki-Heck Type Reaction of Silanols and Organotin Compounds, Bull. Chem. Soc. Jpn., 2000, 73, 1409–1417 CrossRef CAS.
- K. Kuciński and G. Hreczycho, O-Metalation of silanols and POSS silanols over Amberlyst-15 catalyst: A facile route to unsymmetrical siloxanes, borasiloxanes and germasiloxanes, Inorg. Chim. Acta, 2019, 490, 261–266 CrossRef.
- P. A. Di Giorgio, L. H. Sommer and F. C. Whitmore, Di-t-butyl-di-aminoalkyl Silicates, J. Am. Chem. Soc., 1949, 71, 3254–3256 CrossRef CAS.
- M. Igarashi, K. Kubo, T. Matsumoto, K. Sato, W. Ando and S. Shimada, Pd/C-catalyzed cross-coupling reaction of benzyloxysilanes with halosilanes for selective synthesis of unsymmetrical siloxanes, RSC Adv., 2014, 4, 19099–19102 RSC.
- G. Calzaferri, R. Imhof and K. W. Törnroos, Structural and vibrational properties of the octanuclear silasesquioxane C6H13(H7Si8O12), J. Chem. Soc., Dalton Trans., 1994, 3123–3128 RSC.
- Z. Li, J.-G. Liu, W.-P. Zhang and M.-H. Xu, Enantioselective Si–H Insertion of Arylvinyldiazoesters Promoted by Rhodium(I)/Diene Complexes, Adv. Synth. Catal., 2024, 366, 2514–2518 CrossRef CAS.
- T. Aoki, J. Watanabe, Y. Ishimoto, E. Oikawa, Y. Hayakawa and M. Nishida, Synthesis and polymerization of p-pentamethyldisiloxanyl-α,β,β-trifluorostyrene and the oxygen permeability of the polymer, J. Fluor. Chem., 1992, 59, 285–288 CrossRef CAS.
- G. Engelhardt, H. Jancke, M. Mägi, T. Pehk and E. Lippmaa, Über die 1H-, 13C- und 29Si-NMR chemischen Verschiebungen einiger linearer, verzweigter und cyclischer Methylsiloxan-Verbindungen, J. Organomet. Chem., 1971, 28, 293–300 CrossRef CAS.
- J. Ohshita, D. Hamamoto, K. Kimura and A. Kunai, Anodic polymerization of dithienosilole and electroluminescent properties of the resulting polymer, J. Organomet. Chem., 2005, 690, 3027–3032 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data and spectra. See DOI: https://doi.org/10.1039/d4qi01184h |
| This journal is © the Partner Organisations 2024 |