Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring the impact of various reducing agents on Cu nanocluster synthesis
Sourav
Biswas
 a and
Yuichi
Negishi
a and
Yuichi
Negishi
 *ab
*ab
aDepartment of Applied Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: negishi@rs.tus.ac.jp
bResearch Institute for Science & Technology, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan
First published on 11th April 2024
Abstract
The synthesis of copper (Cu) nanoclusters (NCs) has experienced significant advancements in recent years. Despite the exploration of metal NCs dating back almost two decades, challenges specific to Cu NC synthesis arise from the variable oxidation states and heightened reactivity of intermediate Cu complexes, distinguishing it from its analogous counterparts. In this study, we present a comprehensive overview of this newly evolving research domain, focusing on the synthetic aspects. We delve into various factors influencing the synthesis of Cu NCs, with specific emphasis on the role of reducing agents. The impact of the reducing agent is particularly pivotal in this synthetic process, ultimately influencing the formation of model M(0)-containing NCs, which are less readily accessible in the context of Cu NCs. We anticipate that this frontier article will pave the way for accelerated research in the field of Cu NCs. By aiding in the selection of specific reaction conditions and reducing agents, we believe that this work will contribute to a faster-paced exploration of Cu NCs, further advancing our understanding and applications in this exciting area of nanomaterial research.
1. Introduction
The fascinating world of nanoclusters (NCs) has witnessed a transformative journey, especially since the revelation of the well-defined structural architecture of gold (Au) NCs.1 This breakthrough has propelled the trajectory of NC research, marking a pivotal moment in the exploration of these unique entities at the nanoscale.2–4 While nanoparticle (NP) research had previously captured significant attention, the distinct properties of metal NCs swiftly elevated them to the forefront of scientific inquiry. Metal NCs, owing to their size specificity, effectively bridge the gap between larger metal NPs and individual molecular entities.3,5 The allure of these NCs lies in their diminutive size, which imparts a quantized electronic environment, leading to distinctive and intriguing optical properties. The exploration of metal NCs, fueled by their potential applications in various fields, has unveiled a rich tapestry of discoveries, with Au NCs standing as pioneers in this area.6–9In the wake of the Au NCs revelation, the research landscape expanded to encompass other metals, with silver (Ag) and copper (Cu) NCs emerging as notable subjects of study.10–15 Despite the slightly earlier exploration of Ag NCs, the synthesis of various Cu NCs stands as a more recent and captivating development.15–17 The process of synthesizing Cu NCs serves as a testament to the dynamic landscape of NC research, offering distinct hurdles and prospects for researchers to navigate. Although numerous other NCs have been synthesized recently, within the broader context of metal NCs, the elements belonging to Group 11—such as Au, Ag, and Cu—stand out. This preference is rooted in their advantageous electronic configurations, which render them highly conducive to NC formation.2
While Cu shares a similar group with Au and Ag, its abundant availability in the Earth's crust renders it particularly enticing for cost-effective applications. These applications span from catalysis to bioimaging, leveraging their distinct properties for various purposes.18–20 Thus the dynamic interplay between scientific discovery and technological advancement emphasizes the significance of Cu NCs within the broader field of nanoscience. It is noteworthy that the lowest standard reduction potential of Cu (0.52 V) sets it apart from other elements in the same group (Ag: 0.80 V; Au: 1.69 V), posing challenges in its synthesis.21 Nevertheless, numerous approaches have been explored, leading to the successful reporting of various Cu NCs.16 Notably, diverse research groups have employed different techniques to synthesize these NCs, acknowledging the inherent difficulty in this process.16,22–27 Thus, there exists a crucial need to comprehend the synthetic approach for Cu NCs, primarily dictated by the choice of reducing agents. In this article, we aim to provide a comprehensive overview of the impact of various reducing agents on the synthesis of Cu NCs. As we delve into the historical context and synthesis methods of these NCs, a captivating narrative unfolds, shedding light on the intricacies of their formation and the immense potential they hold for shaping the future landscape of nanotechnology.
2. Effect of the various reducing agents
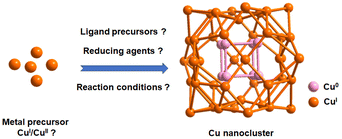
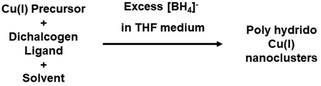
In the domain of NP synthesis, the careful selection and quantity determination of a reducing agent are pivotal for precisely controlling the size, shape, and stability of NPs.28 These choices exert a significant influence on the resulting properties of the NPs. In a broader context, the reducing agent is responsible for catalyzing the reduction of metal ions, a process closely resembling the formation of metal NCs. Despite the absence of comprehensive data on the observed impact, there is a tangible indication of the crucial role played by reducing agents in NC synthesis. This significance is particularly pronounced in the case of Cu NCs, where the variable oxidation states of the metal and the limited availability of Cu(0) complexes present considerable challenges as depicted in Fig. 1.29–32 Given the dynamic nature of the field and the elusive nature of Cu(0) character, the controlled reduction of copper to achieve desired NC structures becomes a pressing concern. To overcome this limitation, a viable approach involves the formation of hydrides during the reduction process, offering optimal stability to the intermediate products. The introduction of hydrides plays a direct role in influencing the cluster nuclearity, exerting a significant impact on both metallic and intermetallic NC growth. Our understandings suggest that the stability achieved through interstitial hydrides preserves the structural integrity, allowing further interaction with a catalyst and ultimately resulting in the formation of Cu(0) characteristics. Thus, the role of the reducing agent emerges as a crucial factor in a multi-step reaction process, its significance varying depending on the nature of the starting precursor material. The intricate interplay between the reducing agent and the formation of hydrides underscores the complexity of the process and highlights the need for a nuanced understanding of the synthetic pathways in Cu NC synthesis.Professor C. W. Liu and his research group conducted an extensive investigation into the synthesis of diverse Cu(I) hydride NCs at the early stage of the exploration of Cu NCs.33,34 Employing a modified Brust method as their synthetic protocol, they utilized an excess of [BH4]− as the reducing agent. Specifically, they opted for readily available [(CH3)4N](BH4), NaBH4 or LiBH4, which facilitated the formation of hydride complexes with Cu(I) as depicted in Fig. 2.35–38 Through the manipulation of ligand structures, they successfully generated a range of Cu(I) hydride NCs.33,38 Remarkably, the research team also achieved the formation of analogous cluster structures without the use of any reducing agents, resulting in clusters devoid of hydrides.33 This observation underscores the significance of designing synthetic protocols that do not rely on reducing agents, as they still yield Cu(I) NCs, typically free from hydrides.
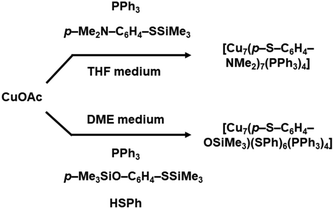
The synthesis of hydride free Cu NCs was then pronounced. Langer et al. synthesized two different Cu NCs just by mixing all the precursors together in a solvent medium.39 Instead of any single type of ligands they utilized both phosphonate and thiolate ligands for their synthesis process. By using these types of diverse ligands, they synthesized [Cu7(p-S-C6H4-NMe2)7(PPh3)4] NCs (Me: methyl; Ph: phenyl) when the precursor was Cu(I) acetate. They optimized the quantity of the mixing of precursor with the ligands in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2
3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (metal precursor (copper acetate (CuOAc))
1 (metal precursor (copper acetate (CuOAc))![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphonate ligand
phosphonate ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thiolate ligand). However, they also achieved a mixed thiolate ligand containing [Cu7(p-S-C6H4-OSiMe3)(SPh)6(PPh3)4] NC by introducing two different thiolate ligands as depicted in Fig. 3. In this step they also optimized the reactants with a weight ratio of 3
thiolate ligand). However, they also achieved a mixed thiolate ligand containing [Cu7(p-S-C6H4-OSiMe3)(SPh)6(PPh3)4] NC by introducing two different thiolate ligands as depicted in Fig. 3. In this step they also optimized the reactants with a weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 (metal precursor
1.1 (metal precursor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphonate ligand
phosphonate ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SPh
SPh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p-S-C6H4-OSiMe3). Although there is no larger difference in the structural architecture of these two NCs, but a little difference in the Cu–Cu distances is observed. The average Cu–Cu distance is 2.7871 Å for the first one and it is modified to 2.8201 Å.
p-S-C6H4-OSiMe3). Although there is no larger difference in the structural architecture of these two NCs, but a little difference in the Cu–Cu distances is observed. The average Cu–Cu distance is 2.7871 Å for the first one and it is modified to 2.8201 Å.
Later on, Jin et al. reported a synthetic protocol by mixing all the precursors together at room temperature to synthesize reducing agent free a recimic mixture of [K(CH3OH)2(18-crown-6)]+[Cu5(S-tert-Bu)6]− NCs (Bu: butyl).40 They utilized cuprous oxide as the metal precursor. Later on, Sun et al. made significant strides in advancing the synthesis process through hydrothermal methods at a temperature of 80 °C as depicted in Fig. 4.41 In their approach, they synthesized [Cu8(tert-BuC6H4S)8(PPh3)4] NC by treating the [tert-BuC6H4SCu] complex with PPh3 and CS2 in a methanol medium. It is crucial to underscore that the synthesis of high nuclear Cu(I) NCs is unattainable in the absence of a reducing agent when commencing from a Cu(I) precursor. Conversely, endeavors to fabricate conventional metal NCs with M(0) characteristics, such as typical Au and Ag NCs, without employing reducing agents have proven futile. This limitation poses a formidable challenge in the field, as the utilization or omission of reducing agents produces divergent outcomes: the inclusion of reducing agents leads to the creation of hydrides Cu(I) NCs, while their omission results in the formation of only diminutive Cu(I) NCs. It is imperative to highlight that, despite these challenges, the realization of Cu(0) characteristics through the use of [BH4]− as a reducing agent remains elusive in ongoing research endeavors.
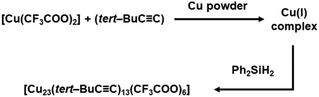
Later on, the gradient reduction strategy emerged as an effective approach for attaining Cu(0) character in Cu NCs synthesis. This strategy involves the sequential use of distinct reducing agents at different stages of Cu reduction. Han et al. implemented this strategy by employing Cu powder and Ph2SiH2 in the synthesis of Cu(0)-containing [Cu23(tert-BuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)13(CF3COO)6] NC.31 In the initial step, Cu powder facilitated the reduction of the Cu(II) precursor [Cu(CF3COO)2], resulting in the formation of Cu(I) species. Subsequently, these Cu(I) species underwent further reduction through the action of Ph2SiH2, ultimately resulting in the formation of NCs containing Cu(0) (Fig. 5). They also noted a sequential color transformation, commencing with colorless, transitioning to light yellow, and culminating in a red color upon the addition of the reducing agents. The authors placed particular emphasis on the effectiveness of employing a gradual reduction approach, employing two distinct reducing agents rather than relying on a single potent reducing agent. Their rationale behind this choice was rooted in the concern that using a single powerful reducing agent might result in direct reduction and the uncontrolled formation of larger Cu NPs. Nevertheless, the use of Ph2SiH2 as a reducing agent, as demonstrated by Lee et al. resulted in a Cu(I) complex when treating Cu(OAc)2 (Ac: acetate) with excess PPh3 (1
C)13(CF3COO)6] NC.31 In the initial step, Cu powder facilitated the reduction of the Cu(II) precursor [Cu(CF3COO)2], resulting in the formation of Cu(I) species. Subsequently, these Cu(I) species underwent further reduction through the action of Ph2SiH2, ultimately resulting in the formation of NCs containing Cu(0) (Fig. 5). They also noted a sequential color transformation, commencing with colorless, transitioning to light yellow, and culminating in a red color upon the addition of the reducing agents. The authors placed particular emphasis on the effectiveness of employing a gradual reduction approach, employing two distinct reducing agents rather than relying on a single potent reducing agent. Their rationale behind this choice was rooted in the concern that using a single powerful reducing agent might result in direct reduction and the uncontrolled formation of larger Cu NPs. Nevertheless, the use of Ph2SiH2 as a reducing agent, as demonstrated by Lee et al. resulted in a Cu(I) complex when treating Cu(OAc)2 (Ac: acetate) with excess PPh3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of Cu to PPh3), indicating that the effectiveness of Ph2SiH2 is limited to a single step of reduction in Cu complex design.42 In a separate study, Nguyen et al. described the presence of partial Cu(0) character in [Cu25H22(PPh3)12]Cl NC synthesized from Cu(OAc) and CuCl with Ph2SiH2 in the presence of PPh3.43 The reduction from Cu(I) to Cu(0) was supported by Ph2SiH2. However, the reaction yielded a mixture of products, with [Cu25H22(PPh3)12]Cl accounting for 25%, [Cu18H17(PPh3)10]Cl for 14%, and 17% being black elemental Cu powder when 13 equivalents of Ph2SiH2 were added to a slurry containing 24 equivalents of Cu(OAc), 1 equivalent of CuCl, and 12 equivalents of PPh3 in a C6H6 medium. This underscores the importance of noting that, although the reducing agent remained constant, it did not fully convert all Cu(I) species to Cu(0), indicating that the effectiveness may depend on concentration of the reducing agents and the reactivity of the associated ligands.
2 molar ratio of Cu to PPh3), indicating that the effectiveness of Ph2SiH2 is limited to a single step of reduction in Cu complex design.42 In a separate study, Nguyen et al. described the presence of partial Cu(0) character in [Cu25H22(PPh3)12]Cl NC synthesized from Cu(OAc) and CuCl with Ph2SiH2 in the presence of PPh3.43 The reduction from Cu(I) to Cu(0) was supported by Ph2SiH2. However, the reaction yielded a mixture of products, with [Cu25H22(PPh3)12]Cl accounting for 25%, [Cu18H17(PPh3)10]Cl for 14%, and 17% being black elemental Cu powder when 13 equivalents of Ph2SiH2 were added to a slurry containing 24 equivalents of Cu(OAc), 1 equivalent of CuCl, and 12 equivalents of PPh3 in a C6H6 medium. This underscores the importance of noting that, although the reducing agent remained constant, it did not fully convert all Cu(I) species to Cu(0), indicating that the effectiveness may depend on concentration of the reducing agents and the reactivity of the associated ligands.
The effective reactivity of the ligands is much prominent when we look into the thiolate NC counter parts. Due to the different reactivities, the gradient reduction process, which is pivotal in various synthesis methods, has not been applied yet to synthesis Cu-thiolate NCs. So, the synthesis of the Cu-thiolate NCs still rely on the modified Burst methods where excess [BH4]− was utilized as the main reducing agent.33 However, as we discussed earlier that using such [BH4]− as a reducing agent generally prefers to produce hydride containing NCs. So, there is an open challenge to the researchers how they optimized the reaction conditions to avoid such integration of the hydrides in their desired NC structure.
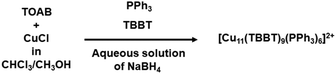
Li et al. detailed the synthesis of [Cu11(TBBT)9(PPh3)6]2+ NC (TBBT: 4-tert-butylbenzenethiolate).44 The process commenced by treating CuCl in a chloroform and methanol medium with tetraoctylammonium bromide (TOAB), initiating the reaction as depicted in Fig. 6. Subsequently, PPh3 and TBBT were introduced directly into the mixture as protecting ligands. Finally, the entire solution underwent treatment with an aqueous NaBH4 solution through rapid incorporation. Despite the introduction of [BH4]− in the reaction, the result was a Cu(I)-thiolate NC devoid of hydride. The investigation into the factors influencing this outcome focused on two key aspects: the selection of specific thiolate ligands and the addition of an aqueous solution of reducing agents. To elucidate the reasons behind this phenomenon, let us consider another example. Following a similar reaction pathway, Ke et al. and reported two different Cu NCs namely [Cu13(SePh)13(Ph3P)4] and [Cu8(SPh)8(Ph3P)4].45 Both of these NCs were hydride-free and employed a similar aqueous NaBH4 solution as reducing agent. However, instead of conducting the reaction at room temperature, they opted for an ice-cold aqueous NaBH4 solution to deliberately slow down the reaction. Revisiting the aforementioned factors, we can now eliminate the possibility of the particular thiolate ligands playing a role, as the same trend is observed across different ligands and even when thiolate is replaced with selenate. According to our understanding, the use of an aqueous solution of [BH4]− leads to the formation of hydride-free Cu thiolate NCs, irrespective of the thiolate ligand structure. The success of this approach prompts further exploration into the underlying mechanisms and opens avenues for investigating alternative strategies in the synthesis of hydride-free Cu(I)-thiolate NCs. However, all the next attempts were resulted hydride integrated Cu(I)-thiolate NCs. As for example, Wang et al. conducted the synthesis of [Cu14(S-tert-Bu)3(PPh3)7H10] NC by treating [Cu(CH3CN)4]BF4 to associated ligands in aqueous NaBH4 solution at room temperature.46 The molar ratio of the metal precursor to the reducing agent in this instance was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.25. Another example, provided by Dong et al., involved the synthesis of [Cu18H(PET)14(PPh3)6(SCN)3] NC starting from CuSCN.47 In this case, an aqueous solution of NaBH4 was utilized with a molar ratio of 1
8.25. Another example, provided by Dong et al., involved the synthesis of [Cu18H(PET)14(PPh3)6(SCN)3] NC starting from CuSCN.47 In this case, an aqueous solution of NaBH4 was utilized with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (metal precursor to reducing agent). Sun et al. employed Cu(OAc)2·H2O as a metal precursor to synthesize [Cu25H10(SPhCl2)18]3− NC.48 The reaction involved the mixing all ligands with the metal precursor in methanol and chloroform at the initial stage, followed by reduction at room temperature using an aqueous solution of NaBH4. The molar ratio of the metal precursor to the reducing agent in this case was 1
4 (metal precursor to reducing agent). Sun et al. employed Cu(OAc)2·H2O as a metal precursor to synthesize [Cu25H10(SPhCl2)18]3− NC.48 The reaction involved the mixing all ligands with the metal precursor in methanol and chloroform at the initial stage, followed by reduction at room temperature using an aqueous solution of NaBH4. The molar ratio of the metal precursor to the reducing agent in this case was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.76. These examples highlight the lack of a generalized rule for the utilization of NaBH4 in achieving a desired Cu NC with a hydride structure. Tang et al. introduced a novel approach, wherein the addition of NaBH4 into the reaction mixture was split into two different steps with two different temperatures, resulting in the formation of hydride-free [Cu75(S-Adm)32]2+ NC (Adm: adamantane).49 In this particular method, the metal precursor [Cu(NO3)2·3H2O] was initially treated with TOAB and HS-Adm at 80 °C in ethanol, producing a Cu(I) complex. Subsequently, the complex underwent reduction with the addition of half-equivalent amount of NaBH4 at room temperature for 12 hours, followed by another half-equivalent amount of NaBH4 at 80 °C for 5 minutes. These diverse approaches demonstrate the ongoing efforts to address challenges related to incorporating hydrides into Cu NCs, with researchers continuously exploring innovative methods to optimize the synthesis process.
4.76. These examples highlight the lack of a generalized rule for the utilization of NaBH4 in achieving a desired Cu NC with a hydride structure. Tang et al. introduced a novel approach, wherein the addition of NaBH4 into the reaction mixture was split into two different steps with two different temperatures, resulting in the formation of hydride-free [Cu75(S-Adm)32]2+ NC (Adm: adamantane).49 In this particular method, the metal precursor [Cu(NO3)2·3H2O] was initially treated with TOAB and HS-Adm at 80 °C in ethanol, producing a Cu(I) complex. Subsequently, the complex underwent reduction with the addition of half-equivalent amount of NaBH4 at room temperature for 12 hours, followed by another half-equivalent amount of NaBH4 at 80 °C for 5 minutes. These diverse approaches demonstrate the ongoing efforts to address challenges related to incorporating hydrides into Cu NCs, with researchers continuously exploring innovative methods to optimize the synthesis process.
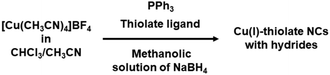
On the other side, we observed Nematulloev et al. synthesized [Cu15(PPh3)6(PET)13]2+ NC (PET: 2-phenylethanethiol) by utilizing methanolic solution of NaBH4.50 This synthesis stands out as a rare instance where the use of a methanolic solution of NaBH4 resulted in the formation of a hydride-free Cu(I)-thiolate NC. The intricate process involved a one-pot synthetic approach. Initially, [Cu(CH3CN)4]BF4 underwent treatment with PET and PPh3 in a medium comprising acetonitrile and chloroform, setting the stage for subsequent reduction using NaBH4 in methanol medium. For example, by following the quite similar one-pot synthetic protocols (as depicted in Fig. 7) [Cu13H10(2-chloro-4-fluorobenzenethiolate)3(PPh3)7],51 [Cu14H10(PPh3)8(dimethylbenzenethiolate)3]+,52 [Cu28(S-c-C6H11)18(PPh2Py)3H8]2+,53 [Cu29(S-tert-Bu)12(PPh3)4Cl6H10]+,54,55 [Cu29(S-Adm)15Cl3(P(Ph-Cl)3)4H10]+,53 [Cu29(S-c-C6H11)18(P(Ph-p-Me)3)4H10]+,53 [Cu32(PET)24H8Cl2]2−,56 [Cu36H10(PET)24(PPh3)6Cl2],57 [Cu57H20(PET)36(PPh3)4]+,58 [Cu58H20(SPr)36(PPh3)8]2+,59 [Cu58H20(PET)36(PPh3)4]2+,60 [Cu61(S-tert-Bu)26S6Cl6H14]+,61 [Cu81H32(SPh)46(tert-BuNH2)10]3+,62 (Pr: propane) has been already synthesized. In each experimental instance, the introduction of the methanolic solution was expedited to prompt a rapid change in color within the reaction mixture. Nevertheless, variations in the thiolate ligand segment and, in certain instances, alterations in the metal precursors led to an observed shift in the molar ratio with the reducing agent. It is noteworthy to emphasize that despite the utilization of potent reducing agents at diverse molar ratios across all scenarios, the manifestation of the Cu(0) character remained absent. Some instances revealed a partial Cu(0) character, yet the precise localization of these Cu(0) characteristics remains undetermined. Notably, Das et al. undertook the synthesis of [Cu18H3(S-Adm)12(PPh3)4Cl2] NC using a protocol similar to previous methods, where the slow addition of a methanolic solution onto the reaction mixture of [Cu(CH3CN)4]BF4 with HS-Adm and PPh3 occurred in acetonitrile and chloroform mediums.63 They observed a distinctive core–shell architecture, wherein the Cu10H3Cl2 core was formed through kernel fusion mediated by a Cu(0) center. The isolation of the Cu(0) character at the center of their NC structure significantly influenced its optical properties. However, unravelling the specific role of NaBH4 in this particular reaction proved challenging, despite the similarities in reaction conditions with previous experiments only the change is slower addition of the reducing agent instead of rapid incorporation.
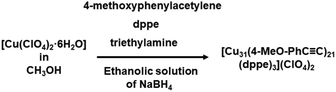
The synthesis of Cu NCs containing Cu(0) species remains a challenging endeavor, despite numerous examples being provided. Nevertheless, there are instances where certain groups have successfully isolated Cu(0) characteristics in their synthesized Cu NCs. Li et al., for instance, detected partial Cu(0) character in their [Cu14(C2B10H10S2)6(CH3CN)8] NC.64 The synthesis of this particular NC involves a one-step process which begins with the reaction of Cu(CF3COO)2 with 1,2-dithiol-o-carborane in a solvent mixture of acetonitrile and tetrahydrofuran. Notably, this reaction represents a unique transformation from Cu2+ to Cu+/Cu0, occurring through a self-reduction process. The manifestation of this process is observed through the distinct color fading of the solution upon the dropwise addition of 1,2-dithiol-o-carborane. This particular example is a rare occurrence in one-step-controlled Cu NC synthesis. Very recently Jia et al. present a synthetic method of Cu(0)-core containing [Cu31(4-MeO-PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)21(dppe)3](ClO4)2 NC (dppe: 1,2-bis(diphenylphosphino)ethane).65 Initially, Cu perchlorate hexahydrate was subjected to treatment with 4-methoxyphenylacetylene, triethylamine, and dppe in a methanol medium as depicted in Fig. 8. Subsequently, an ethanolic solution of NaBH4 was introduced into the mixture, maintaining a specific molar ratio to the metal precursor, precisely at 1
C)21(dppe)3](ClO4)2 NC (dppe: 1,2-bis(diphenylphosphino)ethane).65 Initially, Cu perchlorate hexahydrate was subjected to treatment with 4-methoxyphenylacetylene, triethylamine, and dppe in a methanol medium as depicted in Fig. 8. Subsequently, an ethanolic solution of NaBH4 was introduced into the mixture, maintaining a specific molar ratio to the metal precursor, precisely at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.9. This methodical approach highlights the careful orchestration of reactants and conditions, reflecting the intricate process undertaken to achieve the synthesis of Cu nanoparticles with a Cu(0) core.
3.9. This methodical approach highlights the careful orchestration of reactants and conditions, reflecting the intricate process undertaken to achieve the synthesis of Cu nanoparticles with a Cu(0) core.
3. Conclusions and outlook
In conclusion, our comprehensive exploration of various Cu NCs synthesis methods have shed light on the critical role of the reducing agent in traditional metallic core containing metal NCs synthesis. The intricate balance between reactivity and stability in intermediate Cu complexes poses a significant challenge in achieving the desired structural architecture with Cu(0) character. Throughout our detailed examination of various synthetic protocols, we meticulously identified and addressed the challenges associated with each approach, presenting viable remedial strategies.Our deliberations have culminated in the formulation of a systematic roadmap for the synthesis of new Cu NCs. The key variables that should be meticulously considered in choosing specific reaction conditions include the nature of precursor metal salts, their oxidation states, and the structural architecture of the diverse ligands associated with these clusters. It is imperative to meticulously select the reducing agent and determine its optimal amount, taking into account all the aforementioned factors and the specific reaction conditions.
We anticipate that this roadmap will serve as a valuable guide for researchers seeking to navigate the intricate landscape of Cu NCs synthesis. By offering a clear and informed decision-making framework, our discussion aims to empower researchers to discern the exact route for synthesizing novel Cu NCs successfully. We envision that this comprehensive understanding of the synthesis process will not only expedite current research efforts but also lay the groundwork for future endeavors in the realm of Cu NC synthesis. This collective knowledge will undoubtedly contribute to the advancement of scientific understanding and the exploration of new possibilities in nanomaterial research.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant no. 23H00289 and 22K19012), Scientific Research on Innovative Areas “Aquatic Functional Materials” (grant no. 22H04562), the Yazaki Memorial Foundation for Science and Technology, and the Ogasawara Foundation for the Promotion of Science and Engineering.References
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS PubMed
.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed
.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed
.
- Y. Negishi, Phys. Chem. Chem. Phys., 2022, 24, 7569–7594 RSC
.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526–622 CrossRef CAS PubMed
.
- T. Kawawaki, A. Ebina, Y. Hosokawa, S. Ozaki, D. Suzuki, S. Hossain and Y. Negishi, Small, 2021, 17, 2005328 CrossRef CAS PubMed
.
- W. Kurashige, Y. Niihori, S. Sharma and Y. Negishi, Coord. Chem. Rev., 2016, 320, 238–250 CrossRef
.
- H. Hirai, S. Ito, S. Takano, K. Koyasu and T. Tsukuda, Chem. Sci., 2020, 11, 12233–12248 RSC
.
- X. Kang and M. Zhu, Chem. Soc. Rev., 2019, 48, 2422–2457 RSC
.
- Y. Jin, C. Zhang, X. Y. Dong, S. Q. Zang and T. C. W. Mak, Chem. Soc. Rev., 2021, 50, 2297–2319 RSC
.
- Y. P. Xie, J. L. Jin, G. X. Duan, X. Lu and T. C. W. Mak, Coord. Chem. Rev., 2017, 331, 54–72 CrossRef CAS
.
- S. Biswas, S. Das and Y. Negishi, Coord. Chem. Rev., 2023, 492, 215255 CrossRef CAS
.
- S. Biswas, A. K. Das and S. Mandal, Acc. Chem. Res., 2023, 56, 3721–3727 CrossRef PubMed
.
- R. S. Dhayal, W. E. van Zyl and C. W. Liu, Acc. Chem. Res., 2023, 56, 1838–1849 CrossRef PubMed
.
- A. Baghdasaryan and T. Bürgi, Nanoscale, 2021, 13, 6283–6340 RSC
.
- S. Biswas and Y. Negishi, J. Phys. Chem. Lett., 2024, 15, 947–958 CrossRef CAS PubMed
.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402 CrossRef CAS PubMed
.
- S. Biswas, S. Das and Y. Negishi, Nanoscale Horiz., 2023, 18, 1509–1522 RSC
.
- Y. Liu, J. Yu, Y. Lun, Y. Wang, Y. Wang and S. Song, Adv. Funct. Mater., 2023, 33, 2304184 CrossRef CAS
.
- K. B. Busi, M. Palanivel, K. K. Ghosh, W. B. Ball, B. Gulyás, P. Padmanabhan and S. Chakrabortty, Nanomaterials, 2022, 12, 301 CrossRef PubMed
.
- A. W. Cook, Z. R. Jones, G. Wu, S. J. Teat, S.-L. Scott and T.-W. Hayton, Inorg. Chem., 2019, 58, 8739–8749 CrossRef CAS PubMed
.
- X. Ge, S. Zeng, H. Deng, B. K. Teo and C. Sun, Coord. Chem. Rev., 2024, 503, 215667 CrossRef CAS
.
- T. A. D. Nguyen, Z. R. Jones, D. F. Leto, G. Wu, S. L. Scott and T. W. Hayton, Chem. Mater., 2016, 28, 8385–8390 CrossRef CAS
.
- X. Jia, J. Li and E. Wang, Small, 2013, 9, 3873–3879 CrossRef CAS PubMed
.
- D. Li, Z. Chen, Z. Wan, T. Yang, H. Wang and X. Mei, RSC Adv., 2016, 6, 34090–34095 RSC
.
- C. Zhang, Z. Wang, W.-D. Si, L. Wang, J.-M. Dou, Z.-Y. Gao, C.-H. Tung and D. Sun, ACS Nano, 2022, 16, 9598–9607 CrossRef CAS PubMed
.
- C. Zhang, Z. Wang, W.-D. Si, H. Chu, L. Zhou, T. Li, X.-Q. Huang, Z.-Y. Gao, M. Azam, C.-H. Tung, P. Cui and D. Sun, Nat. Commun., 2023, 14, 6413 CrossRef CAS PubMed
.
- H.-S. Kim, Y. S. Seo, K. Kim, J. W. Han, Y. Park and S. Cho, Nanoscale Res. Lett., 2016, 11, 1–9 CrossRef CAS PubMed
.
- M. E. Moret, L. Zhang and J. C. Peters, J. Am. Chem. Soc., 2013, 135, 3792–3795 CrossRef CAS PubMed
.
- D. S. Weinberger, N. Amin SK, K. C. Mondal, M. Melaimi, G. Bertrand, A. C. Stückl, H. W. Roesky, B. Dittrich, S. Demeshko, B. Schwederski, W. Kaim, P. Jerabek and G. Frenking, J. Am. Chem. Soc., 2014, 136, 6235–6238 CrossRef CAS PubMed
.
- B. L. Han, Z. Liu, L. Feng, Z. Wang, R. K. Gupta, C. M. Aikens, C. H. Tung and D. Sun, J. Am. Chem. Soc., 2020, 142, 5834–5841 CrossRef CAS PubMed
.
- H. Zhao, C. Zhang, B. Han, Z. Wang, Y. Liu, Q. Xue, C. H. Tung and D. Sun, Nat. Synth., 2024 DOI:10.1038/s44160-023-00467-4
.
- P. K. Liao, B. Sarkar, H. W. Chang, J.-C. Wang and C.-W. Liu, Inorg. Chem., 2009, 48, 4089–4097 CrossRef CAS PubMed
.
- P. K. Liao, K. G. Liu, C. S. Fang, C. W. Liu, J. P. Fackler Jr. and Y. Y. Wu, Inorg. Chem., 2011, 50, 8410–8417 CrossRef CAS PubMed
.
- P. K. Liao, C. S. Fang, A. J. Edwards, S. Kahlal, J. Y. Saillard and C. W. Liu, Inorg. Chem., 2012, 51, 6577–6591 CrossRef CAS PubMed
.
- R. S. Dhayal, J. H. Liao, S. Kahlal, X. Wang, Y. C. Liu, M. H. Chiang, W. E. van Zyl, J. Y. Saillard and C. W. Liu, Chem. – Eur. J., 2015, 21, 8369–8374 CrossRef CAS PubMed
.
- R. S. Dhayal, J. H. Liao, Y. R. Lin, P. K. Liao, S. Kahlal, J. Y. Saillard and C. W. Liu, J. Am. Chem. Soc., 2013, 135, 4704–4707 CrossRef CAS PubMed
.
- A. J. Edwards, R. S. Dhayal, P. K. Liao, J. H. Liao, M. H. Chiang, R. O. Piltz, S. Kahlal, J. Y. Saillard and C.-W. Liu, Angew. Chem., Int. Ed., 2014, 53, 7214–7218 CrossRef CAS PubMed
.
- R. Langer, M. Yadav, B. Weinert, D. Fenske and O. Fuhr, Eur. J. Inorg. Chem., 2013, 2013, 3623–3631 CrossRef CAS
.
- Y. Jin, S. Li, Z. Han, B. J. Yan, H.-Y. Li, X. Y. Dong and S.-Q. Zang, Angew. Chem., Int. Ed., 2019, 58, 12143 CrossRef CAS PubMed
.
- P. P. Sun, B. L. Han, H. G. Li, C. K. Zhang, X. Xin, J. M. Dou, Z. Y. Gao and D. Sun, Angew. Chem., Int. Ed., 2022, 61, e202200180 CrossRef CAS PubMed
.
- D. w. Lee and J. Yun, Tetrahedron Lett., 2005, 46, 2037–2039 CrossRef CAS
.
- T. A. D. Nguyen, Z. R. Jones, B. R. Goldsmith, W. R. Buratto, G. Wu, S. L. Scott and T.-W. Hayton, J. Am. Chem. Soc., 2015, 137, 13319–13324 CrossRef CAS PubMed
.
- H. Li, H. Zhai, C. Zhou, Y. Song, F. Ke, W. W. Xu and M. Zhu, J. Phys. Chem. Lett., 2020, 11, 4891–4896 CrossRef CAS PubMed
.
- F. Ke, Y. Song, H. Li, C. Zhou, Y. Du and M. Zhu, Dalton Trans., 2019, 48, 13921–13924 RSC
.
- L. Wang, X. Yan, G. Tian, Z. Xie, S. Shi, Y. Zhang, S. Li, X. Sun, J. Sun, J. He and H. Shen, Dalton Trans., 2023, 52, 3371–3377 RSC
.
- G. Dong, Z. Pan, B. Han, Y. Tao, X. Chen, G.-G. Luo, P. Sun, C. Sun and D. Sun, Angew. Chem., Int. Ed., 2023, 62, e202302595 CrossRef CAS PubMed
.
- C. Sun, N. Mammen, S. Kaappa, P. Yuan, G. Deng, C. Zhao, J. Yan, S. Malola, K. Honkala, H. Häkkinen, B. K. Teo and N. Zheng, ACS Nano, 2019, 13, 5975–5986 CrossRef CAS PubMed
.
- J. Tang, C. Liu, C. Zhu, K. Sun, H. Wang, W. Yin, C. Xu, Y. Li, W. Wang, L. Wang, R. Wu, C. Liu and J. Huang, Nanoscale, 2023, 15, 2843–2848 RSC
.
- S. Nematulloev, R.-W. Huang, J. Yin, A. Shkurenko, C. Dong, A. Ghosh, B. Alamer, R. Naphade, M.-N. Hedhili, P. Maity, M. Eddaoudi, O. F. Mohammed and O. M. Bakr, Small, 2021, 17, 2006839 CrossRef CAS PubMed
.
- X. Lin, J. Tang, C. Zhu, L. Wang, Y. Yang, R. Wu, H. Fan, C. Liu and J. Huang, Chem. Sci., 2023, 14, 994–1002 RSC
.
- S. Zeng, X. Ge, H. Deng, S. Hao, Z. Zhang, B. K. Teo and C. Sun, J. Cluster Sci., 2023, 1–5, DOI:10.1007/s10876-023-02469-w
.
- Y. Bao, X. Wu, B. Yin, X. Kang, Z. Lin, H. Deng, H. Yu, S. Jin, S. Chen and M. Zhu, Chem. Sci., 2022, 13, 14357–14365 RSC
.
- A. K. Das, S. Biswas, A. Pal, S. S. Manna, A. Sardar, P.-K. Mondal, B. Sahoo, B. Pathak and S. Mandal, Nanoscale, 2024, 16, 3583–3590 RSC
.
- J. Sun, X. Yan, L. Wang, Z. Xie, G. Tian, L. Wang, A. He, S. Li, Q. Guo, Chaolumen, J. He and H. Shen, Inorg. Chem., 2023, 62, 9005–9013 CrossRef CAS PubMed
.
- S. Lee, M. S. Bootharaju, G. Deng, S. Malola, W. Baek, H. Häkkinen, N. Zheng and T. Hyeon, J. Am. Chem. Soc., 2020, 142, 13974–13981 CrossRef CAS PubMed
.
- C. Dong, R. W. Huang, C. Chen, J. Chen, S. Nematulloev, X. Guo, A. Ghosh, B. Alamer, M. N. Hedhili, T. T. Isimjan, Y. Han, O. F. Mohammed and O. M. Bakr, J. Am. Chem. Soc., 2021, 143, 11026–11035 CrossRef CAS PubMed
.
- G. G. Luo, Z. H. Pan, B. L. Han, G. L. Dong, C. L. Deng, M. Azam, Y. W. Tao, J. He, C. F. Sun and D. Sun, Angew. Chem., Int. Ed., 2023, 62, e202306849 CrossRef CAS PubMed
.
- S. Biswas, S. Hossian, T. Kosaka, J. Sakai, D. Arima, Y. Niihori, M. Mitsui, D.-e. Jiang, S. Das, S. Wang and Y. Negishi, Chem. Commun., 2023, 59, 9336–9339 RSC
.
- C. Dong, R. W. Huang, A. Sagadevan, P. Yuan, L. Gutiérrez-Arzaluz, A. Ghosh, S. Nematulloev, B. Alamer, O. F. Mohammed, I. Hussain, M. Rueping and O. M. Bakr, Angew. Chem., Int. Ed., 2023, 62, e202307140 CrossRef CAS PubMed
.
- A. Ghosh, R. W. Huang, B. Alamer, E. Abou-Hamad, M.-N. Hedhili, O. F. Mohammed and O. M. Bakr, ACS Mater. Lett., 2019, 1, 297–302 CrossRef CAS
.
- R.-W. Huang, J. Yin, C. Dong, A. Ghosh, M. J. Alhilaly, X. Dong, M. N. Hedhili, E. Abou-Hamad, B. Alamer, S. Nematulloev, Y. Han, O. F. Mohammed and O. M. Bakr, J. Am. Chem. Soc., 2020, 142, 8696–8705 CrossRef PubMed
.
- A. K. Das, S. Biswas, V. S. Wani, A.-S. Nair, B. Pathak and S. Mandal, Chem. Sci., 2022, 13, 7616–7625 RSC
.
- Y. L. Li, J. Wang, P. Luo, X. H. Ma, X. Y. Dong, Z. Y. Wang, C. X. Du, S.-Q. Zang and T. C. W. Mak, Adv. Sci., 2019, 6, 1900833 CrossRef CAS PubMed
.
- T. Jia, Z. J. Guan, C. Zhang, X. Z. Zhu, Y. X. Chen, Q. Zhang, Y. Yang and D. Sun, J. Am. Chem. Soc., 2023, 145, 10355–10363 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |