Low birefringence and low dispersion aliphatic thermosets with a high and tunable refractive index†
Yujin
Jeon‡
a,
Jisung
Choi§‡
a,
Donghwa
Seo¶
a,
Soon Hwa
Jung
b and
Jeewoo
Lim
 *a
*a
aDepartment of Chemistry, College of Sciences, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul, 02447, Korea. E-mail: jeewoo@khu.ac.kr
bPlatform Technology Center, CTO, LG Chem, E7 Block, LG Science Park, 30, Magokjungang 10-ro, Gangseo-gu, Seoul, 07796, Korea
First published on 6th February 2023
Abstract
We herein present a new class of high refractive index polymers prepared through the reaction of bis(2,3-epithiopropyl)sulfide, a series of poly(alkyl polysulfide)s, and elemental sulfur. The addition of an amine catalyst leads to rapid polymerization, resulting in a series of thermosets with high transmittance over the entire visible range. The refractive index of the thermosets can be fine-tuned over the range of 1.700–1.745 (λ = 589 nm) by varying the feed ratios of elemental sulfur and the polysulfide polymers. A wide range of properties, including the birefringence (Δn), optical dispersion, temperature coefficient of refractive index (dn/dT), transmittance, and glass transition temperature (Tg), of the thermosets are presented. The polymers exhibited a very low birefringence (Δn < 0.002), which is attributed to the absence of aromatic functional groups, exceptionally low optical dispersion (VD > 30), Tg > 60 °C, and dn/dT similar to that of PMMA.
Introduction
Transparent and high refractive index polymers (HRIPs)1,2 constitute an integral class of materials for advanced optical components. Applications of HRIPs span from ophthalmic lenses, optical filters, and optical adhesives to high performance display substrates, encapsulants, microlens arrays for image sensors, high reflective/antireflective coatings, and beyond. The advantages, such as low cost, light weight, good impact resistance, and high processability, of HRIPs over their inorganic counterparts have been the major driving force behind the extensive research efforts for the development of novel HRIPs.The Lorentz–Lorenz equation,3,4 which relates the refractive index of a material to its composition, is often used as the basis for designing HRIPs. One form of the equation is
| n = √((1 + 2[R]/V0)/(1 − [R]/V0)) |
While the refractive index (n) of an HRIP is its defining feature, there are other parameters crucial for its wide applicability. HRIPs need low optical dispersion (high Abbe number, VD) and high optical isotropy (low birefringence, Δn).16,17 Both optical dispersion and optical isotropy are related to the polarizability of the repeating unit, with high polarizability leading to high optical dispersion and large anisotropy of the directional components of polarizability (of a repeating unit with respect to the polymer's main chain axis) leading to low optical isotropy. The temperature coefficient of refractive index (dn/dT), which is a measure of changes in a material's refractive index with temperature, is also crucial for the application of HRIPs in advanced electronic applications. Despite the importance of the thermal and optical properties beyond the refractive index, reports presenting such properties are relatively rare.
The vast majority of HRIPs exhibit a nD (refractive index at λ = 589.3 nm) of over 1.6, and the attention has recently shifted to those that have a nD of over 1.7.18 Such HRIPs are yet very scarce and are largely limited to materials with high aromatic group contents.10,14,15,19,20 The presence of conjugated π-systems in HRIPs, however, usually leads to large Δn and low VD.21 Careful monomer design to force aromatic groups into an orthogonal orientation is required to prevent the alignment and π–π interactions of aromatic groups.22,23 Despite such efforts, achieving HRIPs featuring a combination of high nD (>1.70), high VD (>30), and low Δn (<0.002) still remains a formidable challenge.
Polysulfide polymers,24 the structure of which consists of alternating polysulfide and aliphatic chains, are among the polymers with the highest sulfur contents known to date. While the excellent thermal stability, weatherability, and chemical resistance of these elastomeric materials led to their widespread application as sealants, their low Tg and Tm,25 along with their low transparency, make them unsuitable for direct application as HRIPs.
Based on previous reports on linear polymers from sulfur and thiiranes,26,27 along with our recent studies towards the development of functional polysulfide latexes,28,29 we envisioned that fully aliphatic polysulfide polymers and their analogues could be used as starting materials in the preparation of high sulfur content HRIPs under suitable polymerization conditions. Herein, we report a new class of HRIPs, p(PS-BEPS), prepared through the copolymerization of polysulfide polymers, bis(2,3-epithiopropyl)sulfide (BEPS), and elemental sulfur (Scheme 1). Unlike previous HRIPs from episulfides,19,30,31 no thiols are used for the reaction with episulfides. This is potentially advantageous since thiols have relatively poor shelf lives.32 The resulting polymers exhibit high transparency over the visible range and into the NIR region (up to 2200 nm) and, under optimized conditions, a nD of 1.7448, a Δn of 0.0013, and a VD of 30.8. The temperature coefficient of refractive index is −1.52 × 10−4 K−1, which is similar to that of a widely used optical polymer, PMMA. Interestingly, the refractive index could be tuned over the range of 1.7000–1.7448 in a modular fashion, simply by varying the feed ratio of the polysulfide prepolymer and elemental sulfur.
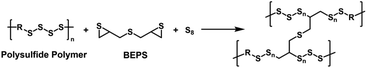 | ||
| Scheme 1 Synthesis of high sulfur content, fully aliphatic HRIPs under thiol-free conditions involving the polysulfide polymer, bis(episulfide) (BEPS), and elemental sulfur. | ||
Experimental
Materials and methods
All reagents were commercially available from Alfa Aesar, Sigma-Aldrich, and TCI. Bis(2,3-epithiopropyl) sulfide was provided by LG Chem. Divinyl sulfone was purified by directly passing through a short column of neutral alumina prior to use. Other chemicals were used as received unless otherwise noted. The preparation of polysulfide stock solutions and polysulfide polymers therefrom was carried out under a dry argon atmosphere using standard Schlenk techniques. S-DIB films were prepared using previously reported methods.33 Films (thickness = 1 mm) of p(PS-BEPS) were prepared by conducting the polymerization in a mold pre-treated with a releasing agent. Fourier transform infrared (FT-IR) spectra from 3500 cm−1 to 400 cm−1 were obtained using a Bruker Invenio-R instrument. The transmittance of the thermosets was measured using a Shimadzu UV-1800 spectrophotometer in the wavelength range of 200–800 nm. Differential scanning calorimetry (DSC) was conducted using a TA Instruments DSC 250. Thermogravimetric analysis was carried out on a TA Instruments SDT Q600 TGA. Elemental analyses (CHNS) of the prepolymers were conducted on a CE Instruments Flash EA1112. The refractive index, birefringence, thermo-optic coefficient, and film thickness were measured on a Sairon Technology SPA-4000 prism coupler using a 632.8 nm He–Ne laser light source. Both GGG and high refractive index rutile prisms were used. Abbe number was obtained using an ATAGO DR-M4 multi-wavelength Abbe refractometer.Synthetic procedures
Results and discussion
Polysulfide polymers were prepared by the reaction of aqueous sodium polysulfides with divinyl sulfone (DVS) and dichloroethane (DCE) (Fig. 1a). DVS was chosen since it readily undergoes a polysulfide–ene29 precipitation polymerization in the aqueous phase to give the corresponding polysulfide polymers under mild conditions and contains the sulfone group, which, while having a slightly smaller molar refraction than functional groups with sulfur atoms in lower oxidation states,2 features very low molar dispersion.17 DCE was chosen to maximize the sulfur content of the polysulfide polymers. An aqueous polysulfide solution was prepared by dissolving elemental sulfur in a solution of sodium sulfide. The stoichiometry was controlled to provide polysulfide solutions having an average sulfur rank of 4. A slight excess of DVS and DCE was used to prevent the formation of thiol chain ends which are prone to chain extension through oxidation.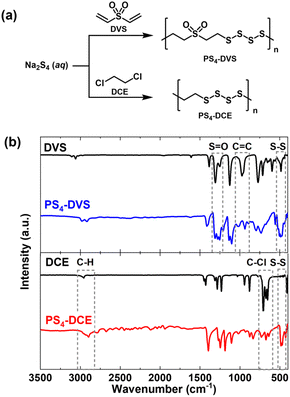 | ||
| Fig. 1 (a) Synthesis of polysulfide polymers used in this work and (b) FT-IR spectra of polysulfide polymers obtained from DVS (top) and DCE (bottom). | ||
The two polysulfide polymers, PS4-DVS and PS4-DCE, showed extremely low solubility in water and in common organic solvents, making characterization through common solution-based methods difficult. FT-IR spectroscopy revealed, along with the emergence of S–S bond vibrations34 in the 450–500 cm−1 region, nearly complete consumption of vinyl groups (for PS4-DVS) and C–Cl bonds (for PS4-DCE) (Fig. 1b). The results of thermogravimetric analysis of PS4-DCE and PS4-DVS (Fig. S1†) were consistent with those of previously reported polysulfide rubbers.25 The differential scanning calorimetry of the two polysulfide polymers (Fig. S2†) indicated melting transition at 67 °C, also consistent with the thermal transitions previously reported for polysulfide rubbers.
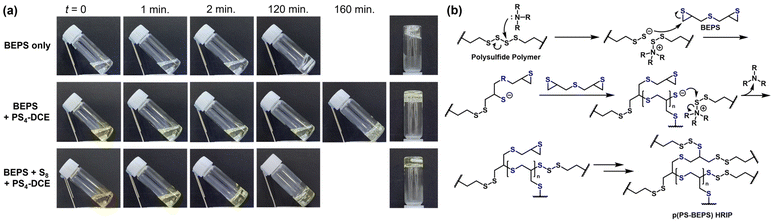
Despite their lack of solubility in common organic solvents, the prepolymers exhibited solubilities of over 15 wt% in BEPS. While the solutions of polysulfide polymers in BEPS did not undergo spontaneous vitrification under ambient conditions, the introduction of a catalytic amount of dicyclohexyl methyl amine led to the vitrification of the solutions to give transparent, glassy polymers (p(PS-BEPS), Fig. 2a). Interestingly, the yellow color of PS4-DCE faded to give a reaction mixture with a pale-yellow tint upon the addition of dicyclohexyl methyl amine. Organic tri- and tetrasulfides have been reported to undergo facile desulfurization by amines35 and phosphines36 through a process initiated by the nucleophilic attack of the S–S bond. The observation that the characteristic yellow color37 of long polysulfides fades upon the addition of the amine suggests that the catalyst reacts with the polysulfide unit of the polysulfide polymer, which would then initiate curing through a proposed mechanism shown in Fig. 2b. Similar curing behavior was observed with other polysulfide polymers. The thermogravimetric analyses of p(PS-BEPS) showed a single stage weight loss, along with a significantly enhanced onset temperature of degradation (Fig. S3†). This observation suggests that the thermosets and homogeneous polymers have high degrees of crosslinking.
With the sulfur contents of BEPS, PS4-DVS, and PS4-DCE at 53.9, 64.5, and 82.1%, respectively, we expected that the refractive indices of p(PS-BEPS)s would be above 1.70. To investigate the possibility, p(PS-BEPS)s were prepared by curing the solutions of polysulfide polymers in BEPS at various concentrations and their refractive index (n632.8nm), birefringence (Δn, measured at 632.8 nm), and optical transmittance were studied (Table S1†).
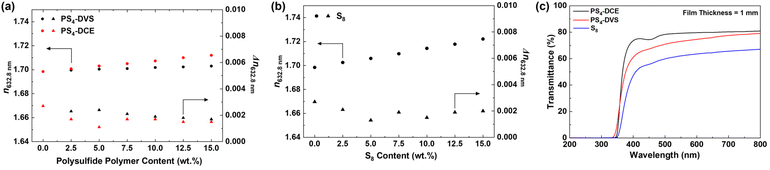
The n632.8nm values of the thermosets prepared from the polymerization of polysulfide polymers and BEPS were shown to increase with increasing polysulfide polymer content (Fig. 3a). Polysulfide polymers bearing DVS units demonstrated a lower degree of enhancement than those bearing DCE units, an observation consistent with the higher sulfur content of PS4-DCE.
The n632.8nm increase was found to show a linear relationship with the polysulfide polymer content, allowing for the slope of the linear fit to be used for quantitative comparison of the impact of each polysulfide polymer on the resulting polymers’ optical properties. This analysis showed that, for PS4-DVS, a 1 wt% increase resulted in a n632.8nm increase of 2.7 × 10−4. At 15 wt% loading, the p(PS-BEPS) polymer from PS4-DVS exhibited a n632.8nm value of 1.7030. The polysulfide polymer from DCE has a stronger impact on n632.8nm increase, with a 1 wt% increase in the PS4-DCE content giving a n632.8nm increase of 9.0 × 10−4. A n632.8nm of 1.712 was measured at a PS4-DCE content of 15 wt%.
Glassy polymers could also be obtained by replacing polysulfide polymers with elemental sulfur (S8) (Table S2† and Fig. 3b). Per wt.% increase in the n632.8nm values of 15.8 × 10−4 was obtained from S8. The n632.8nm value obtained at 15 wt% loading was 1.722 for S8. While all thermosets exhibited high transmittance over the visible range (Fig. 3c and Fig. S4†), p(PS-BEPS), obtained from the reaction of elemental sulfur and BEPS, showed lower transmittance along with a window of transparency starting at a longer wavelength compared to those from polysulfide polymers. This observation is attributed to the presence of longer polysulfide chains in p(PS-BEPS) from sulfur due to higher sulfur contents compared to the polysulfide polymer counterparts.37 In all polymers, extremely low Δn values of below 0.002 were obtained, which is attributed to the absence of highly polarizable functional groups, such as aromatic moieties and halogens (excluding F and Cl), in p(PS-BEPS)s.
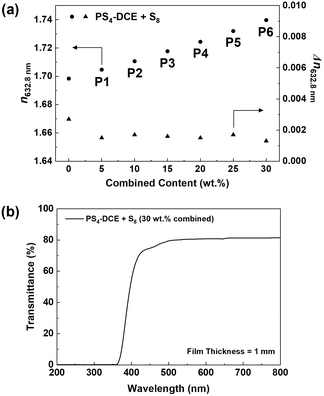
To probe the possibility of increasing the n632.8nm beyond 1.720 while maintaining high optical transmittance and low birefringence, polymerization was conducted using a neat mixture of BEPS and equal amounts of PS4-DCE and S8 to yield p(PS-BEPS)s having a combined PS4-DCE/S8 content of 5.0 to 30.0 wt%. Glassy, transparent polymers (P1–P6) could be obtained, the refractive indices of which increased linearly upon increasing the combined content of PS4-DCE and S8 over the range of 1.71–1.74 (Fig. 4). While attempts to increase the loading of PS4-DCE or S8 each beyond 15 wt% in BEPS led to cloudy reaction mixtures yielding polymers with low transmittance, a glassy, transparent polymer having a n632.8nm approaching 1.74 and a birefringence of below 0.0015 could be obtained at a combined content of 30 wt% (P6).
With a simple, solvent- and thiol-free method for preparing high n, low Δn polymers free of conjugated π-systems in hand, the wavelength-dependent dispersion in the refractive index of the materials was investigated. The trade-off between refractive index and optical dispersion, where materials with high refractive index also exhibit high optical dispersion,38 is well established. The optical dispersion was evaluated by measuring refractive indices at 486, 589, and 656 nm using a multiangle Abbe refractometer and deriving Abbe numbers following the equation
| VD = ((nd − 1)/(nf − nc)) |
| nλ = n∞ + Dλ−2 |
The plots of nf, nd, and nc, along with the respective Cauchy fitting, are shown in Fig. 5, and the relevant optical properties are summarized in Table 1.
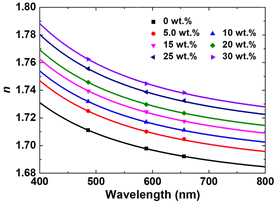 | ||
| Fig. 5 Wavelength-dependent refractive indices of BEPS copolymers prepared with various PS4-DCE/S8 loadings. | ||
| Contenta (wt%) | n d | Δnb | n ∞ | D (μm2) | V D |
|---|---|---|---|---|---|
| a Total content of PS4-DCE and S8, each added in equal weights, in BEPS. b Measured at 632.8 nm from nTE and nTM measurements. | |||||
| 0 | 1.6978 | 0.0027 | 1.6691 | 0.0099 | 36.7 |
| 5.0 (P1) | 1.7101 | 0.0015 | 1.6786 | 0.0110 | 34.9 |
| 10.0 (P2) | 1.7170 | 0.0017 | 1.6853 | 0.0106 | 34.7 |
| 15.0 (P3) | 1.7244 | 0.0016 | 1.6910 | 0.0115 | 33.1 |
| 20.0 (P4) | 1.7297 | 0.0015 | 1.6962 | 0.0121 | 32.6 |
| 25.0 (P5) | 1.7387 | 0.0017 | 1.7032 | 0.0110 | 32.1 |
| 30.0 (P6) | 1.7448 | 0.0013 | 1.7079 | 0.0127 | 30.8 |
The results show that the polymers obtained from the amine-catalyzed polymerization of BEPS with PS4-DCE and elemental sulfur, P1–P6, exhibit high refractive indices of above 1.7, extremely low birefringence values of below 0.0017, and very low optical dispersion as indicated by the VD value of over 30 even at a nd of 1.7448. Reported optical polymers that exhibit a combination of n > 1.7 and VD > 30 are extremely scarce, with thermosets containing polarizable P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se moieties14 and thin films from vapor deposition polymerization involving elemental sulfur being representative examples.39 Variations in the composition of the polymer allowed for a precise control of the refractive index without significant alterations in Δn, suggesting that the results could be utilized as a platform for producing high-n index matching materials.
Se moieties14 and thin films from vapor deposition polymerization involving elemental sulfur being representative examples.39 Variations in the composition of the polymer allowed for a precise control of the refractive index without significant alterations in Δn, suggesting that the results could be utilized as a platform for producing high-n index matching materials.
The n∞ values are the materials’ inherent refractive indexes which exclude contributions from absorptions. The n∞ values for P5 and P6, with the combined PS4-DCE and S8 contents of 25 wt% and 30 wt%, were 1.7032 and 1.7079, respectively, which are higher than those of high-index, amorphous polyimides from thiophene-bearing aromatic diamines.40 The higher n∞ values of p(PS-BEPS) than those of high-index polyimides despite the latter having higher nd values (in the range of 1.748–1.761) are attributed to the low D values resulting from the absence of conjugated π-systems which have high molar dispersion.
The thermo-optical properties of P1–P6 were accessed using variable-temperature prism coupler measurements (Fig. S5† and Table 2). In all cases, dn/dT values in the range of −1.21 to −1.62 × 104 K−1 were observed, similar to those of PMMA41 and amorphous polyimides.42 The linear fit of n vs. T relations revealed a change in the slope between 62 and 64 °C, suggesting a more rapid expansion of the specific volume above the temperature range due to glass transition. The Tg values of P1–P6 were obtained from the intersection of the slopes and were found to be similar regardless of polymer composition. Evaluation of Tg values using differential scanning calorimetry (DSC) was difficult due to weak and broad change in heat flows over the range of 60–90 °C. While we are currently investigating methods for increasing the Tg values of HRIPs obtained using polymerization methods described herein, the current results represent a very simple, solvent-free method of preparing HRIPs with n > 1.7 and Vd > 30 wherein the refractive index could be varied over a wide range without significant alterations in the materials’ birefringence, dn/dT, and Tg.
Finally, with a high sulfur content and the lack of aromatic moieties in p(PS-BEPS)s, it was expected that the material could show high transparency beyond the visible range and into the NIR region. This was of interest since transparency at 1310 and 1550 nm (7634 and 6452 cm−1) is important for telecommunication applications and significant research effort has recently been dedicated to the development of HRIPs with high transmittance at the relevant wavelengths.43 The FT-IR transmittance spectra of a 1 mm-thick sample of P6 was obtained using transmission mode and compared to those of the inverse vulcanization polymer (SDIB), obtained from elemental sulfur and diisopropenyl benzene (DIB), having an identical sulfur content (Fig. 6). The results show that P6 has high transmittance, similar to that of SDIB, in the NIR region with a cut-off wavelength at approximately 2200 nm (4500 cm−1). While the inverse vulcanization product has additional windows of transparency at longer wavelengths, the advantage of p(PS-BEPS) is in the large transparency window in the range of 400 nm–2200 nm, conducive to applications requiring transparency both in the visible and short-wavelength NIR regions.
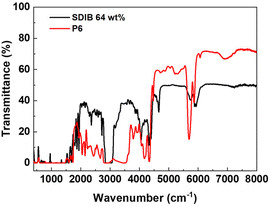 | ||
| Fig. 6 FT-IR transmittance spectra of 1 mm-thick samples of S-DIB prepared from inverse vulcanization and p(PS-BEPS) with 15 wt% each of PS4-DCE and S8. | ||
Conclusions
We have demonstrated a solvent-free method of preparing HRIPs with a high transparency window spanning from 400 to 2200 nm wherein the refractive index (nd) could be systematically varied from 1.700 to over 1.745 without changes in the polymers’ birefringence, Tg, or dn/dT. This simple, thiol-free polymerization method involves the copolymerization of a bis(episulfide), BEPS, with various polysulfide polymers and chalcogenide additives, resulting in HRIPs with a very low optical dispersion (chromatic aberration) with VD exceeding 30 even at a nd of 1.745. The very low birefringence (Δn < 0.0020) and the high intrinsic refractive index (n∞) values, which exceed those reported from high-index polyimides, were observed and attributed to the lack of highly polarizable groups such as aromatic moieties and halides (–Br and –I) in the newly developed HRIPs. The combination of optical properties (n > 1.7, Δn < 0.0020, and VD > 30), along with the modular controllability of n, make the p(PS-BEPS) suitable not only for applications requiring advanced HRIPs but also for applications requiring precise index matching at high-n ranges.Author contributions
JL and SHJ devised the project and the main conceptual ideas. YJ, JC, and DS designed and carried out the synthesis of polysulfide polymers and the thermosets therefrom. YJ and JC carried out the thermal and optical analyses of the materials. YJ, JC, and JL drafted the manuscript and designed the figures. The initial draft was edited and developed through the contribution of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank LG Chem for the financial support which made this work possible. We would also like to acknowledge the support from the NRF of Korea (grant no. 2022R1C1C1013335).References
- J. G. Liu and M. Ueda, J. Mater. Chem., 2009, 19, 8907–8919 RSC.
- T. Higashihara and M. Ueda, Macromolecules, 2015, 48, 1915–1929 CrossRef CAS.
- H. A. Lorentz, Ann. Phys., 1880, 245, 641–665 CrossRef.
- L. Lorenz, Ann. Phys., 1880, 247, 70–103 CrossRef.
- T. Matsuda, Y. Funae, M. Yoshida, T. Yamamoto and T. Takaya, J. Appl. Polym. Sci., 2000, 76, 50–54 CrossRef CAS.
- T. Matsuda, Y. Funae, M. Yoshida, T. Yamamoto and T. Takaya, J. Appl. Polym. Sci., 2000, 76, 45–49 CrossRef CAS.
- R. Okutsu, S. Ando and M. Ueda, Chem. Mater., 2008, 20, 4017–4023 CrossRef CAS.
- M. Briesenick, M. Gallei and G. Kickelbick, Macromolecules, 2022, 55, 4675–4691 CrossRef CAS.
- X. Y. Chen, L. X. Fang, J. J. Wang, F. K. He, X. R. Chen, Y. Q. Wang, J. F. Zhou, Y. Q. Tao, J. Sun and Q. Fang, Macromolecules, 2018, 51, 7567–7573 CrossRef CAS.
- K. Mazumder, H. Komber, E. Bittrich, B. Voit and S. Banerjee, Macromolecules, 2022, 55, 1015–1029 CrossRef CAS.
- B. D. Fairbanks, T. F. Scott, C. J. Kloxin, K. S. Anseth and C. N. Bowman, Macromolecules, 2009, 42, 211–217 CrossRef CAS PubMed.
- S. D. Bhagat, J. Chatterjee, B. H. Chen and A. E. Stiegman, Macromolecules, 2012, 45, 1174–1181 CrossRef CAS.
- M. D. Alim, S. Mavila, D. B. Miller, S. J. Huang, M. Podgorski, L. M. Cox, A. C. Sullivan, R. R. McLeod and C. N. Bowman, ACS Mater. Lett., 2019, 1, 582–588 CrossRef CAS.
- Y. Su, E. B. D. S. Filho, N. Peek, B. H. Chen and A. E. Stiegman, Macromolecules, 2019, 52, 9012–9022 CrossRef CAS.
- L. X. Fang, J. Sun, X. Y. Chen, Y. Q. Tao, J. F. Zhou, C. Y. Wang and Q. Fang, Macromolecules, 2020, 53, 125–131 CrossRef CAS.
- G. S. Liou, P. H. Lin, H. J. Yen, Y. Y. Yu, T. W. Tsai and W. C. Chen, J. Mater. Chem., 2010, 20, 531–536 RSC.
- Y. Suzuki, T. Higashihara, S. Ando and M. Ueda, Macromolecules, 2012, 45, 3402–3408 CrossRef CAS.
- E. K. Macdonald and A. P. Shaver, Polym. Int., 2015, 64, 6–14 CrossRef CAS.
- Y. H. Tang, C. Pina-Hernandez, Q. J. Niu, J. Nie and S. Cabrini, J. Mater. Chem. C, 2018, 6, 8823–8831 RSC.
- M. C. Fu, M. Ueda, S. Ando and T. Higashihara, ACS Omega, 2020, 5, 5134–5141 CrossRef CAS PubMed.
- K. H. Nam, A. Lee, S. K. Lee, K. Hur and H. Han, J. Mater. Chem. C, 2019, 7, 10574–10580 RSC.
- R. Seto, T. Sato, T. Kojima, K. Hosokawa, Y. Koyama, G. I. Konishi and T. Takata, J. Polym. Sci., Polym. Chem., 2010, 48, 3658–3667 CrossRef CAS.
- K. Nakabayashi, T. Imai, M. C. Fu, S. Ando, T. Higashihara and M. Ueda, Macromolecules, 2016, 49, 5849–5856 CrossRef CAS.
- D. Vietti and M. Scherrer, in Kirk-Othmer Encyclopedia of Chemical Technology, ed. Kirk-Othmer, John Wiley & Sons, New York, 2000, DOI:10.1002/0471238961.1615122522090520.a01.
- M. R. Kalaee, M. H. N. Famili and H. Mahdavi, Macromol. Symp., 2009, 277, 81–86 CrossRef CAS.
- S. Penczek, R. Slazak and A. Duda, Nature, 1978, 273, 738–739 CrossRef CAS.
- J.-Y. Chao, T.-J. Yue, B.-H. Ren, G.-G. Gu, X.-B. Lu and W.-M. Ren, Angew. Chem., Int. Ed., 2022, 61, e202115950 CAS.
- J. Lim, U. Jung, W. T. Joe, E. T. Kim, J. Pyun and K. Char, Macromol. Rapid. Commun., 2015, 36, 1103–1107 CrossRef CAS PubMed.
- H. Shin, J. Kim, D. Kim, V. H. Nguyen, S. Lee, S. Han, J. Lim and K. Char, J. Mater. Chem. A, 2018, 6, 23542–23549 RSC.
- H. Morishiri and S. Kobayashi, Jpn. Kokai Tokkyo Koho, 2005, 325274 Search PubMed.
- H. Morishiri and S. Kobayashi, Jpn. Kokai Tokkyo Koho, 2006, 131724 Search PubMed.
- B. R. B. Nasir, S. V. Sai and S. Chandrasekaran, Synlett, 2008, 2684–2688, DOI:10.1055/s-0028-1083517.
- S. Park, D. Lee, H. Cho, J. Lim and K. Char, ACS Macro Lett., 2019, 8, 1670–1675 CrossRef CAS PubMed.
- F. Feher, G. Krause and K. Vogelbruch, Chem. Ber., 1957, 90, 1570–1577 CrossRef CAS.
- Y. Minoura, Rubber Chem. Technol., 1958, 31, 615–617 CrossRef.
- D. N. Harpp and R. A. Smith, J. Am. Chem. Soc., 1982, 104, 6045–6053 CrossRef CAS.
- B. Meyer and K. Spitzer, J. Phys. Chem., 1972, 76, 2274–2279 CrossRef CAS.
- C. J. Yang and S. A. Jenekhe, Chem. Mater., 1995, 7, 1276–1285 CrossRef CAS.
- W. Jang, K. Choi, J. S. Choi, D. Kim, K. Char, J. Lim and S. G. Im, ACS Appl. Mater. Interfaces, 2021, 13, 61629–61637 CrossRef CAS PubMed.
- H. Yeo, J. Lee, M. Goh, B. C. Ku, H. Sohn, M. Ueda and N. H. You, J. Polym. Sci., Polym. Chem., 2015, 53, 944–950 CrossRef CAS.
- Z. Y. Zhang, P. Zhao, P. Lin and F. G. Sun, Polymer, 2006, 47, 4893–4896 CrossRef CAS.
- A. Rosenberg, S. H. Lee, J. S. Shirk and G. Beadie, Opt. Mater. Express, 2018, 8, 2159–2172 CrossRef CAS.
- A. Nishant, K.-J. Kim, S. A. Showghi, R. Himmelhuber, T. S. Kleine, T. Lee, J. Pyun and R. A. Norwood, Adv. Opt. Mater., 2022, 10, 2200176 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py01327d |
| ‡ These authors contributed equally to this work. |
| § Current address: Samsung Electro-Mechanics, 150, Maeyeong-ro, Yeongtong-gu, Suwon-si, Gyeonggi-do, Republic of Korea. |
| ¶ Current address: Semiconductor R&D Center, DS Division, Samsung Electronics, 118 Sinwon-ro, Yeongtong-gu, Suwon, Gyeonggi-do, Republic of Korea. |
| This journal is © The Royal Society of Chemistry 2023 |