Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, structure diversity, and antimicrobial studies of Ag(I) complexes with quinoline-type ligands†
Amal
Yousri
a,
Matti
Haukka
 b,
Morsy A. M.
Abu-Youssef
*a,
Mohammed
Salah Ayoup
*a,
Magda M. F.
Ismail
c,
Nagwan G.
El Menofy
b,
Morsy A. M.
Abu-Youssef
*a,
Mohammed
Salah Ayoup
*a,
Magda M. F.
Ismail
c,
Nagwan G.
El Menofy
 d,
Saied M.
Soliman
d,
Saied M.
Soliman
 a,
Assem
Barakat
a,
Assem
Barakat
 e,
Francoise M.
Amombo Noa
e,
Francoise M.
Amombo Noa
 f and
Lars
Öhrström
f and
Lars
Öhrström
 *f
*f
aDepartment of Chemistry, Faculty of Science, Alexandria University, P.O. Box 426, Ibrahimia, Alexandria 21321, Egypt. E-mail: morsy5@alexu.edu.eg; saied1soliman@yahoo.com
bDepartment of Chemistry, University of Jyväskylä, P.O. Box 35, FI-40014 Jyväskylä, Finland. E-mail: matti.o.haukka@jyu.fi
cDepartment of Pharmaceutical Medicinal Chemistry, Faculty of Pharmacy (Girls), Al-Azhar University, Cairo, Egypt
dDepartment of Microbiology and Immunology, Faculty of Pharmacy (Girls), Al-Azhar University, Cairo, Egypt. E-mail: m.elalfy101@gmail.com
eDepartment of Chemistry, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. E-mail: ambarakat@ksu.edu.sa
fChemistry and Biochemistry, Dept. of Chemistry and Chemical Engineering, Chalmers University of Technology, SE-41296 Göteborg, Sweden. E-mail: mystere@chalmers.se; ohrstrom@chalmers.se
First published on 16th June 2023
Abstract
Compounds [Ag(5NO2Qu)2]BF4 (1) and [Ag(Qu3CN)(H2O)]BF4 (2) were prepared and studied from a structural perspective and screened for antimicrobial activity. The Ag(I) in the monomeric complex 1 is coordinated to two 5-nitroquinoline (5NO2Qu) ligands via the N-atoms of the quinoline rings with equidistant Ag–N bonds (2.146(2) Å) and a N–Ag–N# bond angle of 171.42(8)°. The 2D coordination polymer 2 contains tetracoordinated Ag(I) with two N-atoms (N1 and N2#1) from two quinoline-3-carbonitrile (Qu3CN) ligands and two O-atoms (O1 and O1#1) from two water molecules. The Qu3CN ligand acts as a connector between the Ag(I) sites along the b-direction via two short Ag1–N1 (2.185(4) Å) and Ag1–N2#1 (2.204(4) Å) bonds. In addition, the Ag(I) is coordinated with two symmetry related water molecules which are also acting as connectors between the Ag(I) sites along the a-direction via two longer Ag1–O1 (2.470(4) Å) and Ag1–O1#2 (2.546(4) Å) bonds. Hirshfeld surface analysis confirmed the significance of the polar F⋯H contacts in the molecular packing of 1 (25.9%) and 2 (39.9%). In addition, the crystal packing of 1 showed a significant amount of polar O⋯H (23.5%) contacts. Also, both complexes displayed π–π stacking interactions. The Ag(I) complexes and the free ligand were assessed for their antimicrobial activities. It was found that 1 (MIC = 7.8 μg mL−1) and 2 (MIC = 31.25 μg mL−1) have higher antifungal potency against C. albicans than their free ligands (MIC = 125 μg mL−1). Interestingly, 1 has better antifungal activity than the standard nystatin (15.6 μg mL−1). Also, both Ag(I) complexes and the free ligands as well have better activity against P. mirabilis than the common antibiotic amoxicillin.
1. Introduction
The use of transition metal compounds as therapeutic agents has attracted the attention of researchers for a long time.1–6 The reason is that coordination compounds may be effective in drug design, particularly for the creation of innovative antimicrobial drugs, due to the enormous variety of metals, ligands, and geometries.7 Over a long period of time, considerable interest has been shown in Ag(I) and its complexes. Due to the d10-electron configuration of Ag(I), it forms a wide range of geometries as it lacks stereochemical selectivity, except some preference for linear two-coordinated complexes.The coordination geometry of Ag(I) complexes can thus be linear,8–11 trigonal planar,12–14 T-shaped,15,16 square planar,17,18 tetrahedral,19,20 trigonal bipyramidal,21,22 square pyramidal,23,24 and octahedral.25–27
These complexes show a wide range of biological activities against bacteria, fungi, viruses, and cancerous cells.28–32 Also, Ag(I) and its complexes have potential uses in wound treatment and can be used in anti-infection creams.33–35
On the other hand, quinoline and its derivatives represent a significant category of nitrogen heterocycles36,37 which have interesting structural characteristics capable of influencing the geometry of their metal complexes.
The main reason for such structural properties is the existence of π–π stacking which is one of the significant non-covalent interactions between the atoms of fused polycyclic aromatic rings.38 Also, quinoline based derivatives are considered structural components of many medicines with antibacterial, antimalarial, and anticancer properties.36–46 Generally, quinolines are commonly used in many aspects such as the production of herbicides, corrosion inhibitors, and sensors.36,47 In addition, aromatic compounds with nitro groups, especially nitroquinoline derivatives, are frequently used in the manufacture of drugs, dyes, and explosives.48
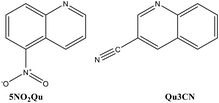
Recently, combinations of N-heterocycles with Ag(I) ions to produce more effective antibacterial agents have been reported.49–52 Quinoline-type ligands are one of the most prominent N-heterocycles which form Ag(I) complexes with versatile structures and interesting biological activity.38 In this work, two new Ag(I) complexes with the 5-nitroquinoline (5NO2Qu) and quinoline-3-carbonitrile (Qu3CN) ligands were synthesized (Fig. 1). The Ag(I) complexes were characterized using several experimental methods including elemental analysis, FTIR, NMR and single crystal X-ray diffraction combined with Hirshfeld calculations. In the light of the remarkable antimicrobial activity of Ag(I) complexes and quinolines as well, the antimicrobial activities of the newly synthesized Ag(I) complexes were assessed.
2. Experimental
2.1 Materials and methods
[Ag(5NO2Qu)2]BF4 (1). AgBF4 (77.9 mg, 0.4 mmol) in 5 mL distilled water was mixed with 5NO2Qu (139.3 mg, 0.8 mmol) in 10 mL ethanol giving a turbid solution. Acetonitrile was added dropwise until the solution became clear. The clear solution was left to slowly evaporate at room temperature giving colorless crystals after five days which were collected from solution by filtration. Yield: 85%; anal. calc. C18H12AgBF4N4O4: C, 39.82; H, 2.23; N, 10.32; Ag, 19.87%. Found: C, 39.60; H, 2.15; N, 10.31; Ag, 19.69%. FTIR cm−1: 3105, 3072, 1624, 1592, 1521, 1341, 1066, 1031. Ligand (5NO2Qu): 3071, 1626, 1593, 1520, 1321 (Fig. S1†). 1H NMR (500 MHz, DMSO-d6), δH: 9.04 (dd, J = 4.0 Hz, 1.5 Hz, 1H, Ar–H), 8.79 (d, J = 8.5 Hz, 1H, Ar–H), 8.40 (t, J = 8.5 Hz, 2H, Ar–H), 7.92 (t, J = 8.0 Hz, 1H, Ar–H), 7.76 (dd, J = 8.0 Hz, 4.5 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δC: 152.5, 147.9, 145.8, 136.5, 131.9, 128.9, 125.2, 124.8, 120.7 (Fig. S2†). [Ag(5NO2Qu)2]BF4 (1): 1H NMR (500 MHz, DMSO-d6), δH: 9.05 (dd, J = 4.0 Hz, 4.0 Hz, 1H, Ar–H), 8.81 (d, J = 8.5 Hz, 1H, Ar–H), 8.42 (t, J = 8.5 Hz, 2H, Ar–H), 7.93 (t, J = 8.0 Hz, 1H, Ar–H), 7.78 (dd, J = 8.5 Hz, 4.5 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δC: 152.7, 147.9, 145.9, 136.5, 132.1, 128.9, 125.4, 124.7, 120.7 (Fig. S3†).
[Ag(Qu3CN)(H2O)]BF4 (2). AgBF4 (77.9 mg, 0.4 mmol) in 5 mL distilled water was mixed with Qu3CN (61.7 mg, 0.4 mmol) in 5 mL ethanol giving a turbid solution. Acetonitrile was added dropwise until the solution became clear. The clear solution was left to slowly evaporate at room temperature giving colorless crystals after five days which were collected from solution by filtration. Yield: 88%; anal. calc. C10H8AgBF4N2O: C, 32.74; H, 2.20; N, 7.64; Ag, 29.40%. Found: C, 32.55; H, 2.13; N, 7.51; Ag, 29.25%. FTIR cm−1: 3354, 3062, 2227, 1621, 1567, 1063, 1033. Ligand (Qu3CN): 3060, 2226, 1618, 1565 (Fig. S4†). 1H NMR (500 MHz, DMSO-d6), δH: 9.13 (d, J = 1.5 Hz, 1H, Ar–H), 9.05 (d, J = 2.5 Hz, 1H, Ar–H), 8.08 (t, J = 9.0 Hz, 2H, Ar–H), 7.94 (t, J = 8.5 Hz, 1H, Ar–H), 7.74 (t, J = 8.0 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δC: 150.7, 148.5, 143.0, 133.5, 129.5, 129.9, 128.9, 126.4, 117.9 (Ar–C), 106.3 (CN) (Fig. S5†). [Ag(Qu3CN)(H2O)]BF4 (2): 1H NMR (500 MHz, DMSO-d6), δH: 9.13 (d, J = 2.5 Hz, 1H, Ar–H), 9.06 (d, J = 1.5 Hz, 1H, Ar–H), 8.08 (t, J = 9.0 Hz, 2H, Ar–H), 7.95 (t, J = 8.5 Hz, 1H, Ar–H), 7.75 (t, J = 7.5 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δC: 150.6, 148.5, 143.1, 133.6, 129.6, 129.4, 128.9, 126.4, 118.0 (Ar–C), 106.3 (CN) (Fig. S6†).
| Complex number | 1 | 2 |
|---|---|---|
| Empirical formula | C18H12AgBF4N4O4 | C10H8AgBF4N2O |
| Formula weight (g mol−1) | 543 | 366.86 |
| Temperature | 170(2) K | 120(2) K |
| Wavelength | 0.71073 Å | 1.54184 Å |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c |
| Unit cell dimensions | a = 9.8173(4) Å | a = 10.0480(2) Å |
| b = 12.9566(6) Å | b = 16.8535(2) Å | |
| c = 14.7156(4) Å | c = 14.5843(2) Å | |
| β = 101.124(2)° | β = 98.505(2)° | |
| Volume | 1836.64(12) Å3 | 2442.60(7) Å3 |
| Z | 4 | 8 |
| Density (calc.) | 1.964 Mg m−3 | 1.995 Mg m−3 |
| Abs. coefficient | 1.175 mm−1 | 13.686 mm−1 |
| F(000) | 1072 | 1424 |
| Crystal size (mm) | 0.237 × 0.202 × 0.122 | 0.095 × 0.040 × 0.018 |
| Theta range | 2.635 to 29.699° | 5.167 to 77.338° |
| Index ranges | −13 ≤ h ≤ 13, −16 ≤ k ≤ 17, −20 ≤ l ≤ 20 | −12 ≤ h ≤ 11, −21 ≤ k ≤ 21, −18 ≤ l ≤ 18 |
| Reflections | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 574 574 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 689 689 |
| Indep. reflections | 2566 [R(int) = 0.0265] | 2596 [R(int) = 0.0851] |
| Completeness | 99.30% | 100.00% |
| Absorption correction | Semi-empirical from equivalents | Gaussian |
| Max. and min. transm. | 0.7459 and 0.6792 | 1.000 and 0.928 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| CCDC | CCDC 2246560 | CCDC 2246561 |
2.2 Hirshfeld surface analysis
Hirshfeld surfaces and 2D fingerprint plots55 were calculated using Crystal Explorer 17.5 software.562.3 Antimicrobial studies
The antimicrobial activity of complexes 1 and 2 as well as the free ligands was screened against some harmful microbes as described in Method S1 (ESI†).573. Results and discussion
3.1 Chemistry and characterization
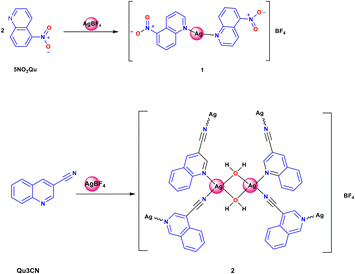
The two target Ag(I) complexes were synthesized using the self-assembly technique by mixing the functional ligand (5NO2Qu or Qu3CN) in ethanol with an aqueous AgBF4 solution, and then acetonitrile was added to dissolve the resulting turbidity (Scheme 1). The clear solution was left to slowly evaporate at room temperature. Complexes 1 and 2 were obtained as colourless crystals after five days, which were found suitable for the X-ray single crystal structure analysis.Their structures were confirmed using FTIR, 1H-NMR, and 13C-NMR. The FTIR spectra of the synthesized complexes compared to those of the corresponding free ligands are presented in Fig. S1 and S2.† The FTIR spectra of complexes 1 and 2 showed expected peaks of the free ligands with some deviations. Symmetric and asymmetric N–O stretches were detected at 1520 and 1321 cm−1 in the free 5NO2Qu while in complex 1, the corresponding values are 1521 and 1341 cm−1.58 In the case of 5NO2Qu, the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and ν(C
N) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) stretching modes appeared at 1626 and 1593 cm−1, respectively, while these appeared at 1618 and 1565 cm−1 for Qu3CN. On the other hand, the ν(C
C) stretching modes appeared at 1626 and 1593 cm−1, respectively, while these appeared at 1618 and 1565 cm−1 for Qu3CN. On the other hand, the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and ν(C
N) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) modes appeared at 1624 and 1592 cm−1, respectively, in 1 while for complex 2, the corresponding values are 1621 and 1567 cm−1. In addition, the peak that appeared at 2227 cm−1 in the free Qu3CN and 2 could be assigned to the ν(C
C) modes appeared at 1624 and 1592 cm−1, respectively, in 1 while for complex 2, the corresponding values are 1621 and 1567 cm−1. In addition, the peak that appeared at 2227 cm−1 in the free Qu3CN and 2 could be assigned to the ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) mode. Also, complex 2 showed the characteristic ν(O–H) mode of coordinated water molecules at 3354 cm−1. In both complexes, the characteristic stretching vibrations of BF4− were detected as broad double split peaks at 1066 and 1031 cm−1 for complex 1, and at 1063 and 1033 cm−1 for complex 2. These peaks are completely absent in the FTIR spectra of the free ligands which sheds light on the possible coordination between Ag(I) and the quinoline ligands in both complexes.
N) mode. Also, complex 2 showed the characteristic ν(O–H) mode of coordinated water molecules at 3354 cm−1. In both complexes, the characteristic stretching vibrations of BF4− were detected as broad double split peaks at 1066 and 1031 cm−1 for complex 1, and at 1063 and 1033 cm−1 for complex 2. These peaks are completely absent in the FTIR spectra of the free ligands which sheds light on the possible coordination between Ag(I) and the quinoline ligands in both complexes.
In addition, the 1H NMR and 13C NMR spectra (see the ESI†) of both complexes showed a characteristic small shift in the signals compared to those of the free ligands which might be evidence for the presence of coordinated ligands in solution38,59,60 However, the exact nature and mix of Ag(I) species in solution remain elusive. Typically these complexes have fast ligand exchange on the NMR scale and the equilibrium may favour substantial proportions of free ligands under stoichiometric conditions, hence the small NMR shift changes and also the noticeably smaller shift changes for 2 that has half the ligand/Ag ratio compared to 1. However, the different species in solution will also have different biological potencies and it is not unlikely that minor species have higher antibacterial activity.38,60
This behaviour is generally known for silver(I) complexes with N-donor ligands, which may have relatively weak Ag–N and Ag–O interactions, although this depends on the exact nature of the nitrogen (i.e. NH3 is different from pyridine).59 Silver(I) complexes with high stability in biological fluids are undesirable for biological applications. In contrast, those with weak Ag–ligand (Ag–N and Ag–O) bonds which have high ability for ligand replacement by biomolecules inside the body are powerful antimicrobial agents.61–64
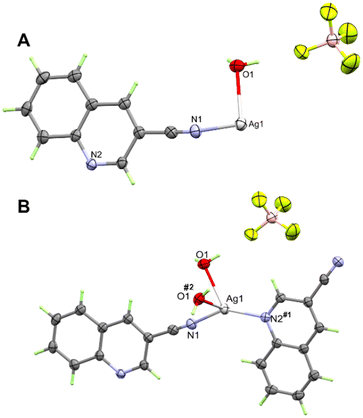
3.2 Single crystal X-ray structures
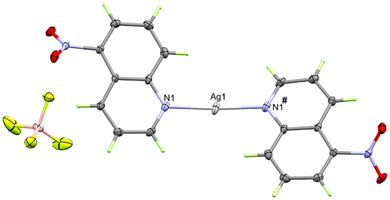
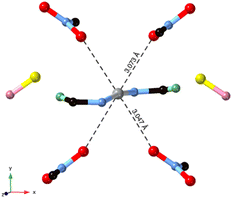
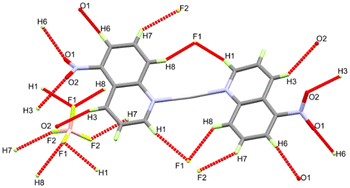
In the cationic inner sphere [Ag(5NO2Qu)2]+, the Ag(I) is coordinated to two symmetry related 5NO2Qu molecules via the quinoline N-atom as a monodentate ligand. The two 5NO2Qu molecules are anti to one another possibly to minimize the steric hindrance between the two bulky quinoline moieties. The two-symmetry related Ag1–N1 and Ag1–N1# bonds are equidistant (2.146(2) Å). The N1–Ag1–N1# angle (171.42(8)°) deviates slightly from the ideal value of 180° possibly due to steric hindrance between the two ligands (H8⋯H1 distance: 3.540(7) Å) or interactions with the counterions. Another possibility is Ag⋯O interactions (see Fig. 3), as both O1⋯Ag1 at 3.073(2) Å and O2⋯Ag1 at 3.047(1) Å fall within the attractive region of Ag⋯O2N–R interactions (Fig. S7† and also Fig. 4). Hence, the coordination geometry of Ag(I) is bent with outer sphere Ag⋯O contacts forming an octahedron. These results agree with the structurally related [Ag(5NO2Qu)2]X complex (X = NO3 or ClO4).38,65
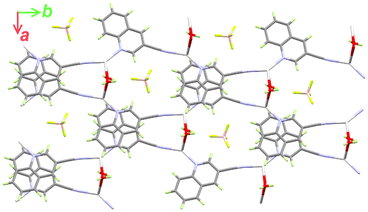
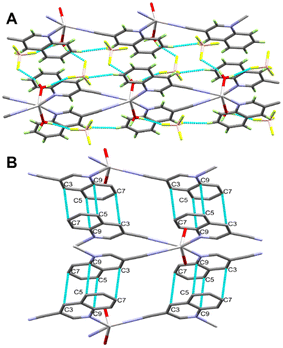
It is not obvious from Fig. 2, but BF4− does not participate in the coordination with Ag(I) (the Ag⋯F1 distance is 3.856(1) Å), although it has a significant contribution to the supramolecular structure of the monomeric [Ag(5NO2Qu)2]BF4 complex (Table 2). There are quite a large number of C–H⋯F non-covalent interactions detected which are presented in Fig. 4. The corresponding geometric parameters are depicted in Table 2. The donor–acceptor distances of the C–H⋯F interactions are in the range of 3.241(3)–3.470(2) Å for the C7–H7⋯F2 and C1–H1⋯F1 interactions. In addition, there are two weak C–H⋯O interactions between the oxygen atoms of the nitro group and the aromatic C–H groups. The donor–acceptor distances of the C3–H3⋯O2 and C6–H6⋯O1 interactions are 3.359(2) and 3.292(2) Å, respectively. A view of the packing of the monomeric complex units via the C–H⋯F and C–H⋯O interactions is shown in Fig. 5A.
| D–H⋯A | D–H (Å) | H⋯A (Å) | D⋯A (Å) | D–H⋯A (°) | Symm. code |
|---|---|---|---|---|---|
| C1–H1⋯F1 | 0.95 | 2.54 | 3.470(2) | 168 | 1/2 + x, 3/2 − y, 1/2 + z |
| C1–H1⋯F3 | 0.95 | 2.48 | 3.258(2) | 139 | 3/2 − x, 3/2 − y, 1 − z |
| C3–H3⋯O2 | 0.95 | 2.34 | 2.889(1) | 116 | x, y, z |
| C3–H3⋯O2 | 0.95 | 2.52 | 3.359(2) | 147 | 1 − x, y, 1/2 − z |
| C6–H6⋯O1 | 0.95 | 2.38 | 3.292(2) | 160 | −x, y, 1/2 − z |
| C6–H6⋯F2 | 0.95 | 2.47 | 2.946(1) | 111 | −1/2 + x, −1/2 + y, z |
| C7–H7⋯F3 | 0.95 | 2.44 | 3.157(1) | 144 | 1/2 − x, 3/2 − y, 1 − z |
| C8–H8⋯F1 | 0.95 | 2.52 | 3.302(2) | 139 | 1/2 − x, 3/2 − y, 1 − z |
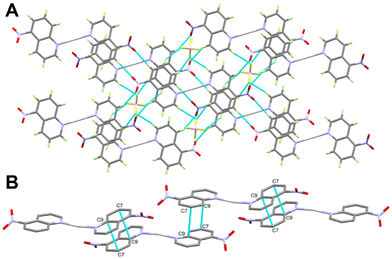 | ||
| Fig. 5 Packing scheme of the [Ag(5NO2Qu)2]BF4 complex via C–H⋯F and C–H⋯O contacts (A) and π–π stacking interactions (B) in the [Ag(5NO2Qu)2]BF4 complex (1). | ||
In addition, the monomeric complex units interact with each other via the C⋯C contacts shown in Fig. 5B. The presence of a short C7⋯C9 contact (3.390 Å) indicated with no doubt the presence of aromatic π–π stacking interactions with the shortest distance of 3.539(2) Å between the quinoline moieties.
It is clear from Fig. 6B that the Ag(I) is tetracoordinated with two N-atoms (N1 and N2) from two Qu3CN ligands. In 2, the Qu3CN ligand acts as a connector between the Ag(1) sites along the b-direction via two short Ag1–N1 (2.185(4) Å) and Ag1–N2#1 (2.204(4) Å) bonds. In addition, the Ag(I) is coordinated with two symmetry related water molecules which also act as connectors between Ag1 sites along the a-direction via two longer Ag1–O1 (2.470(4) Å) and Ag1–O1#2 (2.546(4) Å) bonds. Hence, the structure of this complex could be described as a 2D polymer extended along the ab-plane (Fig. 7). The N(1)–Ag(1)–N(2)#1 and O(1)–Ag(1)–O(1)#2 angles are determined to be 145.81(16) and 77.11(14)°, respectively, while the N–Ag–O angles are in the range of 85.58(16)–126.40(15)° (Table 3). Hence, the AgN2O2 coordination sphere has a highly distorted tetracoordinated system. The distortion τ4 parameter is estimated to be 0.62 indicating an intermediate geometry between the square planar and tetrahedral configurations.66
| Bond | Distance | Bond | Distance |
|---|---|---|---|
| Ag(1)–N(1) | 2.185(4) | Ag(1)–O(1) | 2.470(4) |
| Ag(1)–N(2)#1 | 2.204(4) | Ag(1)–O(1)#2 | 2.546(4) |
| Bonds | Angle | Bonds | Angle |
|---|---|---|---|
| #1 −x + 3/2, y + 1/2, −z + 3/2 #2 −x + 1, y, −z + 3/2. | |||
| N(1)–Ag(1)–N(2)#1 | 145.81(16) | N(1)–Ag(1)–O(1)#2 | 104.65(16) |
| N(1)–Ag(1)–O(1) | 85.58(16) | N(2)#1–Ag(1)–O(1)#2 | 95.26(13) |
| N(2)#1–Ag(1)–O(1) | 126.40(15) | O(1)–Ag(1)–O(1)#2 | 77.11(14) |
In addition, the supramolecular structure of complex 2 is controlled by the O–H⋯F hydrogen bonds and π–π stacking interactions presented in Fig. 8A. The 2D polymeric chains are found connected by O1–H1A⋯F1 and O1–H1B⋯F3 hydrogen bond bridges (Fig. 8B). The H1A⋯F1 and H1B⋯F3 distances are 1.86 and 2.01 Å, respectively, while the respective donor–acceptor distances are 2.693(6) and 2.802(7) Å. The O1–H1A⋯F1 and O1–H1B⋯F3 angles are 173.4 and 154.9°, respectively. In addition, the quinoline moieties are found parallel to each other leading to significant π–π interactions between the stacked aromatic systems. The C3⋯C7 (3.381 Å) and C5⋯C9 (3.348 Å) contacts are the shortest while the distance between the ring centroids is 3.658 Å.
3.3 Hirshfeld surface analysis
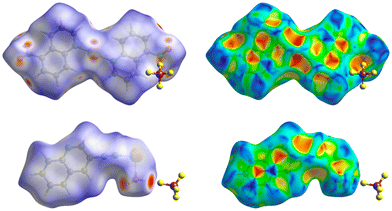
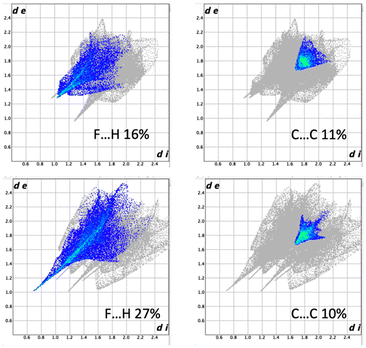
Hirshfeld surfaces were used to demonstrate the possible intermolecular interactions controlling the supramolecular structure of the studied Ag(I) complexes, including hydrogen bonds, π–π stacking, C–H⋯π, and H⋯H interactions.67,68 Also, Hirshfeld surfaces represent a 3D description of close contacts in a crystal, which are equal to or shorter than the van der Waals radii sum of interacting atoms. Also, fingerprint plots can be used to describe these contacts in a quantitative manner.69 The Hirshfeld surfaces of complexes 1 and 2 are depicted in Fig. 9, while Fig. 10 displays the distribution selected intermolecular interactions.The π–π stacking is obvious for both compounds as demonstrated by the triangular pattern of the shape index. For complex 1, the O⋯H (27%) and F⋯H (16%) contacts are the most important interactions. Additionally, there are some Ag⋯O (5%) interactions that contribute to the supramolecular structure. In 2, the other most significant interaction is the F⋯H (27%) contact. In both complexes, all these contacts appeared as red spots in the dnorm map. Additionally, the dark red spots close to the Ag atom in complex 2 are related to the Ag–O and Ag–N bonds which connect the [Ag(Qu3CN)(H2O)] units to form the 2D polymer.
The difference and similarities of 1 and 2 can be seen in Fig. 10 where fingerprint plots of H⋯F and C⋯C contacts can be found. The larger part played by H⋯F interactions in 2 is due to the hydrogen bonding of water to BF4−. As can be seen, the π–π stacking is similar and this is attributed to the relatively low coordinating ability of the BF4− anion, making these weaker interactions more important.
3.4 Antimicrobial studies
The results of the antimicrobial activities in terms of minimum inhibitory (MIC) and minimum bactericidal (MBC) concentrations for the tested compounds are listed in Table 4. The MIC data revealed the better antimicrobial potency of complexes 1 and 2 than the corresponding free ligands for most microbes. It is obvious that complexes 1 (MIC = 7.8 μg mL−1) and 2 (MIC = 31.25 μg mL−1) have higher potency against the fungus C. albicans than the free ligands (MIC = 125 μg mL−1). It is worthy to note that the antifungal activity of 1 is considered better than that of the standard nystatin (15.6 μg mL−1). The MBC results are in good agreement with this conclusion.| Tested compound | 5NO 2 Qu | Complex 1 | Qu3CN | Complex 2 | Control |
|---|---|---|---|---|---|
| a Amoxicillin. b Nystatin. | |||||
| Gram positive bacteria | MIC/MBC (μg mL−L) | ||||
| S. aureus (ATCC 25923) | 125 | 62.5/125 | 125 | 31.25/62.5 | ≤7.8a |
| MRSA (ATCC43300) | 125 | 62.5/125 | 125 | 62.5/250 | >500a |
| MRSA (1) | 125 | 62.5/125 | 125 | 31.2/62.5 | >500a |
| E. faecium (31) | 125 | 31.2/62.5 | 125 | 62.5/125 | >500a |
| Gram negative bacteria | |||||
| E. coli (ATCC 25922) | 125 | 62.5/125 | 125 | 62.5/125 | 62.5a |
| K. pneumonia (ATCC 700603) | 125 | 62.5/125 | 125 | 62.5/125 | >500a |
| P. aeruginosa (ATCC 29853) | 125 | 62.5/125 | 125 | 31.2/125 | 125a |
| A. baumannii (ATCC 19606) | 125 | 31.2/62.5 | 125 | 31.2/62.5 | >500a |
| P. mirabilis | 125 | 7.8/15.6 | 125 | 15.6/62.5 | 125a |
| K. pneumonia (50) | 125 | 62.5/125 | 125 | 62.5/125 | >500a |
| K. pneumonia isolates (R124) | 125 | 31.2/62.5 | 125 | 31.2/125 | >500a |
| P. aeruginosa (5) | 125 | 62.5/125 | 125 | 31.2/62.5 | 500a |
| A. baumannii (8) | 125 | 31.2/62.5 | 500 | 31.2/62.5 | >500a |
| Fungi | |||||
| C. albicans | 125 | 7.8/15.6 | 125 | 31.25/62.5 | 15.6b |
In terms of antibacterial activity, both Ag(I) complexes have better activities than the free ligands against all the studied bacterial strains. Complexes 1 and 2 have the best antibacterial activity against the Gram-negative bacteria P. mirabilis. The MIC values are determined to be 7.8 and 15.6 μg mL−1, respectively, while the MBC values are 15.6 and 62.5 μg mL−1, respectively. Also, both Ag(I) complexes have better activity against this microbe than the common antibiotic amoxicillin. For all Gram-negative bacteria except E. coli (ATCC 25922), the studied compounds have better activity than the common antibiotic amoxicillin. In addition, complex 1 (31.2 μg mL−1) showed better activity against Gram positive bacteria E. faecium (31) than 2 (62.5 μg mL−1). In contrast, complex 2 (31.2 μg mL−1) has better antibacterial activity against MRSA (1) and S. aureus (ATCC 25923) than 1 (62.5 μg mL−1) while both Ag(I) complexes have the same potency against MRSA (ATCC43300). Generally, the studied compounds have better activity against all the studied Gram-positive bacteria (except S. aureus (ATCC 25923)) than amoxicillin.
Neither 1 nor 2 seems to perform better than the silver nicotinate compounds we reported earlier.70
4. Conclusions
Single crystal X-ray diffraction was used to structurally analyze the self-assembled [Ag(5NO2Qu)2]BF4 (1) and [Ag(Qu3CN)(H2O)]BF4 (2) complexes. Complex 1 comprised monomeric [Ag(5NO2Qu)2]BF4 units but in contrast, complex 2 is a 2D coordination polymer via bridged Qu3CN and H2O ligand units. Using Hirshfeld surface analysis, we show that the intermolecular interactions are significantly different in the two compounds. It is also found that complexes 1 and 2 have antimicrobial activities against several bacterial and fungal strains. Complex 1 (MIC = 7.8 μg mL−1) has the best antifungal activity and is even better than the standard nystatin (15.6 μg mL−1). In addition, the MIC and MBC results indicated the better antibacterial activity of 1 and 2 against P. mirabilis than the common antibiotic amoxicillin.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project (RSP2023R64), King Saud University, Riyadh, Saudi Arabia. FMAN and LÖ thank Chalmers University of Technology for financial support.Notes and references
- A. Warra, J. Chem. Pharm. Res., 2011, 3, 951–958 CAS.
- S. Rafique, M. Idrees, A. Nasim, H. Akbar and A. Athar, Microbiol. Mol. Biol. Rev., 2010, 5, 38–45 CAS.
- C. Biot, F. Nosten, L. Fraisse, D. Ter-Minassian, J. Khalife and D. Dive, Parasite, 2011, 18, 207–214 CrossRef CAS PubMed.
- S. Monro, K. L. Colon, H. Yin, J. Roque III, P. Konda and S. Gujar, et al. , Chem. Rev., 2018, 119, 797–828 CrossRef PubMed.
- R. G. Kenny and C. J. Marmion, Chem. Rev., 2019, 119, 1058–1137 CrossRef CAS PubMed.
- M. Claudel, J. V. Schwarte and K. M. Fromm, Chemistry, 2020, 2, 849–899 CrossRef CAS.
- C. N. Morrison, K. E. Prosser, R. W. Stokes, A. Cordes, N. Metzler-Nolte and S. M. Cohen, Chem. Sci., 2020, 11, 1216–1225 RSC.
- C. Shimokawa and S. Itoh, Inorg. Chem., 2005, 44, 3010–3012 CrossRef CAS PubMed.
- A. M. Chippindale, L. E. Head and S. J. Hibble, Chem. Commun., 2008, 3010–3012 RSC.
- Y. Wang, X. F. Wang, T. Okamura, B. Xu, W. Y. Sun and N. Ueyama, Chin. J. Inorg. Chem., 2006, 22, 1487–1490 CAS.
- Y. H. Tan, S. P. Yang, Q. S. Li and Y. L. Tan, Chin. J. Struct. Chem., 2006, 25, 1387–1391 CAS.
- S. V. Ivanov, S. M. Miller, O. P. Anderson and S. H. Strauss, Cryst. Growth Des., 2004, 4, 249–254 CrossRef CAS.
- A. V. Artem'ev, A. Y. Baranov and I. Y. Bagryanskaya, Inorg. Chem. Commun., 2022, 140, 109478 CrossRef.
- H. Wu, S. O. Aderinto, Y. Xu, H. Zhang and Z. Yang, J. Chem. Res., 2016, 40, 492–497 CrossRef CAS.
- G. Kleinhans, A. K. W. Chan, M. Y. Leung, D. C. Liles, M. A. Fernandes and V. W. W. Yam, et al. , Chem. – Eur. J., 2020, 26, 6993–6998 CrossRef CAS PubMed.
- H. Lang, M. Leschke, G. Rheinwald and M. Melter, Inorg. Chem. Commun., 1998, 1, 254–256 CrossRef CAS.
- A. G. Young and L. R. Hanton, Coord. Chem. Rev., 2008, 252, 1346–1386 CrossRef CAS.
- F. Pointillart, P. Herson, K. Boubekeur and C. Train, Inorg. Chim. Acta, 2008, 361, 373–379 CrossRef CAS.
- M. Osawa and M. Hoshino, Chem. Commun., 2008, 6384–6386 RSC.
- C.-Y. Wu, C.-S. Lee, S. Pal and W.-S. Hwang, Polyhedron, 2008, 27, 2681–2687 CrossRef CAS.
- H.-T. Shi, T. Duan, C. Xu and Q.-F. Zhang, Z. Naturforsch., B: J. Chem. Sci., 2009, 64, 204–208 CrossRef CAS.
- C. Liu, P.-K. Liao, C.-S. Fang, J.-Y. Saillard, S. Kahlal and J.-C. Wang, Chem. Commun., 2011, 47, 5831–5833 RSC.
- E. J. Fernández, A. Laguna, J. M. López-de-Luzuriaga, M. Monge, M. Montiel and M. E. Olmos, et al. , Dalton Trans., 2005, 1162–1164 RSC.
- P. J. Malinowski, D. Kurzydłowski and W. Grochala, Dalton Trans., 2015, 44, 19478–19486 RSC.
- H. Yang, J. Lei, B. Wu, Y. Wang, M. Zhou and A. Xia, et al. , Chem. Commun., 2013, 49, 300–302 RSC.
- Q.-H. Wei, L.-Y. Zhang, L.-X. Shi and Z.-N. Chen, Inorg. Chem. Commun., 2004, 7, 286–288 CrossRef CAS.
- Q. L. Ni, F. Zhang, T. H. Huang, X. P. Wu, X. J. Wang and G. M. Liang, et al. , Z. Anorg. Allg. Chem., 2015, 641, 2664–2669 CrossRef CAS.
- A. A. Adeleke, S. J. Zamisa, M. S. Islam, K. Olofinsan, V. F. Salau and C. Mocktar, et al. , Molecules, 2021, 26, 1205 CrossRef CAS PubMed.
- M. S. Altowyan, M. A. El-Naggar, M. A. Abu-Youssef, S. M. Soliman, M. Haukka and A. Barakat, et al. , Crystals, 2022, 12, 356 CrossRef CAS.
- M. A. El-Naggar, M. A. Abu-Youssef, S. M. Soliman, M. Haukka, A. M. Al-Majid and A. Barakat, et al. , J. Mol. Struct., 2022, 1264, 133210 CrossRef CAS.
- M. Cavicchioli, A. C. Massabni, T. A. Heinrich, C. M. Costa-Neto, E. P. Abrão and B. A. Fonseca, et al. , J. Inorg. Biochem., 2010, 104, 533–540 CrossRef CAS PubMed.
- M. Kaloğlu, N. Kaloğlu, S. Günal and İ. Özdemir, J. Coord. Chem., 2022, 74, 3031–3047 CrossRef.
- S. Medici, M. Peana, V. M. Nurchi and M. A. Zoroddu, J. Med. Chem., 2019, 62, 5923–5943 CrossRef CAS PubMed.
- J.-Y. Maillard and P. Hartemann, Crit. Rev. Microbiol., 2013, 39, 373–383 CrossRef CAS PubMed.
- S. Medici, M. Peana, G. Crisponi, V. M. Nurchi, J. I. Lachowicz and M. Remelli, et al. , Coord. Chem. Rev., 2016, 327, 349–359 CrossRef.
- R. I. Khusnutdinov, A. R. Bayguzina and U. M. Dzhemilev, J. Organomet. Chem., 2014, 768, 75–114 CrossRef CAS.
- S. Yamashkin and E. Oreshkina, Chem. Heterocycl. Compd., 2006, 42, 701–718 CrossRef CAS.
- A. A. Massoud, V. Langer, Y. M. Gohar, M. A. Abu-Youssef, J. Jänis and G. Lindberg, et al. , Inorg. Chem., 2013, 52, 4046–4060 CrossRef CAS PubMed.
- A. Garrido Montalban, in Quinolines and Isoquinolines, ed. K. C. Majumdar and S. K. Chattopadhyay, Wiley-VCH Verlag GmbH & Co. KGaA, Germany, 2011, pp. 299–339 Search PubMed.
- V. V. Kouznetsov, C. M. M. Gómez, J. L. V. Peña and L. Y. Vargas-Méndez, in Natural and synthetic quinoline molecules against tropical parasitic pathologies: An analysis of activity and structural evolution for developing new quinoline-based antiprotozoal agents, Discovery and Development of Therapeutics from Natural Products Against Neglected Tropical Diseases, Elsevier, 2019, pp. 87–164 Search PubMed.
- Y. B. Rajesh, in Quinoline heterocycles: synthesis and bioactivity, ed. B. P. Nandeshwarappa and S. O. Sadashiv, 2018 Search PubMed.
- P. M. O'Neill, R. C. Storr and B. K. Park, Tetrahedron, 1998, 54, 4615–4622 CrossRef.
- J. Casal and S. Asís, Austin Tuberculosis: Research & Treatment, 2017, 2, 1007–1010 Search PubMed.
- S. Kumar, S. Bawa and H. Gupta, Mini-Rev. Med. Chem., 2009, 9, 1648–1654 CrossRef CAS PubMed.
- S. Adsule, V. Barve, D. Chen, F. Ahmed, Q. P. Dou and S. Padhye, et al. , J. Med. Chem., 2006, 49, 7242–7246 CrossRef CAS PubMed.
- H. Beyzaei, H. H. Moghadam, G. Bagherzade, R. Aryan and M. Moghaddam-Manesh, Avicenna J. Med. Biochem., 2019, 7, 9–15 CrossRef.
- S. A. Khan, A. M. Asiri, S. H. Al-Thaqafy, H. M. Faidallah and S. A. El-Daly, Spectrochim. Acta, Part A, 2014, 133, 141–148 CrossRef CAS PubMed.
- V. Arjunan, P. Ravindran, T. Rani and S. Mohan, J. Mol. Struct., 2011, 988, 91–101 CrossRef CAS.
- P. Smoleński, S. W. Jaros, C. Pettinari, G. Lupidi, L. Quassinti and M. Bramucci, et al. , Dalton Trans., 2013, 42, 6572–6581 RSC.
- T. P. Andrejević, I. Aleksic, J. Kljun, M. Počkaj, M. Zlatar and S. Vojnovic, et al. , RSC Adv., 2023, 13, 4376–4393 RSC.
- J. K. Aulakh, T. S. Lobana, H. Sood, D. S. Arora, R. Kaur and J. Singh, et al. , RSC Adv., 2019, 9, 15470–15487 RSC.
- M. A. El-Naggar, J. H. Albering, A. Barakat, M. A. Abu-Youssef, S. M. Soliman and A. M. Badr, Inorg. Chim. Acta, 2022, 537, 120948 CrossRef CAS.
- G. M. Sheldrick, Program for empirical absorption correction of area detector data, Sadabs, 1996 Search PubMed.
- (a) A. S. Bruker, Bruker A. Inc., Madison, Wisconsin, USA, 2004; (b) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 467–473 CrossRef.
- F. L. Hirshfeld, Theor. Chim. Acta, 1977, 44, 129–138 CrossRef CAS.
- M. Turner, J. McKinnon, S. Wolff, D. Grimwood, P. Spackman and D. Jayatilaka, et al., CrystalExplorer17, University of Western Australia, 2017, https://crystalexplorer.net/download/ Search PubMed.
- P. Wayne, Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing: 20th informational supplement, CLSI document M100-S20, 2010.
- R. W. Beal and T. B. Brill, Appl. Spectrosc., 2005, 59, 1194–1202 CrossRef CAS PubMed.
- A. N. Khlobystov, A. J. Blake, N. R. Champness, D. A. Lemenovskii, A. G. Majouga and N. V. Zyk, et al. , Coord. Chem. Rev., 2001, 222, 155–192 CrossRef CAS.
- A. M. Alshima'a, Y. M. Gohar, V. Langer, P. Lincoln, F. R. Svensson and J. Jänis, et al. , New J. Chem., 2011, 35, 640–648 RSC.
- K. Nomiya, K. Tsuda, T. Sudoh and M. Oda, J. Inorg. Biochem., 1997, 68, 39–44 CrossRef CAS PubMed.
- K. Nomiya, R. Noguchi and M. Oda, Inorg. Chim. Acta, 2000, 298, 24–32 CrossRef CAS.
- S. Nawaz, A. A. Isab, K. Merz, V. Vasylyeva, N. Metzler-Nolte and M. Saleem, et al. , Polyhedron, 2011, 30, 1502–1506 CrossRef CAS.
- A. A. Isab, S. Nawaz, M. Saleem, M. Altaf, M. Monim-ul-Mehboob and S. Ahmad, et al. , Polyhedron, 2010, 29, 1251–1256 CrossRef CAS.
- M. S. Altowyan, N. H. Al-Shaalan, A. A. Alkharboush, A. Barakat and S. M. Soliman, Symmetry, 2022, 14, 547 CrossRef CAS.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- H. F. Clausen, M. S. Chevallier, M. A. Spackman and B. B. Iversen, New J. Chem., 2010, 34, 193–199 RSC.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 RSC.
- M. A. Abu-Youssef, R. Dey, Y. Gohar, A. A. Massoud, L. Öhrström and V. Langer, Inorg. Chem., 2007, 46, 5893–5903 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2246560 and 2246561. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ce00417a |
| This journal is © The Royal Society of Chemistry 2023 |