Open Access Article
Open Access ArticleInterfacing cells with organic transistors: a review of in vitro and in vivo applications
Andrea
Spanu
 *a,
Laura
Martines
ab and
Annalisa
Bonfiglio
*a,
Laura
Martines
ab and
Annalisa
Bonfiglio
 *a
*a
aDepartment of Electrical and Electronic Engineering, University of Cagliari, Via Marengo, 09123 Cagliari, CA, Italy. E-mail: andrea.spanu@unica.it; annalisa.bonfiglio@unica.it
bDepartment of Bioengineering, Robotics and System Engineering, University of Genoa, Via all'Opera Pia 13, 16145 Genova (GE), Italy
First published on 10th February 2021
Abstract
Recently, organic bioelectronics has attracted considerable interest in the scientific community. The impressive growth that it has undergone in the last 10 years has allowed the rise of the completely new field of cellular organic bioelectronics, which has now the chance to compete with consolidated approaches based on devices such as micro-electrode arrays and ISFET-based transducers both in in vitro and in vivo experimental practice. This review focuses on cellular interfaces based on organic active devices and has the intent of highlighting the recent advances and the most innovative approaches to the ongoing and everlasting challenge of interfacing living matter to the “external world” in order to unveil the hidden mechanisms governing its behavior. Device-wise, three different organic structures will be considered in this work, namely the organic electrochemical transistor (OECT), the solution-gated organic transistor (SGOFET – which is presented here in two possible different versions according to the employed active material, namely: the electrolyte-gated organic transistor – EGOFET, and the solution gated graphene transistor – gSGFET), and the organic charge modulated field effect transistor (OCMFET). Application-wise, this work will mainly focus on cellular-based biosensors employed in in vitro and in vivo cellular interfaces, with the aim of offering the reader a comprehensive retrospective of the recent past, an overview of the latest innovations, and a glance at the future prospects of this challenging, yet exciting and still mostly unexplored scientific field.
Introduction
Being able to understand how the human body works has always been the underlying fire that guided most of the research effort during the past centuries. Starting from a simple “top down” method, the first reported attempts focused on approaching biological systems at the macro-scale, inferring their functioning by considering the most macroscopic and obvious observable aspects. Following these first trials, and thanks to the tremendous technological leap of the last two centuries, modern scientists started to discriminate and slowly understand how the single parts that constitute these systems interact with one another, and the introduction of smaller and more advanced devices (due to the recent rise of microelectronics) has recently allowed these complex systems to be approached in a completely new way. This change of paradigm brought the observer closer to the biological matter, thus making the beginning and the rapid expansion of modern in vitro and in vivo experimental practice possible. Devices such as electrodes for the study of the electrical properties of cellular aggregates and tissues,1 innovative microelectrode arrays (MEAs) for the detection of the electrical activity and for the stimulation of electroactive cell cultures,2 and integrated active devices based on silicon technology3,4 pushed the limits of our knowledge way further than ever happened by allowing different aspects of cellular behavior to be investigated beyond electrical activity, such as metabolic activity,5,6 with an unprecedented resolution. For a more complete literature report on inorganic cellular interfaces, please refer to ref. 7. The use of inorganic semiconductor-based field effect devices has evolved in the following years, giving rise to very interesting devices such as the nanowire transistor, a very promising device with very high sensitivity and excellent spatial and temporal resolution.8,9 The impact of these advancements reverberated in a large number of different biomedical fields such as electrophysiology,10,11 pharmacology,12 and brain machine interfaces.13 However, as the technology and the knowledge progressed, it became clear that, in order to unveil the extraordinarily complicated mechanisms that govern even the simplest biological system, it was of the highest importance to be able to perturb it as little as possible, and to date, despite the tremendous technological advancement that we are witnessing, this crucial aspect still remains an open research issue. An interesting alternative to the existing tools for cellular interfacing is represented by a fairly novel class of materials and devices that are typical of the field of organic electronics, a branch of electronics that deals with carbon-based polymeric conductors and semiconductors.When it comes to interfacing living cells or tissues, both in vitro and in vivo, polymeric materials come in handy to greatly improve the device/tissue coupling thanks to the usually higher effective surface area, which leads to a lower impedance for both recording and stimulating applications,14 and to their convenient mechanical and electrical properties. In fact, since polymers are typically softer than the metals that are usually used in standard cellular applications, the strain mismatch between the tissue and the recording device is dramatically reduced, thus helping in increasing cell viability during in vitro experiments15 or reducing the inflammatory response of the surrounding tissue (especially in vivo). This particular aspect has recently led to a tremendous development of alternative fabrication methods and materials that allow flexible devices to be obtained for in vivo applications, as pointed out by recent reviews on the topic,16–18 as well as innovative 2D and 3D microelectrode arrays.19–23 Regarding the electrical properties, conductive polymers (CPs), such as for instance poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS), may offer the interesting characteristic of being an electric conductor as well as an ionic one,24 thus substantially improving bioelectronic interfaces and opening up interesting solutions in the field of cellular applications (for a comprehensive review on the impact of conductive polymers in the bioelectronic field, please refer to ref. 25). Indeed, these materials appear to be ideal for realizing monitoring systems that fully accomplish the goal of measuring relevant biological parameters without perturbing cell behavior.
This review aims at reporting on the latest approaches that have been proposed for the development of cellular interfaces based on organic active devices, not only for in vitro but also for in vivo applications. In fact, the possibility of exploiting a great number of different materials and structures allowed the development of several interesting organic devices, each of which is able to address specific issues raised by the use of standard devices for cellular interfacing such as, for example, the need for an external reference electrode and the rigidity of the materials that are usually employed. A further advantage is related to the issue of fabrication costs. This aspect could allow the intriguing possibility of having disposable systems for routine testing (for instance in drug testing) to be addressed, with much wider applications, in addition to basic research. In particular, we focus here on 3 particular organic transistor-based devices, namely the organic electrochemical transistor (OECT), two different kinds of solution-gated transistors (SGFETs), namely the electrolyte-gated OFET (EGOFET) and the graphene SGFET (gSGFET), and the organic charge modulated field effect transistor (OCMFET). In the first part of the review, these devices will be introduced in terms of their structure and working principle, while in the second part their more recent applications for in vitro cellular interfacing will be presented and discussed. The third part is devoted to the progress that has been recently obtained for in vivo applications. Surely, this is one of the most intriguing possibilities for organic devices in bioengineering, aiming at opening a future of ambitious challenges in the field of bi-directional brain–machine interfaces, for both biomedical and robotic applications. Together with other devices, such as organic ion pumps, which represent one of the most interesting organic devices in the emerging field of direct electrically-controlled drug delivery26 (and that are comprehensively reviewed in ref. 27), indeed organic transistor-based devices represent the future of organic bioelectronics.
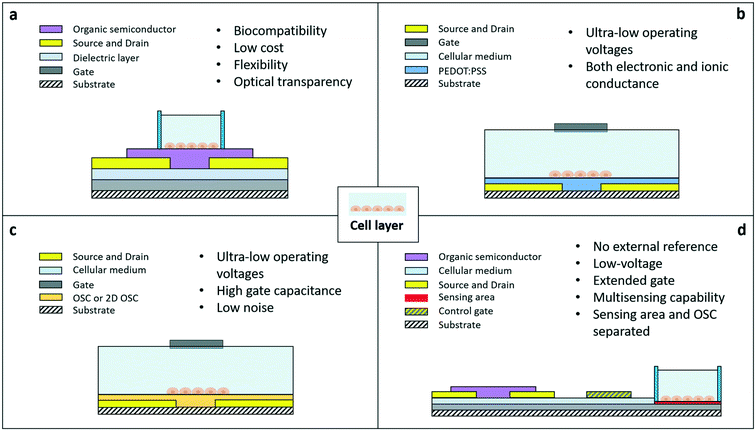
The basic blocks: organic transistors for cell sensing
The organic field effect transistor is one of the most studied and optimized devices in the field of organic electronics. One of its standard configurations (the so-called bottom gate/bottom contact one) is shown in Fig. 1A. The active channel of the device is made of a semiconductor layer included between two contacts, source and drain, where conduction takes place. The voltage applied to the gate, through a purely capacitive effect across the insulating layer, modulates the concentration of charge carriers in the channel, and, as a consequence, the current flow from the source to the drain. The drain current is expressed by two different formulas, according to the working regime of the device. In saturation, the current is independent of the drain voltage and quadratically dependent on the gate voltage, while in the linear regime, the current is linearly dependent on VD and VG. Vth is the minimum gate voltage needed to allow a minimum flow of current inside the channel, i.e. is the threshold value separating the off and the on states of the device.
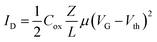
| (1) |
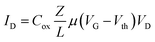
| (2) |
When this standard OFET structure is used for cell-sensing applications, the cells are directly grown on top of the semiconductor. Thanks to the possibility offered by the peculiar materials employed in organic electronics, OFETs can be relatively easily fabricated using biocompatible, flexible, and optically transparent materials, and with large area fabrication techniques that help lower their cost and environmental impact. The first example of OFET-like structures for cellular interfacing has been presented in ref. 28 and 29 with both neural and non-neural cells. The device was mainly employed for electrical stimulation, due to an operating principle based on the formation of an electric field at the interface with the organic semiconductor. In sensing mode, this device is not operated as a standard field effect device, i.e. by exploiting the intrinsic ability of FETs to amplify signals, due to the too high values of voltage needed to switch on the device (in the order of tens of volts): for this reason this kind of application, though interesting, cannot be easily framed within the context of OFETs for cell sensing applications.
As a matter of fact, in cellular applications, standard OFET configurations present several limitations, due particularly to the usually high operating voltages, the instability of some of the most common organic semiconductors if exposed to liquid environments, and the usual need for an external reference electrode immersed directly in the liquid medium where the sensing takes place. Therefore, during the past 10 years, different approaches have been proposed, with the intent of overcoming those limitations and thus being able to fully exploit the outstanding properties of organic semiconductor-based active devices. Lately, three types of organic FETs emerged as promising candidates for the development of new generations of cellular bioelectronic interfaces: the organic electrochemical transistor (Fig. 1b), which exploits the very interesting properties of conducting polymers such as PEDOT:PSS, the solution-gated transistors (Fig. 1c), with their ultra-high gate capacitance and ultra-low operating voltages, and the organic charge modulated FETs (Fig. 1d), with their versatility, convenient structure (which allows keeping the organic semiconductor (OSC) and the sensing area separated), and ability to be operated without an external reference electrode.
The organic electrochemical transistor (OECT)
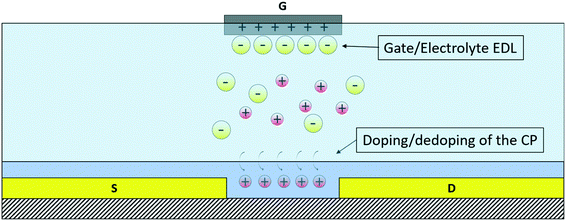
The OECT is a three-terminal device in which two electrodes, source and drain, are connected through an organic active material, while the third, the gate, is separated from the channel by an electrolyte, which consequently is an integral part of the transistor: because of the substantial role played by the electrolyte in the operation of the device, it belongs to the group of organic electrolyte-gated transistors (OEGTs).30 Its working principle is related to the modulation of the doping state of the active material and, consequently, of the channel conductivity caused by the injection of ions from the electrolyte into the organic active material, driven by the application of a suitable voltage VG to the gate as shown in Fig. 2.The organic active materials that are usually employed in these devices are CPs because of their mixed conductivity (both ionic and electronic), chemical tunability, which allows optimizing their structure and characteristics depending on the specific needs, biocompatibility, mechanical flexibility and optical transparency.31 An OECT is therefore able to convert an ionic current into an electronic one, and this makes it a desirable tool for bioelectronics applications, as typically, biological agents (from whole cells to single protein channels) produce electrical signals based on the exchange of ions. The main characteristics of these transistors are the ultra-low operating voltage (below 1 V), which represents an important feature, especially for their application in liquid environments, and the high transconductance.32 For an OECT, the transconductance (gm) in the saturation regime is expressed as follows:33
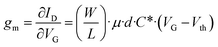 | (3) |
The solution-gated organic field effect transistor (SGOFET)
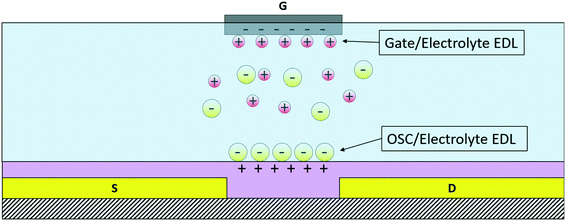
In this review, two types of organic solution-gated transistors will be considered, namely the electrolyte-gated OFET (EGOFET) and the graphene-based solution-gated FET (gSGFET). Although quite different due to the different nature of the active material, both structures, thanks to the formation of an electrical double layer (EDL) at the interface between the gate and the electrolyte and the active material and the electrolyte, are characterized by an extremely high gate capacitance and ultra-low operating voltages, these aspects making them very interesting candidates for biosensing and cellular applications. The SGOFET is structurally quite similar to the OECT, the main difference being the physical principle that is the origin of the modulation of the channel current and the ON/OFF switching mechanism. Indeed, whereas the OECT working principle is based on doping/dedoping of the OSC by ions that penetrate or withdraw (a phenomenon that is driven by the application of a suitable value of the gate voltage), the material employed for the channel of SGOFETs is virtually “impermeable” to ions: as a consequence, the channel carrier concentration is modulated by purely capacitive effects at the interface between the semiconductor and the electrolyte and between the gate electrode and the electrolyte, where an EDL is formed. The EDL capacitance has very high values if compared to that of metal oxides that are usually employed in standard FET structures and this makes the transconductance value, as in the previous case, quite high.Thanks to the peculiar gating mechanism, the SGOFET shows faster response than the OECT, thanks to the nature of the EDL itself, which is able to rapidly re-arrange after a perturbation.35 As previously mentioned, in this review two types of SGOFETs are presented, namely the EGOFET and the graphene solution-gated transistor (gSGFET), which exploit the properties of two different active materials, respectively, organic semiconductors and graphene. In Fig. 3, a schematic representation of an EGOFET's working principle is shown.
In particular, graphene, an allotrope of carbon and the most studied 2D material, has gained considerable attention within the organic electronics community in the past 10 years or so,36,37 and its usage as an active material in liquid-gated transistors is now widely studied for a considerable number of sensing and biosensing applications. The introduction of this device for cellular experiments has been fostered by the remarkable electrical, mechanical and chemical properties of this material, which in fact presents high optical transparency, high mechanical resistance and very high chemical stability.38,39 Also commonly used is reduced graphene oxide, which allows easier fabrication techniques with very similar electric and optical characteristics to those of chemical vapor deposited (CVD) graphene.40–42
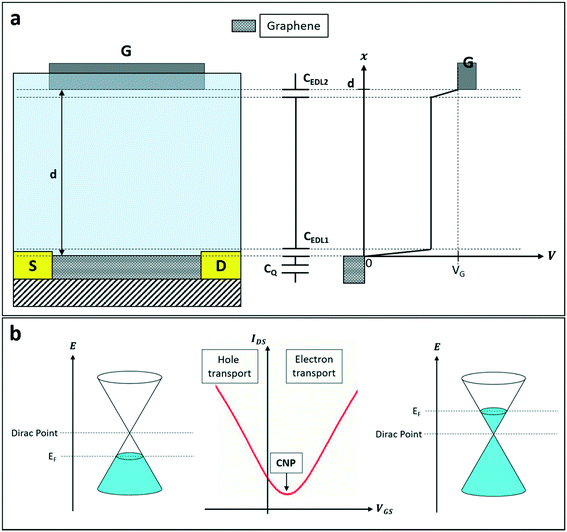
The working principle of gSGFETs (which is presented in Fig. 4) depends on the same phenomenon previously reported for the SGOFET. In this specific case, due to the unique electrical properties of graphene, the device's working mechanism can be described in terms of the modulation of the Fermi level of graphene itself (thus a modulation of the transistor's channel conductance) caused by the application of a gate voltage directly in the electrolyte where graphene is immersed, usually using an Ag/AgCl reference electrode. The main capacitive contributions in this structure (which is shown in Fig. 4) are the two electric double layers at the graphene/electrolyte and gate/electrolyte interfaces (CEDL1 and CEDL2, respectively) and a quantum capacitance CQ. This term can be expressed as 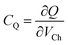 , where Q is the charge density in the graphene channel and VCh is the channel electrostatic potential43 considering the gate as the reference voltage. This capacitive contribution is very high in graphene, therefore this mechanism can be successfully exploited for several kinds of sensing applications, ranging from pH sensing44–47 to biosensing,48–53 thus making this device a versatile and convenient alternative to standard sensors.
, where Q is the charge density in the graphene channel and VCh is the channel electrostatic potential43 considering the gate as the reference voltage. This capacitive contribution is very high in graphene, therefore this mechanism can be successfully exploited for several kinds of sensing applications, ranging from pH sensing44–47 to biosensing,48–53 thus making this device a versatile and convenient alternative to standard sensors.
The organic charge modulated field effect transistor (OCMFET)
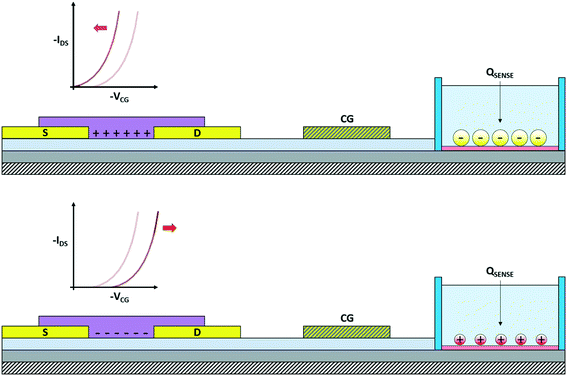
The OCMFET is a double gated OFET that has been specifically designed for sensing applications. The core of the device is the elongated floating gate, at the end of which a sensing area is fabricated by selective functionalization. The second gate, called the control gate, constitutes the contact through which it is possible to set the working point of the transistor, thus making an external reference electrode unnecessary.The working principle of this device is straightforward: the working point is set by applying suitable values of the control gate (VCG) and drain (VDS) voltages. In this situation, if a charge variation occurs in proximity of the sensing area, thanks to its capacitive coupling with the floating gate, it leads to a charge reorganization into the floating gate itself, which as a consequence determines a modulation of the carrier's density in the transistor's channel. In particular, for VS = 0 V the voltage of the floating gate can be expressed as:
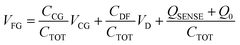 | (4) |
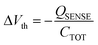 | (5) |
Exploiting this remarkable feature, it has been already employed in several applications, ranging from DNA sensing54,55 to pressure and temperature sensing.56,57 The fact that different kinds of sensors can be obtained using the very same structure by simply modifying the functionalization of the sensing area (while avoiding at the same time exposure of the organic semiconductor to detrimental external agents) is the main advantage of this peculiar design over pre-existing approaches, together with the fact that it does not require any external reference electrode in order to properly function in a liquid environment.
These three organic structures thus present several interesting properties that can be exploited in sensing and biosensing. In particular, considering the particular nature of the applications that we are describing in this review, it is important to synthetize the main requirements that have to be met in order to maximize the success of the device/cell coupling. These are greatly dependent on the specific application and/or cell type or tissue. In general, the principal aspects to consider in cell interfacing are:
i) the absence of cytotoxicity (both in vitro and in vivo). This aspect, although obvious, can be the main cause of failure, especially during in vitro long term experiments. The selection of the materials is therefore of paramount importance in cellular applications, and organic electronics brings to the table the possibility of selecting among a large number of different intrinsically biocompatible polymer-based materials with dielectric, conducting and semiconducting properties.
ii) The mechanical compliance of the materials employed (this aspect is even more important in in vivo applications). In fact, the mechanical mismatch is a common cause of failure for implanted devices, and stems from the formation of scar tissue due to the vastly different Young's modulus between cells/tissues and typical conducting/semiconducting materials (for example, few kPa for brain tissue compared to more than 100 GPa for silicon and the most used metals). Again, organic devices have the advantage of employing materials with relatively low Young's modulus (from a few hundreds of MPa up to a few GPa), and can also be easily integrated onto ultrathin flexible substrates (down to hundreds of nanometers), thus further contributing to the reduction of the mechanical mismatch between the monitoring device and the cell/tissue.58,59
iii) The operating voltages, which must be kept as low as possible in order to avoid the electrolysis of water (around 1.2 V). A non-negligible electric field in proximity of a cell membrane may also alter the electrical behavior of the cell itself, thus low operating voltages are a strict constraint. In the domain of organic devices, obtaining low-voltage devices is not a trivial task, and for this reason only a few particular organic devices are suitable for cellular applications.
Exploring cells in vitro
OECTs
The first organic device that has been used for in vitro applications is the OECT. Thanks to their remarkable features, during the past 10 years OECTs have been extensively applied to the study of the electrical properties of in vitro cultures of both non-electrogenic and electrogenic cells.In particular, the OECT has been used in two different configurations, i.e. the standard – also called vertical configuration – and the so-called planar one. These two structures differ in the positioning of the gate electrode: in particular, in the standard configuration, the source and drain electrodes are coplanar and the gate is usually a wire immersed in the electrolyte solution; in the planar structure, the gate electrode is also coplanar with the source and the drain. The latter structure is then more suitable with low-cost and high-throughput fabrication processes, and has the additional advantage of not using the usually cumbersome Ag/AgCl gate electrode, which turned out to be also toxic for long term cellular applications;60,61 in addition, this configuration is also more convenient for simultaneous optical measurements due to the optical transparency of PEDOT:PSS, which can be used for both the transistor channel and the gate electrode. Another advantage of not having an external gate wire is the possibility of using sealed chambers to maintain the culture under optimal conditions of humidity, CO2 and temperature, thus also allowing long term experiments.62 The two different structures are represented in Fig. 6a (standard configuration) and Fig. 6b (planar configuration).
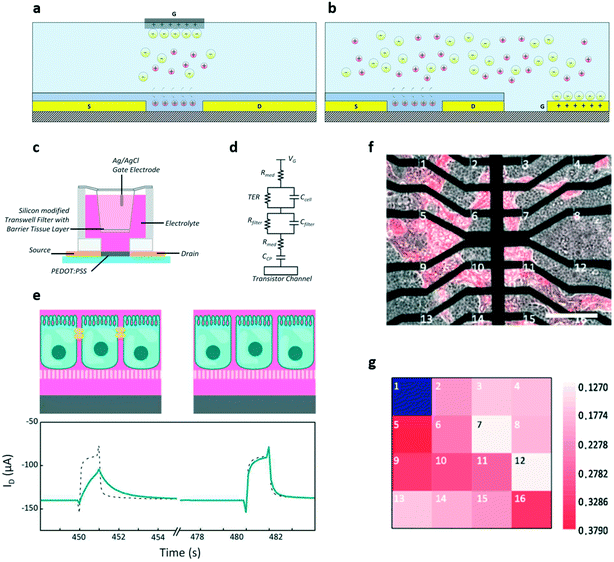 | ||
| Fig. 6 OECT for in vitro monitoring of non-electrogenic cells. a) and b) Standard and planar OECT configuration, respectively. c) Schematic and d) equivalent circuit of a sensor for barrier tissue integrity. e) Sensing mechanism of the device: when the tissue is intact, i.e. with intact tight junctions (left), the ID transient response is smaller and slower than that in the absence of cells (dashed line). When the tissue is damaged, i.e. when the tight junctions are destroyed (right), the transistor response becomes similar to that without cells. In this example, the disruption of tight junctions is caused by the addition of 100 mM of H2O2. f) Optical microscopy image of the co-culture of barrier and cancer tissues plated on an array of 16 OECTs (tumour cells are marked in red with the fluorescent marker mCherry), and g) map of the transconductance value at 100 Hz of each transistor in the array. Lower transconductance values suggest that the transistor is covered by barrier tissue, while higher values indicate cancer cells (OECT 1 was illustrated as blue because it was damaged and thus its signal is unavailable). This result suggests that OECTs can be used to discriminate between different kinds of cells. Panels c–e: Adapted with permission from ref. 64, Copyright 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Panels f and g: Adapted with permission from ref. 70, Copyright 2019 Elsevier B.V. All rights reserved. | ||
The first example of the employment of an OECT for monitoring cell viability was reported in 2010 by Lin et al.63 In their work, two different types of non-electrogenic cells (both cells that form barrier tissue, for example epithelial cells, and non-barrier tissues, i.e. cells that do not form tight junctions) were directly plated onto the transistor channel. By comparing the transfer curves before and after the administration of trypsin (a drug that causes rapid cellular death), it is possible to evaluate the cells' capability to form compact tissues, i.e. cell adhesion and compactness. The cells' effect on the transistor's electrical characteristics can be ascribed to the electrostatic interaction between the double layer surrounding each cell and the conducting polymer, which modulates the potential drop at the cell/semiconductor interface. This potential drop in turn causes the (cell/tissue integrity-dependent) gate voltage shift observed in the transfer curves.
This fascinating in vitro application was pioneered by the group of Dr. Owens, and extensively investigated in the last decade. In a 2012 paper, Caco-2 cells were seeded on Transwell filters suspended between the gate electrode (an Ag/AgCl electrode) and the channel of a PEDOT:PSS OECT, as shown in Fig. 6c (graphical representation) and Fig. 6d (equivalent electrical circuit). The device response time was then evaluated by monitoring the ID current in real time when square voltage pulses were applied through the gate electrode both in the presence of healthy, intact tissue (Fig. 6e, left) and after the addition of ethanol or H2O2 (Fig. 6e, right).64 The results showed that the OECT's response is slower when the cells are healthy, and speeds up after the administration of damaging substances (lower part of Fig. 6e), thus demonstrating the possibility of evaluating tissue integrity continuously over time by monitoring the dynamic properties of the device. Moreover, by using the transistor in this configuration, it is also possible to detect, at a much earlier stage if compared with the state-of-art techniques (30 seconds vs. up to 60 minutes for standard techniques), the damage induced on tissues by toxic compounds.
These preliminary results were examined more in depth in subsequent works,65–67 where they demonstrated how the OECT in this configuration can in fact outperform, in toxicological assays, state-of-the-art methods, such as immunofluorescence, permeability assays, and impedance measurements, in terms of temporal resolution, sensitivity, and specificity.
However promising, an important limitation of standard OECTs lies in their poor performance at high frequency. This limitation depends on the fact that the device's transconductance is high and stable as long as the process of doping/dedoping of the conducting polymer is fast, but dramatically drops at frequencies higher than 1 or 2 kHz without cells – meanwhile, when cells are plated onto the transistor's channel, the cut-off value decreased by about two orders of magnitude depending on the cell type. This limitation can be bypassed by operating the OECT as an impedance sensor, thus obtaining an extension of the frequency response of the device up to 20 kHz (while keeping the stable low frequency response typical of OECTs).68 In 2015 Romeo et al. were able to adapt an OECT device for the specific analysis of cells forming a non-barrier tissue, that, due to the fact that they do not form a compact tissue when cultured in vitro, represent a more challenging cell model.69 In the proposed configuration, the cells are plated on a Transwell membrane, which is kept separated from the transistor channel via a cleft filled with bi-distilled water, where the gate electrode is immersed. The use of bi-distilled water ensures that the only ions that can reach the transistor's channel are those coming from the culture medium after crossing the Transwell membrane; the amount of those ions depends on the permeability of the cell layer, which in turn depends on the cell coverage of the surface of the Transwell. The ions thus modulate the drain current differently, depending on whether the cells are healthy or not.
A step forward in the path toward the use of OECTs in the experimental practise is represented by the development of OECT arrays, which introduced a whole different level of versatility in the field of in vitro applications, by giving the possibility of evaluating not only the global state of a cell culture but also its local characteristics and properties. An interesting example of the application of this approach to the study of non-electrogenic cells is given by the work of Yeung et al., who in 2019 proposed a 16-channel OECT array specifically designed to study the effects of the presence of intruding cancer cells (NCP43) into a layer of healthy ones (Madin–Darby canine kidney MDCK-cells).70 To achieve the relevant task of discriminating between cancer and healthy cells, the authors exploited the dependence of the device's cut-off frequency on the packing level of the cell culture and identified the frequency that better allows one to successfully distinguish between the two kinds of cells (that, due to the structural difference, present different packing levels). Using this technique, the authors eventually have been able to obtain a transconductance map with which it was possible to locate the areas occupied by the two kinds of cells (Fig. 6f and g).
Another interesting aspect of using OECTs for in vitro cellular applications is the possibility of exploiting the device's geometry to tune its electrical characteristics (i.e. transconductance, time response, drain-source current and impedance). To this aim, Yeung et al. tested OECTs with different channel dimensions and characterized them after plating two different kinds of cells (forming both barrier and non-barrier tissues).71 Interestingly, their results indicated that large-sized transistors perform better at lower frequencies (thus can be successfully employed with barrier tissues, which induce slow current variations due to the very good packing among cells), while smaller OECTs, which are characterized by a faster response, are best suitable to monitor non-barrier tissues, which are intrinsically leaky and easily permeable to ions. The possibility of playing with the geometrical features in order to tailor the electrical characteristics of OECTs adds further versatility to this already powerful organic device.
As mentioned at the beginning of this section, another possible OECT configuration is the coplanar. The very first work that reported on a coplanar OECT interfaced with living cells for the control of the growth and adhesion of epithelial Madin–Darby canine kidney (MDCK) cells was presented by Bolin et al. in 2009.72 The goal of this work is unique and quite different from the other applications involving organic transistors coupled with living cells: in fact, the authors aimed at controlling the distribution of cells on a surface, in order to obtain complex patterns. They designed an OECT with PEDOT:tosylate as an active material, whose global oxidation state is controlled by the gate and drain potential (due to the device polarization, the channel is always more oxidized near the source electrode). By appropriately setting their values, thus controlling the oxidation state of the various segments of the transistor's channel, they were able to tune the density and the distribution of cell populations along it: in particular, fewer cells adhere onto the more oxidized portions of the channel, while the increase of gate potential made cells concentrate in the middle area. This demonstrated that mild negative voltages applied to the conducting layer promote the cell adhesion, while more negative potentials have the opposite effect, i.e. inhibit the cell growth and adhesion.
The already discussed advantages of planar OECTs made of optically transparent active materials opened up interesting applications in the field of in vitro cell monitoring. As demonstrated by Ramuz et al.,73 it is in fact possible to correlate optical and electrical data in order to get unprecedented insights into the early stage of tissue formation and to dynamically monitor the tissue's health condition. Thanks to their more compact form factor (i.e. thanks to the absence of the external wire gate electrode), planar OECTs can be easily integrated onto more advanced, multiparametric microfluidic systems that may be used to analyse cell growth in real time and in a physiologically relevant environment.74 With this more versatile approach, it is be possible to obtain more qualitative information on the conformational and structural changes of the cells during tissue formation, a feature that can be quite useful in applications such as the monitoring of tissue healing. In this case, the OECT has been used for producing the wound – by shortening together the source and drain electrodes and applying a voltage square wave pulse between them and the gate electrode – as well as for monitoring the healing under different conditions.
Very recently, Decataldo et al. reported a new OECT-based device specifically designed for cytotoxicity studies.75 In particular, the authors tested the device ability to detect in real time the effect of cytotoxic compounds on different kinds of cells, namely CaCo-2 (which form a barrier tissue) and NIH-3T3 (which in turn form non-barrier tissues), seeding the cells directly on the transistor channel and observing in real time the variation of the OECT's response time to a square voltage wave applied to the gate, when both citrate coated (known to be toxic) or OEG-alkanethiol passivated (non-toxic) silver nanoparticles (AgNPs) were added to the culture medium. The OECT was able to discriminate between the effects of toxic and non-toxic NPs on both tissues, not only giving results in agreement with those obtained by traditional optical assays, but also adding detailed information on the temporal phases of the poisoning process by the citrate coated AgNPs.
Another advantage offered by planar OECTs is the possibility to functionalize the surface of the gate electrode in order to make it sensitive to specific compounds, a feature which is not possible to obtain with standard Ag/AgCl bulky electrodes. This feature has been exploited in recent works by Chen et al.76 and Guo et al.77 In the first paper, which is related to a previous work78 in which the same authors designed a system meant to detect cancer protein biomarkers both in lysated and living cells with a similar biofunctionalized device, the authors investigated the functionalization of the gate of planar OECTs with concanavalin A (Con-A), a carbohydrate-binding protein that specifically binds mannose, an N-glycan present on the surface of human breast cancer cells (MCF-7), whose expression is known to be related to cancer growth and metastasis. Due to concanavalin, MCF-7 cells can be captured on the gate surface in different quantities, depending on the expression of mannose. The obtained OECT-cell device is then turned into an electrochemical sensor by adding specific nanoprobes, functionalized with horseradish peroxidase (HRP), that can bind specific sites onto the cancer cell surface. The biosensor is eventually characterized in PBS solution by adding H2O2, which is reduced by HRP thus leading to a change of the effective gate voltage of the transistor, proportional to the number of cells attached to the gate. To summarize, the cells adhere onto the gate electrode proportionally to the amount of glycans on their surfaces due to the presence of Con-A immobilized onto the gate itself, and thanks to the subsequent addition of HRP-functionalized nanoparticles, a drain current variation (which depends on the cellular superficial concentration on the gate surface) is elicited in the OECT by the addition of H2O2, which is reduced by HPR (Fig. 7a). In particular, the output current variation increases with the number of cells (Fig. 7b) and decreases when the cells are treated with an N-glycan inhibitor (TM), which limits the adhesion of the cells onto the gate (Fig. 7c). Interestingly, the authors demonstrated that their OECT-based cellular biosensor is capable of selectively detecting cancer cells down to a minimum concentration of 10 cells per μL.
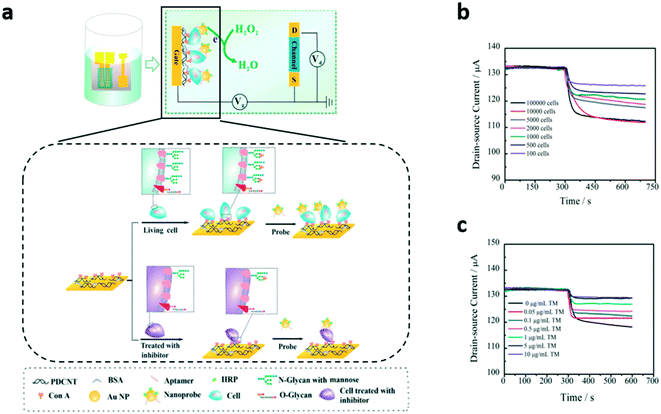 | ||
| Fig. 7 Planar OECT for in vitro monitoring of non-electrogenic cells. a) Scheme and working principle of the device in ref. 76. The variation of ID depends on the amount of HRP-functionalized gold nanoparticles (HPR reduces H2O2 added in solution) captured by the cells adhered onto the gate electrode, and this number depends in turn on the cells' mannose (an N-glycan present on the surface of human breast cancer cells) expression. Thus the ID increases with the number of living cancer cells plated on the device (b), and decreases with the concentration of an inhibitor of mannose expression with which cells are treated (c). Adapted with permission from ref. 76, Copyright 2018 American Chemical Society. | ||
In the second paper, a planar OECT with a functionalized gate has been proposed for the investigation of the cytotoxic effect of nanomaterials such as graphene and Au nanoparticles. In particular, the researchers' goal was to detect the hydrogen peroxide produced by HeLa cells adherent onto a Transwell when stimulated with N-formylmethionyl-leucylphenylalanine (FMLP): since the amount of H2O2 produced by the culture depends on the number of healthy cells, monitoring its variation after the addition of the test nanomaterials to the culture can give information about their cytotoxicity. In order to achieve this result, the authors functionalized the carbon paste gate electrode with a layer of multiwalled carbon nanotubes (MWCNTs) combined with platinum nanoparticles (Pt NPs); indeed, the MWCNTs combined with Pt NPs have the capacity of oxidizing H2O2 (different than the carbon paste constituting the gate electrode), and so their presence has a boosting effect on the sensor's performances. The results have shown that the OECT is able to detect the different levels of toxicity of the different nanoparticles, and the cellular viability is coherent with that obtained with state-of-art methodologies. However, the time response of the OECT is yet to be optimized and this limits, for the moment, the dynamic recording of the hydrogen peroxide produced by cells.
A comparison of the efficacy of the standard and the planar configuration for cell viability applications (with both cells that do form barrier tissues and cells that do not) has been recently proposed by Decataldo et al.79 The authors compared OECTs with the same channel areas but a different configuration of the gate electrode, and evaluated in real time the growth and the detachment of barrier and non-barrier tissues (plated directly on the devices) by measuring the time response of the transistors to a DC potential pulse applied on the gate. It was found that only the planar structure was able to detect the growth and the detachment of both kinds of cells, while the performance of the standard configuration was not influenced by the growth of non-barrier tissue. This effect may be due to the fact that, since in the planar structure the gate is also covered by a PEDOT:PSS layer and the cells, the ions must overcome two cell barriers (the gate/cell and cell/channel interfaces) thus obtaining a measurable increase in the sensitivity of the device.
Although still a very interesting and studied model, cells cultured onto a planar substrate present evident structural differences with respect to the same cells thriving in their physiological environment, and this aspect constitutes the classic “elephant in the room” in the in vitro practice. In order to tackle this problem, in vitro 3D models have gained more and more interest during the past 10 years, due to the potential capacity of giving an unprecedented look on more complex behaviours related to a more physiological arrangement of cellular aggregates, which of course cannot be appreciated with standard planar cellular cultures. With the intention of demonstrating the possibility of using OECTs within this rising field, a device integrating a planar OECT with a PDMS microfluidic has been developed and proposed as an impedance sensor for cellular spheroids.80 To obtain this result, the microfluidic has been designed with a trap – i.e. a cone-shaped profile between the gate and the source/drain – into which the spheroid was blocked (Fig. 8a). The impedance is then extracted starting from the variation of the OECT's transconductance (which depends on the variation in the ion flux when the spheroid is present). The researchers repeated this experiment with spheroids of different dimensions and formed by different kinds of cells and concluded that the spheroid resistance mainly depends on the ion permeability of the cells, as can be seen in Fig. 8b. The effectiveness of the approach for real time applications was demonstrated by dynamically monitoring the resistance of a spheroid after the administration of Triton X (a porogenic molecule). The Triton X addition provoked the loss of 90% of the initial resistance in about 35 minutes, while the control experiment (without Triton X) maintained the same resistance (Fig. 8c).
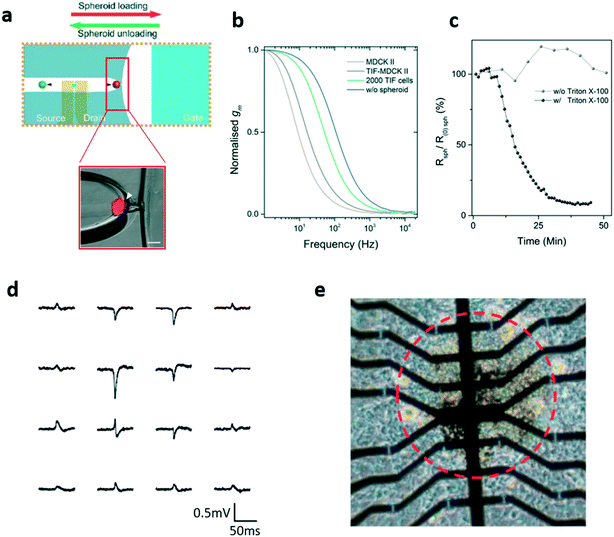 | ||
| Fig. 8 Planar OECT for in vitro monitoring of 3D cellular spheroids. a) Representation of the impedance sensing platform proposed in ref. 80 comprising a planar OECT and a microtrap to block the spheroid (inset). b) The transconductance of the OECT varies with the spheroids' composition, suggesting the idea that it depends on the permeability of cells, and c) real time measurement of the spheroid resistance variation upon the administration of a porogenic surfactant (Triton-X). d) Cardiac spheroid action potentials (APs) recorded by the OECT array; it is possible to note different AP shapes corresponding to the different spheroid areas. e) Optical image of the 16-OECT array used in ref. 84 for the detection of the electrical activity of 3D cardiac cell microtissues. The red dashed circle separates the centre of the cardiac spheroid from the area where it expanded after seeding. Panels a–c: Adapted with permission from ref. 80, Copyright The Royal Society of Chemistry 2018. Panels d and e: Adapted with permission from ref. 84, Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
More recently, a novel OECT structure, namely the “tubistor” has been designed and proposed as an interesting tool for monitoring 3D cellular aggregates.81 This device consists of a T-shaped tube, the uniaxial ends of which are the inlet and outlet ports, while the source and drain electrodes are inserted through the central opening. The gate electrode is a platinum wire and the active channel is made of a 3D, porous PEDOT:PSS scaffold formed in situ by a freeze-drying process. By tuning the principal characteristics of the scaffold, like the pore size and density or the PEDOT:PSS formulation and the material/dimension of the gate electrode, it is possible to adjust both the transistor performances and the scaffold properties, to fit specific applications. Interestingly, the tubistor has been able to reliably monitor cell attachment and growth distinguishing between 2 different cell lines, plated onto a 3D scaffold. In particular, it allows real-time monitoring of cell growth, from the early stage to the complete development of the tissue: in order to do this, cells were plated in situ (i.e. when the scaffold is positioned inside the tube) and perfused with a continuous flow of medium. The system was observed through the variations of transistor transconductance and through imaging techniques; the results were coherent with those obtained with the monitoring of tissue formation with 2D OECTs (i.e. the transconductance decreases as the density of the cells cultured onto the scaffold increases). The use of such a peculiar structure allowed the importance of the perfusion of fresh medium in the growth of 3D cell cultures to be experimentally confirmed.
While a great deal of effort has been put into the study of non-electrogenic cells in vitro using OECT-based sensors, very few applications concerning in vitro electrogenic cells have been developed so far. The first example of an in vitro interface between an OECT and excitable cells was proposed by Yao et al. in 2015.82 In this example, an array of PEDOT:PSS OECTs produced both on rigid (i.e. glass) and flexible (i.e. PET) substrates has been utilized to successfully monitor the electrical activity of HL-1 cells (a cardiac cell line), plated directly onto the active material. In this experiment, after setting VDS and VGS, the ion flow caused by an action potential modulates the output current. In both cases (flexible and rigid substrate), the arrays recorded well defined spikes with good SNR. Other examples of OECT arrays for the detection of the electrical activity of cardiac cellular cultures have been proposed shortly after by Gu et al.83 In this work, the authors were able to record the activity of primary neonatal Sprague–Dawley rat cardiomyocytes with a good signal-to-noise ratio from several channels up to 42 days without significant attenuation of the recorded signal, demonstrating the stability and robustness of the device. In a more recent work, authors from the same group validated their OECT array with 3D cultures of primary cardiomyocyte spheroid “microtissues”,84 being able to observe some features of the action potentials that usually don't appear in primary cardiomyocyte monolayer recordings, such as different shapes of action potentials in different parts of the array, corresponding to different areas of the spheroid, as shown in Fig. 8d: one shape corresponds to the centre of the spheroid, another one to the periphery, where it expanded after being seeded, and the third corresponds to the boundary of the two areas (Fig. 8e). In the same paper, the researchers proposed also the proof-of-concept of a 64-channel array able to record the electrophysiological activity with a better space resolution.
In 2017, an array of 16 OECTs with interdigitated source/drain electrodes in a common source configuration was also utilized85 to record signals generated from HL-1 cardiac cells (after 3 days in vitro) when the cells are treated with the cardio-stimulant drug norepinephrine. Shortly after, a 27-channel, flexible array of OECTs with interdigitated source and drain electrodes has been proposed to record the activity of cardiomyocyte-like HL-1 cells.86 The device showed good performance in terms of transconductance, IDS, on/off ratio, stability and SNR. Shortly after, the same group examined the performances of interdigitated transistors (called iOECTs) with different geometrical characteristics, such as the number, the width and the length of the electrodes' fingers, as well as the channel length,87 by monitoring their output current and transconductance, in order to identify the set of parameters that maximize them. They found that the transconductance does not scale with the channel width-to-length ratio (W/L), but is limited on the value of the source–drain series resistances (Rsd) and the channel resistance (Rch). To prove the reliability of the obtained design rules, the authors recorded the electrical activity of HL-1 cells using an array of 27 optimized OECTs, obtaining signal to-noise-ratios up to 18 (considerably higher than those in their first work).
Interestingly, OECTs have been also employed for the in vitro testing of a delaminating depth probe88 designed for in vivo applications. The experiments have been performed on hippocampus preparations, with a device characterized by a flexible, conformal probe attached to a sacrificial layer called shuttle that is designed in order to give to the electrode sufficient mechanical strength to penetrate the brain, and is then extracted, leaving the electrode in the targeted brain region (Fig. 9a). Although this probe has been designed to be implanted in the cortex of a rat, in order to prove the biocompatibility of the materials, the recording and stimulation capability of this device have been tested in completely extracted hippocampus preparations, i.e. a preparation that maintains the whole 3D hippocampal architecture (Fig. 9b and c), thus effectively counting as an in vitro experiment.
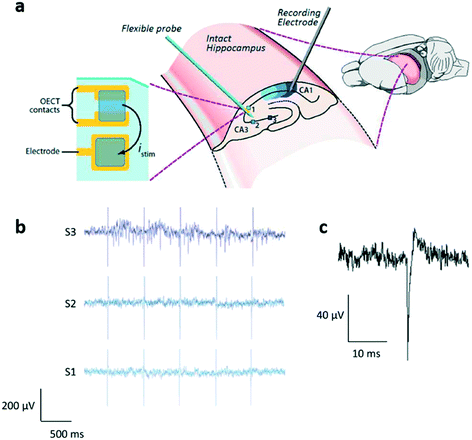 | ||
| Fig. 9 OECT for neural in vitro applications. a) The OECT array on a delaminating probe proposed by Williamson et al. The device (which contains both OECTs and PEDOT:PSS electrodes and that was designed to be used in vivo) has been preliminarily characterized in vitro on intact hippocampus preparations: stimulations are applied to 3 different sites of region CA3 (identified as 1, 2 and 3), and the activity of the rightmost part of CA1 is recorded. b) Results of this experiment: only the stimuli to site 3 (rightmost region of CA3) evoked a response in the recorded section of CA1; c) shape of a single evoked spike in CA1. Reproduced with permission from ref. 88, Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
A distinct group of in vitro applications involving the OECT consists of monitoring analytes produced or consumed as a consequence of the cellular metabolic activity, such as lactate or glucose. In these applications, the culture medium is usually collected from the cell culture and transferred onto the recording OECT device. Although these cannot be considered as real cellular interfaces, these attempts clearly also show a growing interest in exploring novel solutions involving organic active devices for monitoring the metabolic activity of cellular aggregates. An example of this kind of application is the PEDOT:PSS-PVA based OECT structure biofunctionalized for lactate and glucose monitoring proposed by Strakosas et al. in 2017.89 This device has been validated using complex media where kidney epithelial cells (MDCK II) had been cultured, showing marked differences between signals recorded from healthy cells and from cells treated with a nephrotoxic drug. A second example of metabolic sensor, designed for lactate detection in tumoral cell culture with intrinsic subtraction of the background noise, has been presented by Braendlein and co-workers.90 This device is a reference-based sensor circuit that consists of two planar all-PEDOT:PSS OECTs in a Wheatstone bridge configuration: only one transistor is functionalized through lactate oxidase (LOx) enzyme to monitor lactate variations, while the other is the reference. This circuit has been tested using complex media derived from healthy peripheral blood mononuclear cells (PBMCs) and from non-Hodgkin's lymphoma cells. These experiments highlighted a higher lactate production of cancer cells, thus confirming their intrinsic enhanced metabolic activity.
Very recently, a new OECT structure consisting of a nanosized, needle-type OECT (i.e. an OECT in which the gate, source and drain electrodes are spearhead carbon nanoelectrodes, with the source and drain connected through a thin film of PEDOT:PSS) has been proposed.91 This transistor, which has been only preliminarily characterized as a dopamine sensor, thanks to its peculiar shape and high aspect ratio that allows its precise placement, could offer unprecedented temporal and spatial resolution, thus representing a very new powerful tool for in vivo applications.
In conclusion, the OECT is so far the most mature example of an organic device employed to measure several different aspects of the cell activity, ranging from the control of cell distribution, to the viability assessment of non-electrogenic cells, to the electrophysiological activity of excitable cells. The OECT-based sensors for these applications have been created exploiting advantages like extremely high transconductances, biocompatibility, and stability in aqueous media. In addition, starting from the two principal architectures (the standard and the planar), different geometries – such as for example the already mentioned tubistor – and sensing mechanisms have been designed and tested in novel and promising cellular applications, such as neuromorphics, another rising field that is of extreme interest in organic bioelectronics.92–94
SGOFETs
SGOFETs have been extensively studied as promising tools for in vitro cellular applications, especially for the study of electrogenic cells. However, there are only a few examples of EGOFETs employed in this field. In 2013, Cramer et al.95 realized a device for in vitro recording and stimulation of the global electrical activity of neuronal networks using an ultra-thin pentacene-based EGOFET device. In this configuration, murine neural stem cells have been directly cultured on the pentacene layer, without any additional layer of cell-adhesive molecules, and the transistor current has been monitored for 7 days during the differentiation of stem cells into neurons and the formation of the mature neuronal network. After the complete differentiation of the cells, the device was able to stimulate and record the global activity of the neuronal network. More recently, an EGOFET for the electrophysiological monitoring of human pluripotent stem cell-derived cardiomyocytes has been reported96 (in Fig. 10a, a representation of the device is presented). The authors, using a blend of a small molecules (diF-TES-ADT) and polystyrene (an insulating molecule) as the active layer, were able to record the cardiomyocyte activity for 10 days, thus demonstrating the stability of the device. In addition, they were able to modulate the cell activity using drugs such as norepinephrine and verapamil, measuring highly reproducible spike shapes, even though different from the expected shape of the cardiac myocyte extracellular potential (this unusual effect is still under investigation), as shown in Fig. 10b.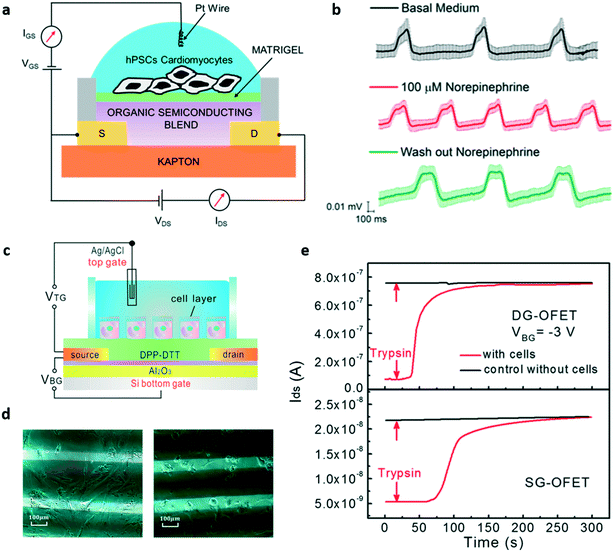 | ||
| Fig. 10 EGOFETs for in vitro applications. a) Representation of the EGOFET-based sensor for the in vitro study of hPSC-derived cardiomyocytes proposed by Kyndiah et al. b) Signals recorded with the device during basal activity, after the addition of norepinephrine and after the wash out of the drug. c) The double-gated EGOFET proposed by Zhang et al. for the detection of the detachment of human mesenchymal stem cells. d) Optical images of the human mesenchymal stem cells plated onto the device before (left) and after (right) trypsin addition. e) Transistor response, in terms of output current (IDS) variation, after the addition of trypsin, of the double gated EGOFET (DG-OFET, on top) compared to that of a single-gated one (SG-OFET, bottom). Noteworthily, the IDS current response of the DG-OFET is larger and faster if compared with the SG-OFET. Panels a and b: Adapted with permission from ref. 96, Copyright 2019 Elsevier B.V. all rights reserved. Panels c–e: Adapted with permission from ref. 98. Copyright 2017 American Chemical Society. | ||
In order to set the optimal working point of the transistor, and hence to obtain maximum amplification, using VGS values that are safe for cells (i.e. lower than 0.3 V to not elicit damage or rupture of the lipidic membrane due to undesired electrochemical processes97), Zhang and colleagues98 designed a double-gated EGOFET to which it is possible to add a bottom gate bias that allows high output current and high transconductance values with low top gate voltage to be reached, enabling precise control of the device, the schematic of which is shown in Fig. 10c. They tested this double-gated OFET as a sensor for the detachment of human mesenchymal stem cells (Fig. 10d), obtaining a substantial improvement of the sensitivity, as represented in Fig. 10e. In the same work, the authors also applied the same dual gate structure to an OECT, and repeated the same experiment performed with the DG-EGOFET; in this case the dual gated-device also showed better response if compared to the single-gated one.
Recently, an interesting EGOFET-based device called Electrolyte Gated Organic Synapstor (EGOS) has been proposed by Desbief et al.99 A synapstor (short name for synapse transistor) is a device that simulates neuronal short term plasticity (STP), a phenomenon observed in neuronal aggregates in which the activity of a certain synapse strongly depends on the pre-synaptic activity (either excitatory or inhibitory). The synapstor shows this behaviour thanks to the memory effect that is conferred by the charging/discharging of gold nanoparticles (NPs) added at the organic semiconductor/gate dielectric interface. The EGOS showed marked STP at low-voltage spike stimulation (down to 50 mV) with a good dynamic response (few tens of ms) and a very good biocompatibility (it was in fact tested with two neural cell lines to ensure the actual usability with cell cultures). This device, although only preliminarily, demonstrates how EGOFETs can be in principle employed to study/implement complex neuronal behaviour, thus extending the applicability of such organic devices in the neuroscientific field.
Different than EGOFETs, whose employment in cellular applications is still in its preliminary stage, graphene transistors have been extensively tested in the field, thanks to the peculiar properties of graphene, which is characterized, as previously reported, by very good chemical stability and much higher mobility if compared to standard organic semiconductors (such as, for example, P3HT). The first reported example of gSGFETs for in vitro cellular interfacing dates back to 2010, when Cohen-Karni et al. proposed a graphene transistor specifically tailored for monitoring the electrical activity of spontaneously beating embryonic chicken cardiomyocytes,100 obtaining interesting results in terms of the signal-to-noise-ratio (SNR > 4). Shortly after, Hess et al. introduced an optimized gSGFET with CVD deposited graphene and high transconductance,101 which paved the way to the introduction of the first gSGFET array for in vitro monitoring of electroactive cells.102 In particular, in their work, Hesse et al. deeply investigated and assessed the actual advantages offered by graphene transistor arrays over standard microelectrodes and silicon-based devices in terms of transconductance, signal-to-noise-ratio (SNR), and stability in aqueous solutions. In particular, the high SNR directly depends on the low 1/f noise of graphene-based devices, which stems from the peculiar characteristics of this 2D material.103 Moreover, the facile integration of the device with flexible substrates makes graphene-based electronics even more appealing for cellular applications. Further studies on graphene transistor arrays led to a model that demonstrated the feasibility of using these devices for the detection of the activity of voltage-gated potassium ion channels.104 In particular, the proposed model takes into account the increase of the ionic strength in the cleft occurring during cellular activity and used experimental data to modify the standard point-contact model.105
Another peculiar cellular application of gSGFETs was proposed by Ang et al. in 2011, who developed a graphene transistor-based biosensor for the label-free electrical detection of malaria-infected red blood cells (RBCs).106 In this interesting example, graphene was functionalized using specific receptors that allowed the selective capture of malaria-infected RBCs in a flow-catch-release microfluidic system. The binding of the infected cells to the functionalized graphene lead to a modulation of the drain–source conductivity of the graphene channel (thanks to a local doping induced on the graphene by the charged protrusions on the cell surface), thus allowing their direct electrical detection. In addition to the high stability and sensitivity of graphene FETs, the device optical transparency is also an important aspect, allowing the simultaneous electrical and optical monitoring of the binding-release process. This gave the possibility of finely monitoring the cell velocity and the mechanical properties (since these two aspects are correlated), through which it was possible to gather information on the progression of the infection and to differentiate between healthy and unhealthy cells. Although this example is not precisely an in vitro cellular sensor, this work represents the perfect example of the remarkable versatility of gSGFET-based sensors.
As previously mentioned, an advantage of gSGFETs (if compared to standard silicon transistors) is the fact that they can be fabricated onto flexible substrates without any loss in transconductance, this peculiarity making them interesting tools for ex vivo and in vitro cardio-electrophysiology applications, as demonstrated by Kireev et al.107 The same group also demonstrated the possibility of using gSGFET arrays for neural in vitro recording, and proposed a new passivation approach called “feedline follower”, which consists of passivating only the metallic feedlines instead of the whole surface of the array, in order to improve the cell/graphene coupling by reducing the gap distance between the cell and the gate.108 In Fig. 11a and b, respectively, a healthy culture of cardiac cells cultured on the recording area of the array and two minutes of cardiac electrical activity recorded with a graphene transistor within the array are shown. Another example of a gSGFET-based in vitro neural interface is represented by the work of Veliev et al.,109 who demonstrated how flexible and transparent gSGFETs provide a highly-sensitive, biocompatible, transparent, and chemically stable platform for neurophysiological applications. In their work, they were able to successfully record the spontaneous activity of hippocampal neurons (Fig. 11c and d) thanks to sensitivities up to 4 mS V−1 and ultra-low noise levels (down to 10−22 A2 Hz−1). In a more recent work, the same group further demonstrated the remarkable properties of this kind of device by recording the activity of single neuronal ion channels110 (a possibility that was already demonstrated using graphene electrodes111). The monitoring of single channels' activity is possible thanks to the already mentioned low noise of graphene transistors and to the inevitable presence of grain boundaries on (CVD grown) graphene,112 which act as ultra-high-sensitive sensing sites. In particular, the ionic currents flowing through a single ion channel positioned over a grain boundary are able to tune the Fermi level of the grain boundary itself, thus altering the conductivity of the transistor (the mechanism is depicted in Fig. 11e and f). This constitutes a very interesting and unique capability that can be of great importance in the development of novel tools for the study of neurodegenerative diseases. Besides ionic currents, gSGFETs can be also used for the detection of neurotransmitters, which represent another important aspect when dealing with neuronal cultures. Recently, a reduced graphene oxide (rGO)-based transistor functionalized with synthesized glutamate receptors has been used for the real time monitoring of glutamate from primary neuronal cultures,113 thus confirming the impressive versatility of gSGFETs.
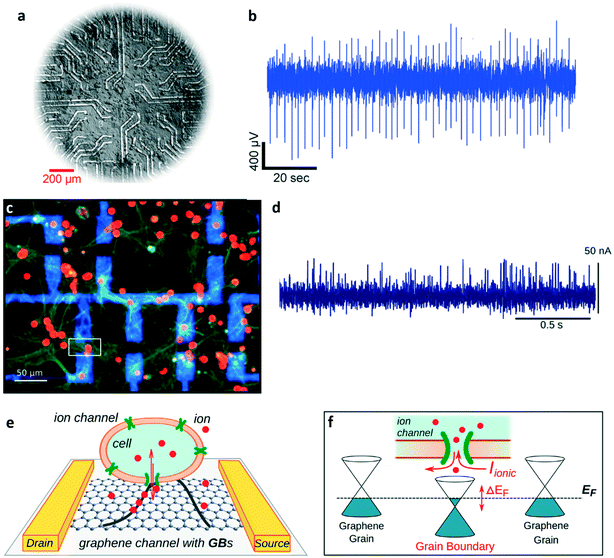 | ||
| Fig. 11 Graphene transistors for in vitro applications. a) Example of a HL-1 cell culture on top of the graphene-transistor chip (G-FET) proposed by Kireev et al. (optical micrograph). b) Two-minute recording of the activity of a HL-1 culture recorded with a graphene transistor in the G-FET device. c) Immuno-fluorescence image of healthy neurons cultured for 21 days on a G-FET, stained with DAPI (red – neuronal soma) and anti-synapsin (green – synaptic vesicles). It is possible to observe the metal contact leads (blue) and the channel area of a graphene transistor (white square). d) Example of neuronal activity measured with a G-FET. Graphene transistors, due to the presence of grain boundaries (GBs) on CVD graphene layers, are suitable for the detection of single channels' activity. e) Graphical representation of an electroactive cell on the channel of a graphene transistor. f) Transduction mechanism of the activity of a single membrane channel. When an ion channel aligns randomly on a grain boundary, the ionic currents flowing through it can tune the Fermi level of the grain boundary itself, thus modulating the transistor's channel conductivity. Panels a and b: Reproduced with permission from ref. 108 (open access article distributed under the terms of the Creative Commons CC BY license). Panels c and d: Reproduced with permission from ref. 109 (open access article distributed under the terms of the Creative Commons CC BY license). Panels e and f: Adapted with permission from ref. 110, copyright 2018 IOP publishing ltd. | ||
OCMFETs
The OCMFET exploits the principle of amplifying, by means of an organic field effect transistor, the potential modulation induced by the electrical signal produced by cells seeded on top of a floating metal surface, which is capacitively coupled with the transistor channel (Fig. 12a). With the metal surface being physically separated from the channel, the OSC is not affected by the harsh environmental conditions typical of cell cultures and the amplifying ability of the transistor structure is fully preserved and stable in time. The first design of an OCMFET for in vitro electrophysiology was proposed in 2013.114 In this case, the sensing area was reduced in order to match that of common microelectrode arrays (MEAs), and the biocompatibility of the device was successfully tested with cultures of cardiomyocytes. In the same work, the possibility of using the OCMFET in electrophysiological application was preliminarily investigated using synthetic cardiac extracellular signals directly transmitted through a platinum electrode into the solution containing the recording area. Soon after, the same group reported the first actual electrophysiological measurements that were performed with a single OCMFET with cardiac HL-1 line cells (Fig. 12b).115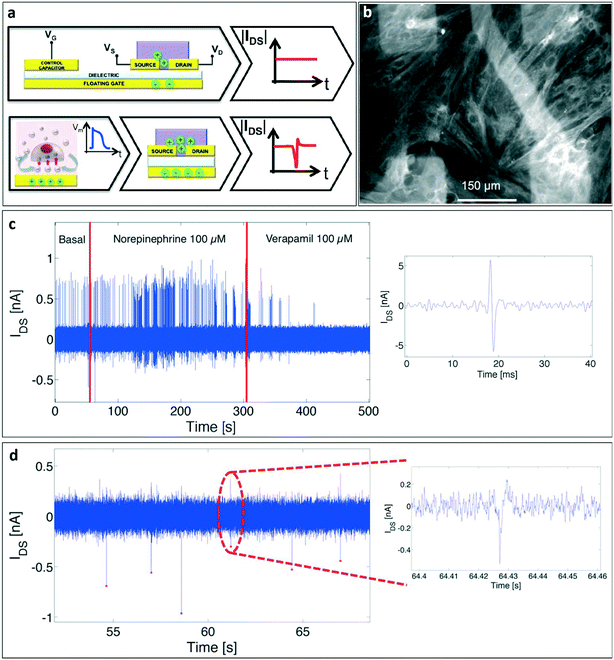 | ||
| Fig. 12 OCMFET for electrical recordings of electroactive cells' activity. a) Working principle of an OCMFET for the detection of cellular electrical activity. The working point of the device is set by applying appropriate control gate and drain voltages; the cellular electrical activity over the sensing area determines a charge re-distribution inside the floating gate, which modulates the charge carriers density inside the organic semiconductor, thus inducing a variation of the output current of the transistor. b) A healthy culture of rat primary cardiomyocytes (fixed after 8 days in vitro and immunostained for the sarcomeric protein tropomyosin) cultured on the recording area of an OCMFET array. c) Pharmacological modulation of cellular electrical activity: the spontaneous activity was accelerated by the administration of norepinephrine (a cardio-stimulant that acts on b-adrenergic receptors), and then suppressed with a high dose of verapamil (a calcium blocker that acts as a cardio-relaxant). c (Inset): A single extracellular cardiac action potential recorded with an OCMFET. d) Example of neuronal action potentials (striatal cells from a rat embryo – 21 DIV) recorded with an OCMFET (inset: single neuronal action potential). Reproduced with permission from ref. 116 (open access article distributed under the terms of the Creative Commons CC BY license). | ||
The signals recorded with the OCMFET were coherent with those recorded on the same type of culture with standard MEAs, thus confirming the conclusions of the previous work. As already underlined, this double-gated organic device can be used for sensing a variety of chemical/physical parameters in a liquid environment, provided that the sensing area is properly functionalized with the right “receptor”. Thus, the main opportunity offered by this technology is that of fabricating an array of devices including different kinds of sensors.
In 2015, an array of up to 16 OCMFETs (called MOA, Multi OCMFET Array) was extensively tested with rat cardiomyocytes (Fig. 12c) and with rat primary neuronal cultures (Fig. 12d). Interestingly, its functionality in the transduction of the cellular electrical activity has been confirmed by comparing the OCMFETs' output to those of standard microelectrodes fabricated within the same recording area.116 The matrix configuration also allowed recording signals from several OCMFETs simultaneously, thus further validating the suitability of using this device for applications such as pharmacology and, in principle, in vitro neurophysiology. More recently, the previously only envisioned possibility of monitoring the metabolic activity of a cellular culture with an OCMFET117 has also been demonstrated. In fact, thanks to a simple physical functionalization of the sensing area, the OCMFET can be turned into a reference-less and supernerstian pH sensor with a sensitivity of up to 1.4 V per pH.118 The amplification effect that allows achievement of Vth variations higher than those predicted by the Nernst limit (i.e. 59 mV per pH at 25 °C) stems from the capability of the OCMFET to directly transduce the charge that is present on the sensing area, an effect that can be finely tuned by simply modifying the transistor's geometrical parameters (such as the extension of the sensing area, the control gate area and the channel area). A similar effect has been already observed in other dual-gate devices.119,120 Such a high pH sensitivity is of great importance in cellular application, due to the fact that very small pH variations are induced in the extracellular medium by modifications of cell metabolic activity. As a consequence, a very sensitive device is needed in order to record such modifications. When integrated into a MOA, these devices allowed the monitoring of the metabolic activity of rat primary neurons, as reported in a recent work121 (Fig. 13), thus demonstrating the interesting potential of using OCMFET-based devices for the realization of multi-sensing tools for cellular applications, able to record at the same time, different useful parameters.
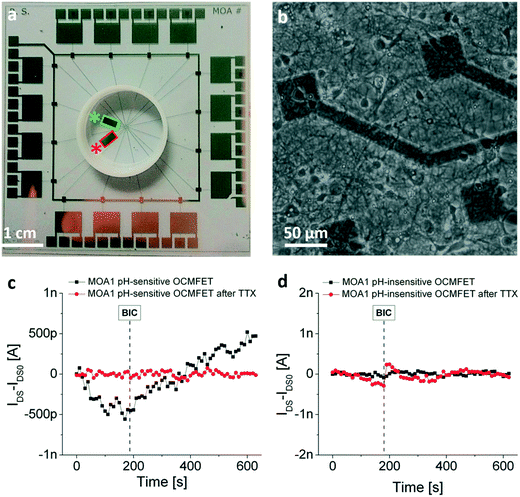 | ||
| Fig. 13 OCMFET for neuronal metabolic activity monitoring. a) Example of a multi-sensing OCMFET-based chip for the detection of both the electrical and the metabolic activity of neuronal cells. The red and the green rectangles are respectively an ultra-sensitive pH sensor and a control pH-insensitive identical OCMFET that serves as a control for the metabolic experiments. b) Healthy primary hippocampal neurons cultured on the device recording area. c) Response of the pH-sensitive OCMFET to the addition of bicuculline (BIC) before (black) and after (red) the addition of a high dose of tetrodotoxin (TTX). Before the TTX-induced cell death, the OCMFET is able to discriminate between the pre-BIC “low-metabolic” state and the post-BIC “high-metabolic” state. d) The same experimental protocol recorded within the same device with the pH-insensitive OCMFET. Reproduced with permission from ref. 121 (open access article distributed under the terms of the Creative Commons CC BY license). | ||
In vivo applications
Though more recent, the application of cellular sensors based on organic devices to the in vivo practice is indeed a very intriguing possibility for this emerging technology, as it allows some unique electrical and mechanical characteristics of the employed materials and devices to be fully exploited. The development of seamless brain machine interfaces is undoubtedly the field that will benefit the most from these favourable features, which can, however, also bring important advances to other fields such as pharmacology and diagnostics.OECTs
The first use of an electrochemical transistor in the in vivo practise was reported by Khodagholy et al. in 2013, when the authors designed and fabricated a highly conformable array consisting of 17 PEDOT:PSS OECTs (and 8 PEDOT:PSS passive electrodes) specifically designed for electrocorticography (ECoG).122 In Fig. 14a and b respectively, the flexible device structure and the same device positioned on a rat's cortex are shown. Comparing the signals recorded with three different kinds of electrodes (namely OECTs, PEDOT:PSS passive electrodes and standard penetrating electrodes), the authors were able to validate the efficacy of the OECT array, which showed a higher SNR if compared to passive planar electrodes, and similar features to those typically obtained using penetrating electrodes but with the advantage of not damaging tissues with invasive probes (Fig. 14c).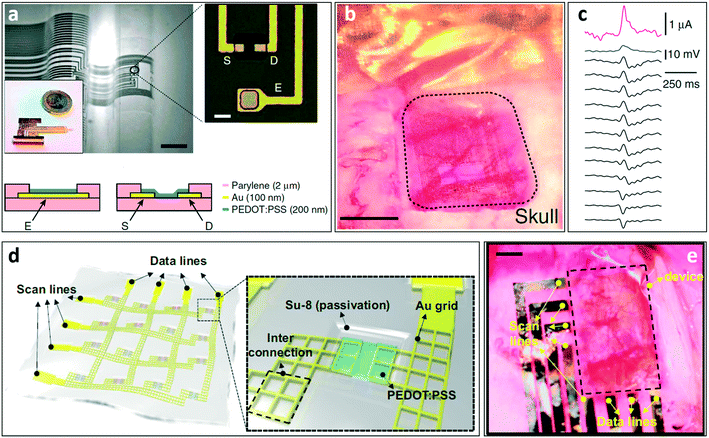 | ||
| Fig. 14 OECT for in vivo applications. a) Optical micrograph of the neural probe proposed by Khodagholy et al. Thanks to the ultrathin substrate it can easily conform onto non-flat surfaces. Scale bar, 1 mm. The transistor/electrode array is connected to the external electronics through a zero insertion force (ZIF) connector. In the inset (scale bar, 10 μm), a magnification of the recording area of the device, which contains both OECTs and PEDOT:PSS surface electrodes, is shown (S = source, D = drain, E = electrode), while in the bottom the schematic layouts of the OECT and the PEDOT:PSS electrode are presented. b) The same neural probe depicted in (a) positioned onto the cortex of an anesthetized rat, and c) recording of an epileptiform spike (induced by the administration of bicuculline) from an OECT (pink), a PEDOT:PSS electrode (blue) and 12 Ir-penetrating electrodes (black) that were used to validate the OECT recordings. Interestingly, the OECT outperforms both the surface electrode and the penetrating electrodes in terms of the signal amplitude. d) Representation of the transparent electrophysiology OECT array proposed by Lee et al. (Inset) OECT recording site: the peculiar source and drain grid pattern ensures the transparency of the whole recording area (which is shown in (e)) after the positioning on the cortex of an optogenetic rat (scale bar: 1 mm). Panels a and b: Reproduced with permission from ref. 122 (open access article distributed under the terms of the Creative Commons CC BY license). Panels d and e: Reproduced with permission from ref. 124 Copyright 2017 National Academy of Sciences. | ||
An interesting advantage of OECTs is the fact that it is possible to tune their performance by controlling the channel thickness without changing the lateral dimensions,123 a feature that is particularly interesting for all applications requiring small yet high-performing recording sites. Another aspect that is particularly relevant for in vivo applications is the fact that OECT arrays are optically transparent (as the active layer has a typical thickness of a few tens of nm). Optical transparency in fact gives the possibility of, for example, checking the right positioning of the device over the cortex and/or conducting optogenetic experiments. An example that sums up all these features is the work of Lee et al.,124 who were able to record the evoked activity of cortical neurons of optogenetic rats (i.e. the electrical activity induced by an external optical stimulation due to the expression of light-sensitive membrane channels) using a fully transparent OECT array. Thanks to the peculiar structure of the device, the authors were able to reconstruct the cortical map of activation of an optogenetic rat, a task that is not easy to perform using standard ECoG electrodes. The structure of the device is shown in Fig. 14d.
The authors designed a transparent (transparency higher than 60%) system made from a matrix of OECTs interconnected by an Au grid (Au grid dimension of 3 um). The sheet resistance of the grid is 3 Ω sq−1 (a low value of sheet resistance is fundamental to minimize the voltage drop in the wire and consequently to properly operate the OECT without increasing the voltage applied on the contact pads). The array is then positioned on the cortical surface of the brain of an optogenetic rat (as shown in Fig. 14e), and the ECoG is recorded after inducing an optical stimulation through the transparent matrix.
In 2018, a flexible matrix of PEDOT:PSS-based OECTs was designed to record ECG signals from the heart surface of living rats.125 The stretchability of the device, which is particularly important in applications involving contractile tissues, has been obtained with a particular honeycomb structure that allows the matrix to retain its electrical characteristics even upon the application of 15% of strain. In addition, the device is coated with an antithrombotic (PMC3A) agent that doesn't influence the electrical properties of the device but, avoiding the adhesion of platelets, increases the stability of the OECTs: this allows for long-term ECG signal recordings onto the bleeding surface of the heart with an SNR of 52 dB (while the non-coated matrix was unable to record even after 30 minutes from the implant), thus outperforming standard recording techniques.
The need for an electrolyte solution as a fundamental part of the OECT structure brings also some limitations. In particular, in OECT arrays, the presence of a common gate does not allow each transistor to be independently addressed; moreover, the time response of the device depends on the diffusion of the ions from the electrolyte and the channel, thus intrinsically limiting the bandwidth.126 In order to overcome these limitations, a new architecture called internal ion-gated organic electrochemical transistor (IGT) has been recently presented. In this peculiar structure, mobile ions are embedded directly inside the conducting polymer (thanks to a combination of PEDOT:PSS and D-sorbitol, which creates an “ion reservoir” distributed through the entire bulk of the polymer), and in an amount sufficient to dope/de-dope the semiconductor in response to the applied gate voltage; therefore this device does not need an external shared electrolyte to exchange ions with the solution.127 In addition, to allow independent gating of each transistor, an ion membrane of chitosan is introduced between each transistor channel and the gate electrode, to guarantee efficient ionic (but not electronic) conduction. The device has been initially validated with EEG recordings, but it also represents a possible interesting solution for other cellular applications since it offers the possibility of addressing each OECT independently (in a matrix configuration) and can provide a more stable interaction between the device and the cells. To fill the gap with standard integrated circuits, which are typically based on both depletion (normally ON, like the standard IGT device) and enhancement mode transistors (normally OFF), an enhancement mode IGT, called e-IGT, has been recently developed.128 The e-IGT showed its applicability in a wide range of biomedical applications, ranging from EEG recording to intracranial encephalography in freely moving rats, thanks to its high transimpedance and volumetric capacitance, a fast response time, long-term stability, and biocompatibility.
Within the domain of in vivo bioelectronics, the monitoring of neurotransmitters can also provide important information to better understand the brain functioning. In most studies in this field, commercially available neurotransmitters are used as benchmark compounds to design OECT-based sensors for several molecules such as glutamate, acetylcholine129 or dopamine with high selectivity.130,131 Recently, Xie et al.132 developed an array of planar OECTs specifically designed for dopamine detection for in vivo applications. The researchers developed an OECT with a platinum gate electrode onto which some molecules, such as the catecholamine neurotransmitter (CA-NT) dopamine, can be oxidized into quinone with the release of two electrons. These electrons are thus transferred to the gate (generating a faradic current) and ultimately increase the effective gate voltage. With this device and using a controlled local stimulation, the authors were able to successfully record the real-time dopamine release simultaneously in different brain regions. These examples show a clear trend towards future applications of OECT-based devices in the in vivo cellular domain.
SGOFETs
EGOFETs for in vivo applications have not been realized yet. However, a preliminary feasibility study was published in 2018.133 In their work, Lago et al. simulated the operation of a back gated EGOFET that in principle should be able to simultaneously stimulate and record cell activity without the need for any external reference electrode. In this device, the stimulations are delivered to the cells by applying voltage pulses to the back gate, which induce small perturbations at the cell/semiconductor interface. The modulation of the channel of the transistor occurs by means of “self-polarization” through the source and drain electrodes that polarizes the solution. The so-called reference-less EGOFET (RL-EGOFET) is quite promising, as suggested by both simulations and proof-of-concept experiments.The aforementioned characteristics of graphene transistors (biocompatibility, high transconductance, and suitability for flexible electronics applications) have also been recently exploited for in vivo applications. In particular, Blaschke et al.134 presented a gSGFET array employed to record cortical activity (low frequency spontaneous oscillations, as well as pharmacologically-induced pre-epileptic discharges and visually evoked events) from anesthetized rats with high SNR, thanks to the intrinsic low noise of these flexible graphene-based devices (Fig. 15a–d). These very important features, namely flexibility, optical transparency and excellent electronic performance, have been further developed and exploited in recent in vivo applications involving flexible gSGFET microtransducers for electrocorticography135 and high resolution epicortical and intracortical mapping of brain activity.136 In particular, the latter work (showed in Fig. 15e–h) gives an interesting perspective on the outstanding low frequency performance of graphene transistors. More recently, Garcia-Cortadella et al. provided new insights on the possibility of further improving the gSGFET's SNR by accurately modeling harmonic distortions and non-ideal frequency response of the device (induced by the dependence of the transconductance on, respectively, the gate voltage and the frequency of the acquired signal) that lead to the distortion of the output signal, and compensating for it.137 This in depth study of such an important aspect of graphene transistors gives the possibility of improving the already impressive performance of graphene transistors, thus making them ideal candidates for in vivo cellular interfacing.
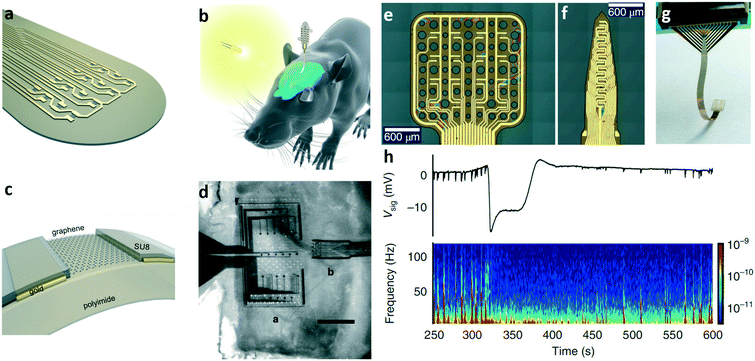 | ||
| Fig. 15 Graphene transistors for in vivo applications. a) and b) Recording area of the graphene transistor-based implant proposed by Blaschke et al., and representation of the implant placed on the surface of the optogenetic rat's brain, respectively. c) Cross section of a graphene transistor from the implant with materials. d) Actual image of the graphene device (b) positioned onto the rat's brain next to a MEA with Pt electrodes (a). Optical images of the flexible 4 × 4 gSGFET array (e), the 15-channel intracortical array (f), and the whole neural probe after being introduced into a zero insertion force connector (g) proposed by Masvidal-Codina et al. h) (top) Output signal of a cortical spreading depression (CSD) event recorded by a gSGFET and (bottom) the relative spectrogram through which it is possible to appreciate the effectiveness of the proposed device in the detection of both low- and high-frequency cellular signals. Panels a–d: Adapted with permission from ref. 134, Copyright 2017 IOP Publishing Ltd. Panels e–h: Adapted with permission from ref. 136, Copyright 2018 Springer Nature. | ||
Conclusions and outlook
The multifaceted domain of cellular interfacing has tremendously evolved in the past decades, and still represents a fast-expanding research area with numerous possible repercussions in important fields such as fundamental biology, neuroscience, pharmacology and toxicology, but also brain machine interfaces and studies on neuronal functional connectivity. Organic electronics, which has been only recently considered for application in this field, rapidly took over the scene, and nowadays represents one of the most promising technological approaches, being able to successfully tackle some of the most critical issues with the introduction of innovative devices, fabrication techniques, and materials. More specifically, the new families of organic transistors that have been considered in this review (namely the OECT, the SGFET, and the OCMFET) thanks to their peculiar structures and electrical characteristics are now able to provide convenient methods for the assessment of the integrity of tissue-like cellular cultures, as well as for the monitoring of cellular metabolic and, when excitable cells are considered, electrical activity. Moreover, these modern OFET-based devices allow new approaches for the stimulation of the chemical and electrical activity of cells, and for the multiparametric analysis of the extracellular environment, both in vitro and in vivo.The fundamental advantage of using devices based on organic materials to interface living cells and tissues lies in the similarity between the physical properties of the two components of the bio-interface, which has now the possibility of becoming truly “seamless” thanks to more compatible mechanical (relatively low Young's modulus and, in some cases, even the capacity for self-healing) and electrical properties (some organic polymers can in fact combine electronic and ionic conduction), and to the capability of targeting the biological domain from the subcellular level to cell aggregates and tissues or organs. In other words, interfacing the body at the multiscale seems to be the potential rising future for organic electronics.
Although already impressive, this perspective will be even more realistic as soon as more performing organic conductors and semiconductors are developed. Graphene is already a good example of how obtaining top-notch electrical performances, together with improved mechanical properties such as flexibility, may have a substantial impact on this kind of application. Therefore, however challenging the path may seem, we should be ready to witness an unprecedented richness of novel tools and approaches, which in fact have just started entering clinical practice. Following these promising results, organic electronics-based devices are bound to become important players in the investigation of complex aspects of cellular systems, thus helping the advancement of several biomedical fields, ranging from the development of fast and reliable pharmacological and toxicological assays, to the improvement of the ways we study the human brain and the early stage of life-threatening diseases.
Authors contribution
Andrea Spanu contributed to writing the paper and supervised the writing procedure. Laura Martines contributed to writing the paper. Annalisa Bonfiglio contributed to writing the paper and supervised the writing procedure.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the italian project TEX-STYLE ARS01_00996 (Nuovi tessuti intelligenti e sostenibili multi-settoriali per design creativo e stile made-in-Italy, PNR 2015-2020), and the sardinian project POR FESR Sardegna 1C-177 (BIOMED “Piattaforma elettronica per la rilevazione diagnostica e prognostica di marker tumorali”) for the financial support.References
- C. A. Thomas, P. A. Springer, G. E. Loeb, Y. Berwald-Netter and L. M. Okun, A miniature microelectrode array to monitor the bioelectric activity of cultured cells, Exp. Cell Res., 1972, 74, 61–66 CrossRef.
- M. Grattarola and S. Martinoia, Modeling the Neuron-Microtransducer Junction: From Extracellular to Patch Recording, IEEE Trans. Biomed. Eng., 1993, 40, 158–165 CrossRef.
- P. Bergveld, J. Wiersma and H. Meertens, Extracellular Potential Recordings by Means of a Field Effect Transistor Without Gate Metal, Called OSFET, IEEE Trans. Biomed. Eng., 1976, 23, 136–144 CAS.
- P. Fromherz and A. Offenhausser, A Neuron-Silicon Junction: A Retzius Cell of the Leech on an Insulated-Gate Field-Effect Transistor, Science, 1991, 252, 1290–1293 CrossRef CAS.
- S. Martinoia, et al., Development of ISFET array-based microsystems for bioelectrochemical measurements of cell populations, Biosens. Bioelectron., 2001, 16(9–12), 1043–1050 CrossRef CAS.
- A. Poghossian, et al. Field-effect devices for detecting cellular signals, Semin. Cell Dev. Biol., 2009, 20, 1 CrossRef.
- P. Massobrio, G. Massobrio and S. Martinoia, Interfacing cultured neurons to microtransducers arrays: a review of the neuro-electronic junction models, Front. Neurosci., 2016, 10, 282 Search PubMed.
- Q. Qing, et al., Nanowire transistor arrays for mapping neural circuits in acute brain slices, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(5), 1882–1887 CrossRef CAS.
- Y. Zhao, et al., Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording, Nat. Nanotechnol., 2019, 14(8), 783–790 CrossRef CAS.
- F. Morin, et al., Constraining the connectivity of neuronal networks cultured on microelectrode arrays with microfluidic techniques: a step towards neuron-based functional chips, Biosens. Bioelectron., 2006, 21(7), 1093–1100 CrossRef CAS.
- P. Massobrio, et al., In Vitro Studies of Neuronal Networks and Synaptic Plasticity in Invertebrates and in Mammals Using Multielectrode Arrays, Neural Plast., 2015, 2015, 196195 Search PubMed.
- G. Xiang, et al., Microelectrode array-based system for neuropharmacological applications with cortical neurons cultured in vitro, Biosens. Bioelectron., 2007, 22(11), 2478–2484 CrossRef CAS.
- M. A. Lebedev and M. A. L. Nicolelis, Brain-machine interfaces: From basic science to neuroprostheses and neurorehabilitation, Physiol. Rev., 2017, 97(2), 767–837 CrossRef.
- R. M. Owens and G. G. Malliaras, Organic electronics at the interface with biology, MRS Bull., 2010, 35(6), 449–456 CrossRef CAS.
- N. Lago and A. Cester, Flexible and organic neural interfaces: a review, Appl. Sci., 2017, 7(12), 1292 CrossRef.
- M. Lee, et al., Soft high-resolution neural interfacing probes: Materials and design approaches, Nano Lett., 2019, 19(5), 2741–2749 CrossRef CAS.
- W. Lee and T. Someya, Emerging trends in flexible active multielectrode arrays, Chem. Mater., 2019, 31(17), 6347–6358 CrossRef CAS.
- E. Song, et al., Materials for flexible bioelectronic systems as chronic neural interfaces, Nat. Mater., 2020, 19(6), 590–603 CrossRef CAS.
- S. P. Lacour, et al., Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces, Med. Biol. Eng. Comput., 2010, 48(10), 945–954 CrossRef.
- L. D. Garma, et al., Inkjet-printed PEDOT: PSS multi-electrode arrays for low-cost in vitro electrophysiology, Lab Chip, 2019, 19(22), 3776–3786 RSC.
- Y. Liu, et al., Soft conductive micropillar electrode arrays for biologically relevant electrophysiological recording, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(46), 11718–11723 CrossRef CAS.
- D. A. Soscia, et al., A flexible 3-dimensional microelectrode array for in vitro brain models, Lab Chip, 2020, 20(5), 901–911 RSC.
- A. Spanu, et al. A three-dimensional micro-electrode array for in-vitro neuronal interfacing, J. Neural Eng., 2020, 17, 3 Search PubMed.
- M. Berggren and G. G. Malliaras, How conducting polymer electrodes operate, Science, 2019, 364(6437), 233–234 CAS.
- D. Ohayon and S. Inal, Organic Bioelectronics: Organic Bioelectronics: From Functional Materials to Next-Generation Devices and Power Sources(Adv. Mater. 36/2020), Adv. Mater., 2020, 32(36), 2070267 Search PubMed.
- J. Isaksson, et al., Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump, Nat. Mater., 2007, 6, 673–679 CrossRef CAS.
- T. A. Sjöström, et al., A decade of iontronic delivery devices, Adv. Mater. Technol., 2018, 3, 1700360 CrossRef.
- V. Benfenati, et al., A transparent organic transistor structure for bidirectional stimulation and recording of primary neurons, Nat. Mater., 2013, 12(7), 672–680 CrossRef CAS.
- A. I. Borrachero-Conejo, et al. Electrical stimulation by an organic transistor architecture induces calcium signaling in nonexcitable brain cells, Adv. Healthcare Mater., 2019, 8(3), 1801139 CrossRef.
- S. H. Kim, et al., Electrolyte-gated transistors for organic and printed electronics, Adv. Mater., 2013, 25(13), 1822–1846 CrossRef CAS.
- X. Strakosas, M. Bongo and R. M. Owens, The organic electrochemical transistor for biological applications, J. Appl. Polym. Sci., 2015, 132(15), 41735 CrossRef.
- L. Kergoat, et al., Advances in organic transistor-based biosensors: from organic electrochemical transistors to electrolyte-gated organic field-effect transistors, Anal. Bioanal. Chem., 2012, 402(5), 1813–1826 CrossRef CAS.
- J. Rivnay, et al., Organic electrochemical transistors, Nat. Rev. Mater., 2018, 3(2), 1–14 Search PubMed.
- Y. Wen and X. Jingkun, Scientific Importance of Water-Processable PEDOT–PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review, J. Polym. Sci., Part A: Polym. Chem., 2017, 55(7), 1121–1150 CrossRef CAS.
- M. Fahlman, et al., Interfaces in organic electronics, Nat. Rev. Mater., 2019, 4(10), 627–650 CrossRef CAS.
- S. Pang, et al., Graphene as transparent electrode material for organic electronics, Adv. Mater., 2011, 23(25), 2779–2795 CrossRef CAS.
- X. Wan, et al., Graphene–a promising material for organic photovoltaic cells, Adv. Mater., 2011, 23(45), 5342–5358 CrossRef CAS.
- I. Heller, et al., Influence of electrolyte composition on liquid-gated carbon nanotube and graphene transistors, J. Am. Chem. Soc., 2010, 132(48), 17149–17156 CrossRef CAS.
- L. H. Hess, et al. High-transconductance graphene solution-gated field effect transistors, Appl. Phys. Lett., 2011, 99(3), 033503 CrossRef.
- S. Stankovich, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide, Carbon, 2007, 45(7), 1558–1565 CrossRef CAS.
- S. Gilje, et al., A chemical route to graphene for device applications, Nano Lett., 2007, 7(11), 3394–3398 CrossRef CAS.
- J. D. Fowler, et al., Practical chemical sensors from chemically derived graphene, ACS Nano, 2009, 3(2), 301–306 CrossRef CAS.
- F. Yan, M. Zhang and J. Li, Solution-Gated Graphene Transistors for Chemical and Biological Sensors, Adv. Healthcare Mater., 2014, 3(3), 313–331 CrossRef CAS.
- P. K. Ang, et al., Solution-gated epitaxial graphene as pH sensor, J. Am. Chem. Soc., 2008, 130(44), 14392–14393 CrossRef CAS.
- Y. Ohno, et al., Electrolyte-gated graphene field-effect transistors for detecting pH and protein adsorption, Nano Lett., 2009, 9(9), 3318–3322 CrossRef CAS.
- B. Mailly-Giacchetti, et al. pH sensing properties of graphene solution-gated field-effect transistors, J. Appl. Phys., 2013, 114(8), 084505 CrossRef.
- I.-Y. Sohn, et al., pH sensing characteristics and biosensing application of solution-gated reduced graphene oxide field-effect transistors, Biosens. Bioelectron., 2013, 45, 70–76 CrossRef CAS.
- N. Mohanty and V. Berry, Graphene-based single-bacterium resolution biodevice and DNA transistor: interfacing graphene derivatives with nanoscale and microscale biocomponents, Nano Lett., 2008, 8(12), 4469–4476 CrossRef CAS – [BIOINTERFACCIA – Chemical Modified Graphene – CMG].
- C.-H. Lu, et al., A graphene platform for sensing biomolecules, Angew. Chem., Int. Ed., 2009, 48(26), 4785–4787 CrossRef CAS.
- C. Xiong, et al., ZIF-67 derived porous Co3O4 hollow nanopolyhedron functionalized solution-gated graphene transistors for simultaneous detection of glucose and uric acid in tears, Biosens. Bioelectron., 2018, 101, 21–28 CrossRef CAS.
- Y. Huang, et al., Graphene-based biosensors for detection of bacteria and their metabolic activities, J. Mater. Chem., 2011, 21(33), 12358–12362 RSC.
- S. Li, et al., Highly sensitive solution-gated graphene transistors for label-free DNA detection, Biosens. Bioelectron., 2019, 136, 91–96 CrossRef CAS.
- M. Ma, et al., Non-invasive detection of glucose via a solution-gated graphene transistor, Analyst, 2020, 145(3), 887–896 RSC.
- C. Napoli, et al. Electronic Detection of DNA Hybridization by Coupling Organic Field-Effect Transistor-Based Sensors and Hairpin-Shaped Probes, Sensors, 2018, 18(4), 990 CrossRef.
- S. Lai, et al., Ultralow voltage, OTFT-based sensor for label-free DNA detection, Adv. Mater., 2013, 25(1), 103–107 CrossRef CAS.
- A. Spanu, et al., A high-sensitivity tactile sensor based on piezoelectric polymer PVDF coupled to an ultra-low voltage organic transistor, Org. Electron., 2016, 36, 57–60 CrossRef CAS.
- F. A. Viola, et al., Ultrathin, flexible and multimodal tactile sensors based on organic field-effect transistors, Sci. Rep., 2018, 8(1), 1–8 CrossRef CAS.
- J. Wang, et al., Thickness dependence of elastic modulus and hardness of on-wafer low-k ultrathin polytetrafluoroethylene films, Scr. Mater., 2000, 42(7), 687–694 CrossRef CAS.
- Z. Ao and S. Li, Temperature-and thickness-dependent elastic moduli of polymer thin films, Nanoscale Res. Lett., 2011, 6(1), 1–6 CrossRef.
- C. Greulich, et al., Studies on the biocompatibility and the interaction of silver nanoparticles with human mesenchymal stem cells (hMSCs), Langenbeck's Arch. Surg., 2009, 394(3), 495–502 CrossRef CAS.
- S. Kittler, et al., Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions, Chem. Mater., 2010, 22(16), 4548–4554 CrossRef CAS.
- M. Ramuz, et al., Combined optical and electronic sensing of epithelial cells using planar organic transistors, Adv. Mater., 2014, 26(41), 7083–7090 CrossRef CAS.
- P. Lin, et al., The application of organic electrochemical transistors in cell-based biosensors, Adv. Mater., 2010, 22(33), 3655–3660 CrossRef CAS.
- L. H. Jimison, et al., Measurement of barrier tissue integrity with an organic electrochemical transistor, Adv. Mater., 2012, 24(44), 5919–5923 CrossRef CAS.
- S. A. Tria, et al. Validation of the organic electrochemical transistor for in vitro toxicology, Biochim. Biophys. Acta, Gen. Subj., 2013, 1830(9), 4381–4390 CrossRef CAS.
- S. Tria, et al., Sensing of EGTA mediated barrier tissue disruption with an organic transistor, Biosensors, 2013, 3(1), 44–57 CrossRef CAS.
- S. A. Tria, et al., Dynamic monitoring of Salmonella typhimurium infection of polarized epithelia using organic transistors, Adv. Healthcare Mater., 2014, 3(7), 1053–1060 CrossRef CAS.
- J. Rivnay, et al. Organic electrochemical transistors for cell-based impedance sensing, Appl. Phys. Lett., 2015, 106(4), 043301 CrossRef.
- A. Romeo, et al., Drug-induced cellular death dynamics monitored by a highly sensitive organic electrochemical system, Biosens. Bioelectron., 2015, 68, 791–797 CrossRef CAS.
- S. Y. Yeung, et al. Organic electrochemical transistor array for monitoring barrier integrity of epithelial cells invaded by nasopharyngeal carcinoma, Sens. Actuators, B, 2019, 297, 126761 CrossRef CAS.
- S. Y. Yeung, et al., Engineering organic electrochemical transistor (OECT) to be sensitive cell-based biosensor through tuning of channel area, Sens. Actuators, A, 2019, 287, 185–193 CrossRef CAS.
- M. H. Bolin, et al., Active control of epithelial cell-density gradients grown along the channel of an organic electrochemical transistor, Adv. Mater., 2009, 21(43), 4379–4382 CrossRef CAS.
- M. Ramuz, et al., Optimization of a Planar All-Polymer Transistor for Characterization of Barrier Tissue, ChemPhysChem, 2015, 16(6), 1210–1216 CrossRef CAS.
- V. F. Curto, et al. Organic transistor platform with integrated microfluidics for in-line multi-parametric in vitro cell monitoring, Microsyst. Nanoeng., 2017, 3, 17028 CrossRef.
- F. Decataldo, et al. Organic Electrochemical Transistors for Real-Time Monitoring of In Vitro Silver Nanoparticle Toxicity, Adv. Biosyst., 2020, 4(1), 1900204 CrossRef CAS.
- L. Chen, et al., Organic electrochemical transistors for the detection of cell surface glycans, ACS Appl. Mater. Interfaces, 2018, 10(22), 18470–18477 CrossRef CAS.
- X. Guo, et al., Organic Electrochemical Transistor for in Situ Detection of H2O2 Released from Adherent Cells and Its Application in Evaluating the In Vitro Cytotoxicity of Nanomaterial, Anal. Chem., 2019, 92(1), 908–915 CrossRef.
- Y. Fu, et al. Highly sensitive detection of protein biomarkers with organic electrochemical transistors, Adv. Mater., 2017, 29(41), 1703787 CrossRef.
- F. Decataldo, et al. Organic Electrochemical Transistors: Smart Devices for Real-Time Monitoring of Cellular Vitality, Adv. Mater. Technol., 2019, 4(9), 1900207 CrossRef CAS.
- V. F. Curto, et al., A planar impedance sensor for 3D spheroids, Lab Chip, 2018, 18(6), 933–943 RSC.
- C. Pitsalidis, et al. Transistor in a tube: A route to three-dimensional bioelectronics, Sci. Adv., 2018, 4(10), eaat4253 CrossRef CAS.
- C. Yao, et al., Rigid and flexible organic electrochemical transistor arrays for monitoring action potentials from electrogenic cells, Adv. Healthcare Mater., 2015, 4(4), 528–533 CrossRef CAS.
- X. Gu, et al., 16-Channel Organic Electrochemical Transistor Array for In Vitro Conduction Mapping of Cardiac Action Potential, Adv. Healthcare Mater., 2016, 5(18), 2345–2351 CrossRef CAS.
- X. Gu, et al. Organic electrochemical transistor arrays for in vitro electrophysiology monitoring of 2D and 3D cardiac tissues, Adv. Biosyst., 2019, 3(2), 1800248 CrossRef.
- F. Hempel, et al., PEDOT: PSS organic electrochemical transistor arrays for extracellular electrophysiological sensing of cardiac cells, Biosens. Bioelectron., 2017, 93, 132–138 CrossRef CAS.
- Y. Liang, et al. High performance flexible organic electrochemical transistors for monitoring cardiac action potential, Adv. Healthcare Mater., 2018, 7(19), 1800304 CrossRef.
- Y. Liang, et al. Tuning Channel Architecture of Interdigitated Organic Electrochemical Transistors for Recording the Action Potentials of Electrogenic Cells, Adv. Funct. Mater., 2019, 29(29), 1902085 CrossRef.
- A. Williamson, et al., Localized neuron stimulation with organic electrochemical transistors on delaminating depth probes, Adv. Mater., 2015, 27(30), 4405–4410 CrossRef CAS.
- X. Strakosas, et al. Catalytically enhanced organic transistors for in vitro toxicology monitoring through hydrogel entrapment of enzymes, J. Appl. Polym. Sci., 2017, 134(7), 44483 CrossRef.
- M. Braendlein, et al. Lactate detection in tumor cell cultures using organic transistor circuits, Adv. Mater., 2017, 29(13), 1605744 CrossRef.
- F. Mariani, et al. Needle-type organic electrochemical transistor for spatially resolved detection of dopamine, Microchim. Acta, 2020, 187, 378 CrossRef CAS.
- S. T. Keene, et al., A biohybrid synapse with neurotransmitter-mediated plasticity, Nat. Mater., 2020, 19(9), 969–973 CrossRef CAS.
- M. Giordani, et al. Neuromorphic Organic Devices that Specifically Discriminate Dopamine from Its Metabolites by Nonspecific Interactions, Adv. Funct. Mater., 2020, 2002141 CrossRef CAS.
- Y. van de Burgt, et al. A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing, Nat. Mater., 2017, 16(4), 414–418 CrossRef CAS.
- T. Cramer, et al., Organic ultra-thin film transistors with a liquid gate for extracellular stimulation and recording of electric activity of stem cell-derived neuronal networks, Phys. Chem. Chem. Phys., 2013, 15(11), 3897–3905 RSC.
- A. Kyndiah, et al. Bioelectronic Recordings of Cardiomyocytes with Accumulation Mode Electrolyte Gated Organic Field Effect Transistors, Biosens. Bioelectron., 2020, 150, 111844 CrossRef CAS.
- D. A. Bernards, et al. Gating of an organic transistor through a bilayer lipid membrane with ion channels, Appl. Phys. Lett., 2006, 89(5), 053505 CrossRef.
- Y. Zhang, et al. Liquid–solid dual-gate organic transistors with tunable threshold voltage for cell sensing, ACS Appl. Mater. Interfaces, 2017, 9(44), 38687–38694 CrossRef CAS.
- S. Desbief, et al., Electrolyte-gated organic synapse transistor interfaced with neurons, Org. Electron., 2016, 38, 21–28 CrossRef CAS.
- T. Cohen-Karni, et al., Graphene and nanowire transistors for cellular interfaces and electrical recording, Nano Lett., 2010, 10(3), 1098–1102 CrossRef CAS.
- L. H. Hess, et al. High-transconductance graphene solution-gated field effect transistors, Appl. Phys. Lett., 2011, 99(3), 033503 CrossRef.
- L. H. Hess, et al., Graphene transistor arrays for recording action potentials from electrogenic cells, Adv. Mater., 2011, 23(43), 5045–5049 CrossRef CAS.
- A. A. Balandin, Low-frequency 1/f noise in graphene devices, Nat. Nanotechnol., 2013, 8(8), 549–555 CrossRef CAS.
- L. H. Hess, et al., Electrical coupling between cells and graphene transistors, Small, 2015, 11(14), 1703–1710 CrossRef CAS.
- R. Weis, B. Müller and P. Fromherz, Neuron adhesion on a silicon chip probed by an array of field-effect transistors, Phys. Rev. Lett., 1996, 76(2), 327 CrossRef CAS.
- P. K. Ang, et al., Flow sensing of single cell by graphene transistor in a microfluidic channel, Nano Lett., 2011, 11(12), 5240–5246 CrossRef CAS.
- D. Kireev, et al., Graphene field-effect transistors for in vitro and ex vivo recordings, IEEE Trans. Nanotechnol., 2016, 16(1), 140–147 Search PubMed.
- D. Kireev, et al., Graphene transistors for interfacing with cells: towards a deeper understanding of liquid gating and sensitivity, Sci. Rep., 2017, 7(1), 1–12 CrossRef CAS.
- F. Veliev, et al. Recording spikes activity in cultured hippocampal neurons using flexible or transparent graphene transistors, Front. Neurosci., 2017, 11, 466 CrossRef.
- F. Veliev, et al. Sensing ion channel in neuron with graphene field effect transistors, 2D Mater., 2018, 5(4), 045020 CrossRef CAS.
- Y. Y. Wang, et al., Charging the quantum capacitance of graphene with a single biological ion channel, ACS Nano, 2014, 8(5), 4228–4238 CrossRef CAS.
- M. Seifert, et al. Role of grain boundaries in tailoring electronic properties of polycrystalline graphene by chemical functionalization, 2D Mater., 2015, 2(2), 024008 CrossRef.
- Y.-T. Li, et al., Receptor-Mediated Field Effect Transistor Biosensor for Real-Time Monitoring of Glutamate Release from Primary Hippocampal Neurons, Anal. Chem., 2019, 91(13), 8229–8236 CrossRef CAS.
- M. Demelas, et al., Charge sensing by organic charge-modulated field effect transistors: Application to the detection of bio-related effects, J. Mater. Chem. B, 2013, 1(31), 3811–3819 RSC.
- A. Spanu, et al. Organic FET device as a novel sensor for cell bioelectrical and metabolic activity recordings, 2013 6th International IEEE/EMBS Conference on Neural Engineering(NER). IEEE, 2013 Search PubMed.
- A. Spanu, et al. An organic transistor-based system for reference-less electrophysiological monitoring of excitable cells, Sci. Rep., 2015, 5, 8807 CrossRef CAS.
- A. Spanu, et al. Bioelectrical and metabolic activity recordings by means of organic field effect transistors, 2015 XVIII AISEM Annual Conference. IEEE, 2015 Search PubMed.
- A. Spanu, et al., A reference-less pH sensor based on an organic field effect transistor with tunable sensitivity, Org. Electron., 2017, 48, 188–193 CrossRef CAS.
- O. Knopfmacher, et al., Nernst limit in dual-gated Si-nanowire FET sensors, Nano Lett., 2010, 10(6), 2268–2274 CrossRef CAS.
- M. Spijkman, et al., Beyond the Nernst-limit with dual-gate ZnO ion-sensitive field-effect transistors, Appl. Phys. Lett., 2011, 98(4), 043502 CrossRef.
- A. Spanu, et al. An organic neurophysiological tool for neuronal metabolic activity monitoring, APL Bioeng., 2018, 2(4), 046105 CrossRef CAS.
- D. Khodagholy, et al. In vivo recordings of brain activity using organic transistors, Nat. Commun., 2013, 4, 1575 CrossRef.
- J. Rivnay, et al. High-performance transistors for bioelectronics through tuning of channel thickness, Sci. Adv., 2015, 1(4), e1400251 CrossRef.
- W. Lee, et al., Transparent, conformable, active multielectrode array using organic electrochemical transistors, Proc. Natl. Acad. Sci. U. S. A., 2017, 114(40), 10554–10559 CrossRef CAS.
- W. Lee, et al. Nonthrombogenic, stretchable, active multielectrode array for electroanatomical mapping, Sci. Adv., 2018, 4(10), eaau2426 CrossRef CAS.
- D. A. Bernards and G. G. Malliaras, Steady-state and transient behavior of organic electrochemical transistors, Adv. Funct. Mater., 2007, 17(17), 3538–3544 CrossRef CAS.
- G. D. Spyropoulos, J. N. Gelinas and D. Khodagholy, Internal ion-gated organic electrochemical transistor: A building block for integrated bioelectronics, Sci. Adv., 2019, 5(2), eaau7378 CrossRef CAS.
- C. Cea, et al. Enhancement-mode ion-based transistor as a comprehensive interface and real-time processing unit for in vivo electrophysiology, Nat. Mater., 2020, 1–8 Search PubMed.
- L. Kergoat, et al., Detection of glutamate and acetylcholine with organic electrochemical transistors based on conducting polymer/platinum nanoparticle composites, Adv. Mater., 2014, 26(32), 5658–5664 CrossRef CAS.
- I. Gualandi, et al. Selective detection of dopamine with an all PEDOT: PSS organic electrochemical transistor, Sci. Rep., 2016, 6, 35419 CrossRef CAS.
- N. Wang, et al., AC measurements using organic electrochemical transistors for accurate sensing, ACS Appl. Mater. Interfaces, 2017, 10(31), 25834–25840 CrossRef.
- K. Xie, et al. Organic electrochemical transistor arrays for real-time mapping of evoked neurotransmitter release in vivo, eLife, 2020, 9, e50345 CrossRef CAS.
- N. Lago, et al., Simultaneous stimulation and recording of cell activity with reference-less sensors: Is it feasible?, Org. Electron., 2018, 62, 676–684 CrossRef CAS.
- B. M. Blaschke, et al. Mapping brain activity with flexible graphene micro-transistors, 2D Mater., 2017, 4(2), 025040 CrossRef.
- C. Hébert, et al. Flexible graphene solution-gated field-effect transistors: efficient transducers for micro-electrocorticography, Adv. Funct. Mater., 2018, 28(12), 1703976 CrossRef.
- E. Masvidal-Codina, et al. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors, Nat. Mater., 2019, 18(3), 280 CrossRef CAS.
- R. Garcia-Cortadella, et al. Distortion-Free Sensing of Neural Activity Using Graphene Transistors, Small, 2020, 16(16), 1906640 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |