Dynamic pneumatic rails enabled microdroplet manipulation†
Renchang
Zhang
abc,
Chang
Gao
ab,
Lu
Tian
ad,
Ronghang
Wang
ab,
Jie
Hong
ab,
Meng
Gao
ac and
Lin
Gui
 *ab
*ab
aKey Laboratory of Cryogenics, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, 29 Zhongguancun East Road, Haidu District, Beijing 10019, China. E-mail: lingui@mail.ipc.ac.cn; Tel: +86 10 8254 3483
bUniversity of Chinese Academy of Sciences, 19 Yuquan Road, Shijingshan District, Beijing 100039, China
cResearch Center for Internet of Things, Advanced Institute of Information Technology, Peking University, 311215, Hangzhou, Zhejiang, P. R. China
dBeijing Smart-Chip Microelectronics Technology Company, Ltd., Beijing 100192, China
First published on 23rd November 2020
Abstract
This study presented a convenient method of gathering, splitting, merging, and sorting microdroplets by dynamic pneumatic rails in double-layered microfluidic devices. In these devices, the pneumatic rails were placed below the droplet channel, with a thin elastic polydimethylsiloxane (PDMS) film between them. The PDMS film would sag down to the rail channel, forming a groove pattern at the bottom of the droplet channel, when the fluid pressure in the droplet channel was higher than the air pressure in the rail channel. The groove could capture the flattened droplets and guide the flow path of them due to the lowered surface energy when they extended into the groove. We have designed different components consisting of pneumatic rails to split, merge and sort droplets, and demonstrated that the components maintained good performance in manipulating droplets only by controlling the air pressure. Furthermore, a pneumatic rail-based sorter has been successfully used to sort out single-cell droplets. The pneumatic rail can be integrated into pneumatic valve-based microfluidic devices to be a flexible tool for droplet-based biological and chemical analysis.
Introduction
In the last decades, droplet microfluidic devices, where droplets serve as independent reaction chambers, have been widely applied in biological analysis,1–4 drug discovery,5,6 and disease diagnosis.7–9 The droplet reactors can be as small as sub-nanoliter, which should save reactants and make single-cell analysis possible.10–13 Through the droplet manipulation technology, the desired droplets can be easily acquired, such as the number and volume of droplets and the types of reactants encapsulated in droplets. The operation units of droplet microfluidics usually contain droplet generation, merging, mixing, sorting, and splitting. A simple and flexible droplet manipulation technology would make it easier for non-experts to use droplet microfluidics.First, the addition of the desired reagents into target droplets is one of the valuable steps for a multistep reaction and high throughput screening. To induce merging droplets by hydrodynamic force, the target droplets coming from different inlets should first meet at a specific position, and then overcome the surface tension to merge together. The direct method is to control the droplet generation frequency and flow speed to match with the droplet pair.14,15 In this method, the operator should be skilled enough to generate droplets in the microchannel. An easier operation way is that the front droplet is stopped or decelerated by pillars to wait for the subsequent droplet.16,17 The droplet size should be large enough to be blocked by the pillars. Second, droplet splitting is also a practical method to increase the production of microdroplets.18 For pressure-driven droplets, as the droplet moves into a bifurcating microchannel, it can be split into two daughter droplets due to the pressure forces.19,20 The size of the two daughter droplets is related to the hydrodynamic resistances of the branches. Multiple splitting of droplets is an efficient method to increase the production rate.21 Third, sorting the desired droplets from a large population is a common step for biochemical analysis.22 Hydrodynamic droplet sorting has been demonstrated to separate out desired droplets based on the different properties, e.g. droplet size.23,24 Compared to the passive hydrodynamic methods, active methods achieved by external intervention are more flexible and accurate. The external intervention would be performed by pneumatic,25,26 laser,27 electric,28 acoustic devices,29 or magnetic30–32 devices.
If the droplets are smaller than the width of the microchannel, the trajectory of the moving droplet is highly affected by the distribution of the flow field and will not be stable when the flow field is not stable. Any disturbance of the flow field will make the droplet fail to move precisely as desired. For example, when the trajectory of the droplet changes, the merging target droplets cannot meet accurately at the desired merging point, or the splitting droplet cannot flow to the bifurcation point precisely to make a perfect splitting. In ref. 33, a grooved rail was etched at the top of the microchannel to control the movement of the flattened droplets. When a flattened droplet enters into the groove, its surface energy decreases, which makes the droplet likely to remain in the groove and flow steadily along with it.34 The droplets would be aligned when they flow along a linear grooved rail. Xu et al. achieved error-free droplet fusion with a railroad-like channel network and guiding rails.35 The mismatched droplet pairs from upstream were synchronized by the railroad-like channel and induced to merge by an electrical device. In addition, the droplets were directly guided to the selective sites by a grooved rail, and then a laser was used to sequentially sort the droplets into 6 rails to make droplet arrays.36
In this study, a “dynamic” pneumatic rail-based method was proposed to split, merge, and sort droplets. A groove pattern was formed when the PDMS film above the rail channel sagged down due to the fluid pressure change in the droplet channel. The groove could not only guide droplet flow to the target sites but also control the droplets by changing the air pressure in the rail channel. We have demonstrated that the pneumatic rails could be used in splitting, merging or sorting droplets precisely. To demonstrate its sorting application, a pneumatic rail-based sorter was designed to pick out cells from a large number of void droplets.
Manipulating droplets by pneumatic rails
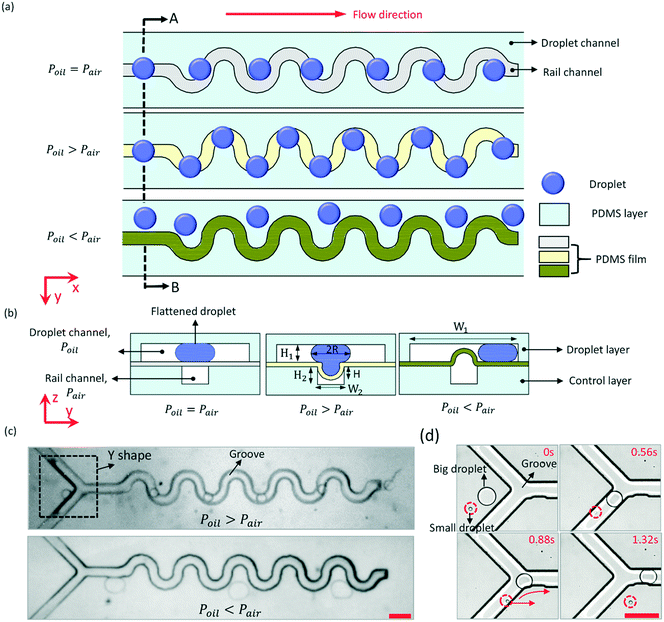
The basic way of manipulating droplets by pneumatic rails is to form groove patterns on the bottom of the droplet channel. When the height (H1) of the droplet channel is smaller than the spherical droplets, the droplet is flattened by the top and bottom walls of the droplet channel. As the flattened droplet expands into the groove, its surface energy releases a little bit and thus reaches a lower state, which makes the droplet willing to stay with the groove and be guided by the direction of the groove.As shown in Fig. 1, a sinusoidal rail pattern was designed to control the trajectory of microdroplets. The double-layered microfluidic chip consisted of a droplet layer and a control layer. The droplet channel and rail channel were fabricated on the droplet layer and control layer separately and bonded face-to-face with a PDMS film between them. The PDMS film has good flexibility, and is easy to deform under pressure. First, when the fluid pressure (Poil) above the PDMS film is equal to the air pressure (Pair) in the rail channel, the PDMS film remains flat, and the droplets move straight forward in the original direction due to the pressure drag (Fd) of the fluid in the droplet channel, as shown in Fig. 1(a). The drag force rises as the flow rate (Voil) of the fluid increases. Then, when Poil > Pair, the PDMS film sagged downward to the rail channel, forming a groove pattern at the bottom of the droplet channel, as shown in Fig. 1(b). The groove could capture the flattened droplets and guide the moving droplets. Thus, the trajectory of the droplet followed with the rail channel, and Fig. 1(c) and ESI† Movie S1 show the experimental results. When the droplet partially entered into the groove, it was trapped in the groove by a trapping force, Fγ, to maintain lower surface energy. The condition whereby droplets can be anchored or guided by the groove is Fd < Fγ. Third, when Poil < Pair, the PDMS film will protrude upward and divide the droplet channel. The droplets were confined to one side of the channel by the protruded PDMS film, and the experiment result is shown in Fig. 1(c).
The working principle of using the pneumatic rail to switch trapping or repelling droplets is to change Fγ or Fd. Fγ was estimated as γΔA/d, where ΔA is the difference in the total surface area of the droplet between the droplet entering and leaving the groove.31γ is the interfacial energy of the droplet, and was kept constant in this work. d is the characteristic length scale over which the surface area changes. For a droplet trapped in the groove, ΔA decreases with the decrease of the depth (H) of the PDMS film depression. Enlarging Pair would decrease H. Fγ is positively correlated with ΔA. So, the value of Fγ can be changed by controlling the air pressure in the pneumatic rail. If Fγ decreases to less than Fd, the trapped droplet would be dragged out from the groove due to Fd. Then, the magnitude scale of Fd is μ0VoilR2/H1, where μ0 is the viscosity of the oil, and R is the radius of the droplet in the droplet channel.34 When H is certain, Voil has a critical value (V*) that satisfies Fd = Fγ. When Voil < V* (namely Fd < Fγ), the droplet would be trapped in the groove due to Fγ. In contrast, when Voil > V* (namely Fd > Fγ), the droplet would be pulled out from the groove due to large Fd. Voil would be changed by controlling the air pressure in the rail channel to make the PDMS film protrude upward or sag downward.
The basic condition for the droplet to be manipulated by the pneumatic rail is 2R > H1. If 2R ≤ H1, the droplet would remain spherical in the droplet channel, and ΔA equals zero when the droplet flows through the groove. The trajectory of the small droplet wouldn't be affected by the groove. As shown in Fig. 1(d), at the front of the sinusoidal rail is a Y-shaped structure. It is used to capture droplets from the upstream and then, guide the droplets to the sinusoidal rail. When H1 = 50 μm, a big droplet with a diameter of 213 μm was captured by the rail, and flowed along the rail. However, a small droplet with a diameter of 37 μm couldn't be captured, and moved straight forward. This structure can be used as a droplet sorter to pick up those large droplets. For 2R > H1, whether the droplet can be manipulated by the pneumatic rail depends on Fd and Fγ. For example, when Fγ is constant, the smaller the Voil, the larger the droplet that the pneumatic rail can manipulate. Therefore, the maximum size of droplets that can be manipulated by the rail can be very large only if Voil is small enough.
Experimental methods
Microfluidic device fabrication
We fabricated the PDMS microfluidic device by standard multilayer soft lithography technology. First, SU-8 2050 and SU-8 2025 (MicroChen, MA, USA) were separately used to manufacture the wafer molds of the droplet channel and rail channel. The droplet microchannel and the rail channel were 50 μm and 20 μm high, separately. Second, the Sylgar 184 silicone elastomer (a mixture of a base and curing agent at a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight, Dow Corning, MI, USA) was poured onto these two wafer molds, and cured on a hot plane at 65 °C for 2.5 hours. Then, both PDMS layers were peeled off from the wafer molds. Thus, the droplet channel layer and rail channel layer are ready for bonding. To fabricate a 22 μm-thick PDMS film, PDMS was again poured onto a wafer and spin-coated at 3000 rpm for 60 s, and cured on the hot plane at 75 °C for 0.5 h. Then the droplet layer was bonded to the 22 μm-thick PDMS film by air plasma treatment (plasma cleaner, YZD08-2C, Tangshan Yanzhao Technology, China) and peeled off from the wafer together with the PDMS film. Holes were introduced at the end of the droplet channel and holes for air input were introduced at the certain position of the assembly. Finally, the droplet channel layer with a PDMS film was bonded with the control layer face to face to achieve the whole chip. To reduce the hydrophilicity of the surface of the droplet channel, the microfluidic device was baked at 95 °C for 48 hours.
1 by weight, Dow Corning, MI, USA) was poured onto these two wafer molds, and cured on a hot plane at 65 °C for 2.5 hours. Then, both PDMS layers were peeled off from the wafer molds. Thus, the droplet channel layer and rail channel layer are ready for bonding. To fabricate a 22 μm-thick PDMS film, PDMS was again poured onto a wafer and spin-coated at 3000 rpm for 60 s, and cured on the hot plane at 75 °C for 0.5 h. Then the droplet layer was bonded to the 22 μm-thick PDMS film by air plasma treatment (plasma cleaner, YZD08-2C, Tangshan Yanzhao Technology, China) and peeled off from the wafer together with the PDMS film. Holes were introduced at the end of the droplet channel and holes for air input were introduced at the certain position of the assembly. Finally, the droplet channel layer with a PDMS film was bonded with the control layer face to face to achieve the whole chip. To reduce the hydrophilicity of the surface of the droplet channel, the microfluidic device was baked at 95 °C for 48 hours.
Device operation
In this work, we used silicone oil (Xilong Chemical Co., Ltd., Shantou, China) and deionized (DI) water (Merck Chemical Technology, Ltd., Shanghai, China) as the continuous phase and dispersed phase separately. Black ink was used to visualize the liquid in the droplet sorting part and fusion part. The human renal epithelial cell suspended solution was used to generate single-cell droplets. A pneumatic valve below the T junction was used to generate water-in-oil droplets on demand. By regularly switching on and off the valve, it was very handy and steady to control the droplet generation rate, droplet length, and distance between droplets.37 A microfluidic control system (MFCSTM-EZ, FLUIGENT, France) was used to control the pressure of the continuous phase and the dispersed phase. In addition, a programmable multi-channel pump (Suzhou Wenhao Microfluidic Technology Co., Ltd., Suzhou, China) was used to control the air pressure in the rail channel and the pneumatic valve. A microscope (Axio Observer Z1, Carl Zeiss) was used to record the experimental details.Results and discussion
Splitting
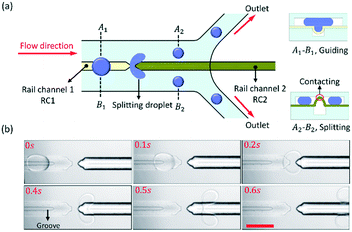
As shown in Fig. 2, we designed two linear rail channels (RC1 and RC2) to split the droplets. The rail channels were aligned in a line, and the distance between them was 50 μm. The principle of droplet splitting is shown in Fig. 2(a). The PDMS film above RC1 would sag downward to capture droplets and align them with RC2, and then the PDMS film above RC2 would bulge up and divide the droplet channel into two “sub” microchannels from its centre. Thus, the oil in the droplet channel was divided by the protruded PDMS film. When a droplet was guided by RC1 to RC2, the continuous phase continuously pulls it forward, and then splits the droplet into two daughter droplets by RC2. The widths of the droplet channel and rail channel were equal to 600 μm and 100 μm, respectively.The experimental result of the droplet splitting is shown in Fig. 2(b) and ESI† Movie S2. As the droplet flowed along RC1 to approach RC2, the front end of the droplet started to deform at 0.2 s. Then, two lobes were developed until droplet break-up occurred due to the shear stress from 0.4 s to 0.5 s. Finally, the droplet was split into two small droplets at 0.6 s. The ratio of the daughter droplet size was dependent on the hydraulic resistance of the “sub” microchannels at both sides of RC2. The rail channels were aligned to the centre of the droplet channel, so the hydraulic resistance of both sides of RC2 was equal, and the daughter droplet size of both sides was equal too. If Fγ > Fd, the big droplets from the upstream are captured and guided to RC2 precisely by the grooved rail, and stably divided into daughter droplets of uniform size.
The front of RC2 was a sharp tip that made it easier to split droplets and improved the droplet division efficiency. In addition, the distance between the two rail channels should be as small as possible to ensure that the trajectory of the droplet would not change after the droplet left RC1.
Merging
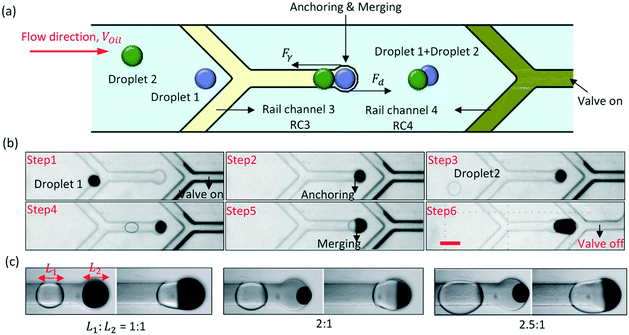
The groove can also be an anchor to hold droplets when Fγ > Fd. We have designed an anchor channel (RC3) for the droplet fusion, and the principle of the droplet fusion is shown in Fig. 3(a). The end of RC3 was a circle, which was the fusion site. The droplets were guided to flow to the circle in turn, and were anchored there, and then merged together. To prevent the droplets from being pulled out of the circle by Fd, the rail channel 4 (RC4) at the downstream was used as a valve to control the flow rate (Voil) of the oil. Fd was positively rated to Voil. When the PDMS film above RC4 protruded, the hydraulic resistance in the droplet channel increased. Voil and Fd would decrease. By controlling the air pressure in RC4, the PDMS film would either protrude (recorded as valve on) or sag (recorded as valve off). The width of RC3 and RC4 were both 100 μm and the diameter of the circle was 150 μm.The process of droplet fusion by pneumatic rails is shown in Fig. 3(b) in ESI† Movie S3. During this process, the air pressure in RC3 remained equal to 0 bar. First, the air pressure in RC4 increased to turn on the valve, and that caused the flow rate of an ink-in-oil droplet from the upstream to decrease to about 194 μm s−1. The slow droplet was captured by the grooved rail and flowed directionally to the circle (the fusion site). Then, because Fγ > Fd, the droplet was anchored at the fusion site to wait for the subsequent droplet. After a few seconds, a water-in-oil droplet was also guided to the fusion site accurately by RC3, and fusion occurred. When the subsequent droplet extended into the larger groove, its surface was disturbed, and that increased the efficiency of the fusion. Finally, after the droplet fusion, the air pressure in RC4 decreased to turn off the valve to increase Voil, and the mixture was pulled out from the fusion site by Fd. For droplets of different lengths, it was easy to change Voil for anchoring the droplets at the fusion site by controlling the air pressure in RC4. As shown in Fig. 3(c), when the ratio of the droplet length was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2.5
1 or 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the two droplets were stably merged together at the fusion site.
1, the two droplets were stably merged together at the fusion site.
Sorting
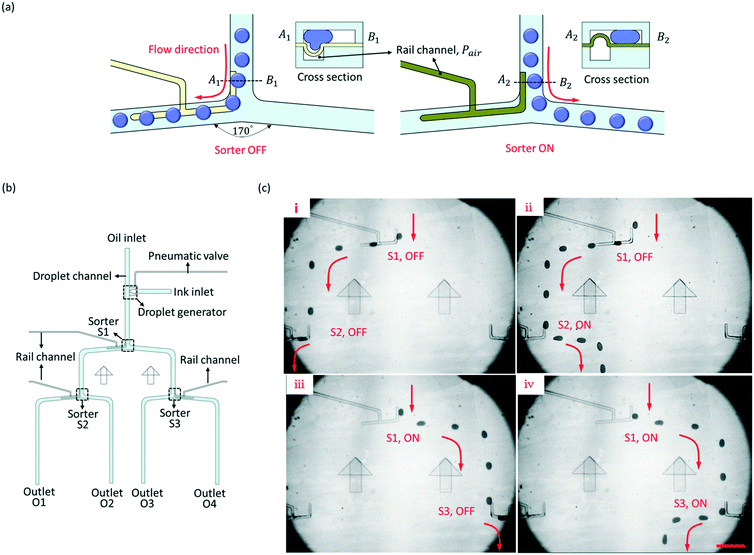
We have designed a rail channel to direct the flowing paths of droplets for droplet sorting, and the principle of the sorter is shown in Fig. 4(a). A rail channel is aligned along one side of the fork of the droplet channel. When the PDMS film sags downward to form a grooved rail, the droplets from the upstream are guided to flow along the groove to the left branch. The status of the sorter is recorded as OFF. Then, protruding the PDMS film upward would increase the hydraulic resistance of the left branch, and the droplets flow to the right branch, where the hydraulic resistance is less. The status of the sorter is recorded as ON. Before the droplet flows into the fork, its trajectory has been changed by the rail channel, and the droplet shifted to one side of the droplet channel. Thus, the droplets can flow into the sorting junction instead of being split. The widths of the rail channel and the droplet channel were 50 μm and 200 μm, separately. The cross angle of the fork was 170°.Three pneumatic rail-based sorters (S1, S2, and S3) were integrated into one microfluidic chip to direct the droplets from one inlet to four outlets (O1, O2, O3, and O4) in turn. The schematic diagram of the microfluidic chip is shown in Fig. 4(b). At each fork, a rail channel was accurately aligned to one side of the droplet channel, and each sorter can independently control the flow direction of droplets. The experiment result was shown in Fig. 4(c) and ESI† Movie S4. When S1 and S2 were both OFF, the droplets were directed to O1. Then, S2 turned ON, and the droplets flowed to O2. When S1 was ON and S3 was OFF, the flow direction of droplets was immediately changed, and the droplets moved to O3. Similarly, after S3 turned ON, the droplets flowed to O4. The oil pressure in the droplet channel decreases along the flow due to hydraulic resistance. If the sorter, e.g. S2 and S3, is too far away from the inlet, the oil pressure above the rail channel may be too small to sag the PDMS film downward completely. The shallower the groove is, the smaller Fγ is. It is hard for a shallow groove to direct the path of the droplets. Therefore, increasing the oil pressure of the inlet would improve the performance of S2 and S3.
To study the long-term reliability of the PDMS film during the repeatable bending upward and downward, fatigue tests of 22 μm and 12 μm thick PDMS films were carried out. The film was bent upward and downward every 0.5 s. After 5000 cycles, the 22 μm thick film was maintained well without any damage, and it was still stable for sorting droplets. But, for the 12 μm thick film, because the film was too thin, air bubbles overflowed from the rail channel to the droplet channel through the film when the film bent from upward to downward, and the film lost flexibility and failed to bend after 5000 cycles. The invalidation would reduce the life of the film for long-term manipulation. More details about the experiment results are shown in the ESI.†
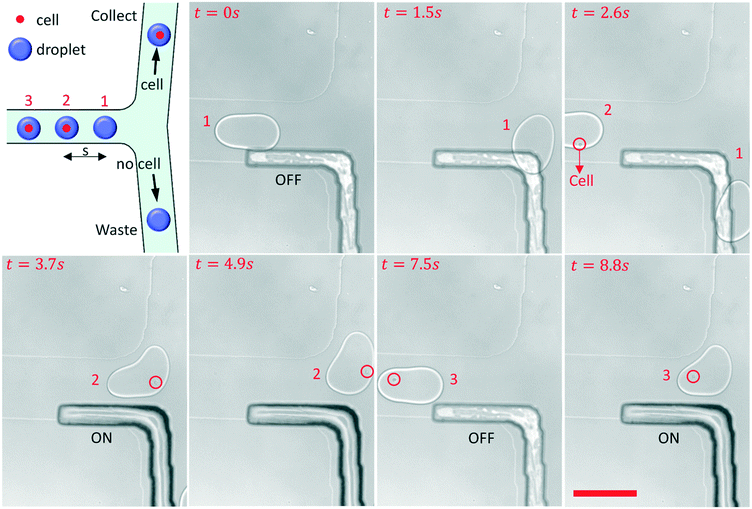
Cell sorting
An experiment on the sorting single-cell droplets by a pneumatic rail has been conducted, as shown in Fig. 5 and ESI† Movie S5. The cell concentration in the suspended solution was approximately 105 cells per ml. During the experiment, all the droplets containing single cells inside were actively sorted and gathered into one collection channel.When the sorter turned to OFF, from 0 s to 2.6 s, droplet 1 without cells was guided to flow into the waste channel by the grooved rail. As a single cell droplet flowed into the sorter area, the sorter turned to ON immediately by increasing the air pressure in the rail channel, and droplet 2 was pushed to the collection channel from 3.7 s to 4.9 s. After droplet 2 passed the fork, the air pressure decreased to 0 bar again, and the sorter turned to OFF at 7.5 s. The PDMS film would be damaged due to long-term expansion, so it is better to return the PDMS film to its original state. Then, another single-cell droplet encountered the fork, and the sorter turned to ON again to direct droplet 3 to the collection channel. The response time (t) is about 30 ms when the sorter turned to ON from OFF, and it depends on the performance of the pump and the compressibility of air. So the flow rate of droplets that can be sorted should be less than s/t, where s is the distance between the droplets. The pneumatic rail based sorter can be integrated with the fluorescence detection system for single-cell droplet sorting in the future.
Conclusion
In summary, a novel pneumatic rail-based method was proposed to split, merge, and sort droplets in microfluidic devices. When Poil > Pair, the PDMS film sagged down to the top surface of the rail channel due to the stress, and a groove pattern was formed at the bottom of the droplet channel. The grooved rail could anchor or guide the flattened droplets when Fd < Fγ. The rail could control the trajectory of microdroplets by changing Pair. First, a droplet division component consisting of two straight rail channels was designed to split the droplets. The droplets were guided precisely to the protruded rail by the grooved rail, and the droplets were stably split into two daughter droplets of uniform size. Then, a circular groove was used to anchor droplets, and another rail channel was used as a valve to create suitable fluid conditions for droplet fusion. The target droplets were guided to the fusion spot in turn and then merged with following droplets. Finally, three rail channels were aligned along one side of the fork of the droplet channel separately to sort droplets from one inlet to four outlets. Furthermore, we conducted an experiment to sort single cells by a pneumatic rail-based sorter.Author contributions
Conceptualization, R. Zhang and L. Gui; data curation, R. Zhang, L. Tian and M. Gao; methodology, R. Zhang, L. Gui, L. Tian, C. Gao, R. Wang, and M. Gao; software, R. Zhang; validation, R. Zhang and L. Gui; formal analysis, R. Zhang and L. Tian; investigation, R. Zhang; original draft preparation, R. Zhang; writing-review and editing, L. Gui; supervision, L. Gui; funding acquisition, L. Gui.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key Research and Development Program of China (2019YFB2204903): “The Study of medical pressure sensor, micropump and chip-level heterogeneous integration” and the Science and Technology Program from State Grid Corporation of China (No. 5700-201955318A-0-0-00): “Principle, Structural Design and Functional Verification of Multi-parameter Parallel Sensing with Liquid Metals” and the National Natural Science Foundation of China (No. 31427801).Notes and references
- L. Shang, Y. Cheng and Y. Zhao, Chem. Rev., 2017, 117, 7964–8040 CrossRef CAS.
- H. Song, D. L. Chen and R. F. Ismagilov, Angew. Chem., Int. Ed., 2006, 45, 7336–7356 CrossRef CAS.
- T. D. Rane, C. M. Puleo, K. J. Liu, Y. Zhang, A. P. Lee and T. H. Wang, Lab Chip, 2010, 10, 161–164 RSC.
- T. S. Kaminski and P. Garstecki, Chem. Soc. Rev., 2017, 46, 6210–6226 RSC.
- G. T. Vladisavljevic, N. Khalid, M. A. Neves, T. Kuroiwa, M. Nakajima, K. Uemura, S. Ichikawa and I. Kobayashi, Adv. Drug Delivery Rev., 2013, 65, 1626–1663 CrossRef CAS.
- M. Courtney, X. Chen, S. Chan, T. Mohamed, P. P. Rao and C. L. Ren, Anal. Chem., 2017, 89, 910–915 CrossRef CAS.
- D.-K. Kang, M. Monsur Ali, K. Zhang, E. J. Pone and W. Zhao, TrAC, Trends Anal. Chem., 2014, 58, 145–153 CrossRef CAS.
- D. Sun, F. Cao, L. Cong, W. Xu, Q. Chen, W. Shi and S. Xu, Lab Chip, 2019, 19, 335–342 RSC.
- I. Hajji, M. Serra, L. Geremie, I. Ferrante, R. Renault, J. L. Viovy, S. Descroix and D. Ferraro, Sens. Actuators, B, 2020, 303 Search PubMed.
- H. S. Moon, K. Je, J. W. Min, D. Park, K. Y. Han, S. H. Shin, W. Y. Park, C. E. Yoo and S. H. Kim, Lab Chip, 2018, 18, 775–784 RSC.
- N. Nitta, T. Sugimura, A. Isozaki, H. Mikami, K. Hiraki, S. Sakuma, T. Iino, F. Arai, T. Endo, Y. Fujiwaki, H. Fukuzawa, M. Hase, T. Hayakawa, K. Hiramatsu, Y. Hoshino, M. Inaba, T. Ito, H. Karakawa, Y. Kasai, K. Koizumi, S. Lee, C. Lei, M. Li, T. Maeno, S. Matsusaka, D. Murakami, A. Nakagawa, Y. Oguchi, M. Oikawa, T. Ota, K. Shiba, H. Shintaku, Y. Shirasaki, K. Suga, Y. Suzuki, N. Suzuki, Y. Tanaka, H. Tezuka, C. Toyokawa, Y. Yalikun, M. Yamada, M. Yamagishi, T. Yamano, A. Yasumoto, Y. Yatomi, M. Yazawa, D. Di Carlo, Y. Hosokawa, S. Uemura, Y. Ozeki and K. Goda, Cell, 2018, 175, 266–276 CrossRef CAS , e213.
- N. Shembekar, H. Hu, D. Eustace and C. A. Merten, Cell Rep., 2018, 22, 2206–2215 CrossRef CAS.
- M. T. Chung, K. Kurabayashi and D. Cai, Lab Chip, 2019, 19, 2425–2434 RSC.
- S. Zeng, B. Li, X. Su, J. Qin and B. Lin, Lab Chip, 2009, 9, 1340–1343 RSC.
- L. Mazutis and A. D. Griffiths, Lab Chip, 2012, 12, 1800–1806 RSC.
- X. Niu, S. Gulati, J. B. Edel and A. J. deMello, Lab Chip, 2008, 8, 1837–1841 RSC.
- X. Chen, A. Brukson and C. L. Ren, Microfluid. Nanofluid., 2017, 21 CAS.
- D. R. Link, S. L. Anna, D. A. Weitz and H. A. Stone, Phys. Rev. Lett., 2004, 92, 054503 CrossRef CAS.
- A. M. Leshansky, S. Afkhami, M. C. Jullien and P. Tabeling, Phys. Rev. Lett., 2012, 108, 264502 CrossRef CAS.
- Z. Li, L. Li, M. Liao, L. He and P. Wu, Biomicrofluidics, 2019, 13, 024112 CrossRef.
- A. R. Abate and D. A. Weitz, Lab Chip, 2011, 11, 1911–1915 RSC.
- H. N. Joensson, M. Uhlen and H. A. Svahn, Lab Chip, 2011, 11, 1305–1310 RSC.
- A. C. Hatch, A. Patel, N. R. Beer and A. P. Lee, Lab Chip, 2013, 13, 1308–1315 RSC.
- M. Li, M. van Zee, K. Goda and D. Di Carlo, Lab Chip, 2018, 18, 2575–2582 RSC.
- Y. Chen, Y. Tian, Z. Xu, X. Wang, S. Yu and L. Dong, Appl. Phys. Lett., 2016, 109, 143510 CrossRef.
- M. R. Raveshi, S. N. Agnihotri, M. Sesen, R. Bhardwaj and A. Neild, Sens. Actuators, B, 2019, 292, 233–240 CrossRef CAS.
- Z. G. Li, K. Ando, J. Q. Yu, A. Q. Liu, J. B. Zhang and C. D. Ohl, Lab Chip, 2011, 11, 1879–1885 RSC.
- S. S. Schutz, T. Beneyton, J. C. Baret and T. M. Schneider, Lab Chip, 2019, 19, 2220–2232 RSC.
- K. Mutafopulos, P. Spink, C. D. Lofstrom, P. J. Lu, H. Lu, J. C. Sharpe, T. Franke and D. A. Weitz, Lab Chip, 2019, 19, 2435–2443 RSC.
- Z. Long, A. M. Shetty, M. J. Solomon and R. G. Larson, Lab Chip, 2009, 9, 1567–1575 RSC.
- S. Jiang, Y. Hu, H. Wu, Y. Zhang, Y. Wang, Y. Zhang, W. Zhu, J. Li, D. Wu and J. Chu, Adv. Mater., 2019, 31, 1807507 CrossRef.
- S. Zhu, Y. Bian, T. Wu, C. Chen, Y. Jiao, Z. Jiang, Z. Huang, E. Li, J. Li, J. Chu, Y. Hu, D. Wu and L. Jiang, Nano Lett., 2020, 20, 5513–5521 CrossRef CAS.
- P. Abbyad, R. Dangla, A. Alexandrou and C. N. Baroud, Lab Chip, 2011, 11, 813–821 RSC.
- R. Dangla, S. Lee and C. N. Baroud, Phys. Rev. Lett., 2011, 107, 124501 CrossRef.
- L. Xu, H. Lee, R. Panchapakesan and K. W. Oh, Lab Chip, 2012, 12, 3936–3942 RSC.
- E. Fradet, C. McDougall, P. Abbyad, R. Dangla, D. McGloin and C. N. Baroud, Lab Chip, 2011, 11, 4228–4234 RSC.
- S. Zeng, B. Li, X. Su, J. Qin and B. Lin, Lab Chip, 2009, 9, 1340–1343 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0lc00805b |
| This journal is © The Royal Society of Chemistry 2021 |