Supramolecular fluorescent hydrogelators as bio-imaging probes
Nabila
Mehwish†
a,
Xiaoqiu
Dou†
a,
Yong
Zhao
 *b and
Chuan-Liang
Feng
*b and
Chuan-Liang
Feng
 *ab
*ab
aState Key Lab of Metal Matrix Composites, School of Materials Science and Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240, China. E-mail: clfeng@sjtu.edu.cn
bHenan Univ, Collaborat Innovat Ctr Nano Funct Mat & Applicat, Key Lab Special Funct Mat, Kaifeng 475004, People's Republic of China
First published on 31st October 2018
Abstract
Supramolecular fluorescent hydrogels (SFH) have shown great potential as detecting probes for biomedical applications such as cell imaging, disease diagnosis, bio-sensing etc. This review aims to summarize the recent developments and trends in SFH, investigations of their preparation methods, fluorescent properties of SFH, their prospective applications and interactions involved in SFH based bio-imaging, along with the perspectives on future opportunities and the remaining challenges confronting this research field. After a brief introduction, the categories of SFH, such as fluorescent gelators (self-assembly) and dyes diffused into the matrix of gelators (co-assembly), are illustrated. Various possible uses of SFH as a bio-imaging tool have been realized by their non-covalent interactions like aromatic–aromatic interactions, hydrogen bonding, and hydrophobic interactions etc. More importantly, the uses of SFH in helping address major questions about the consequences of the fluorescent molecules, comprising enzymes and bola-amphiphiles, are also described. This review will serve as a rationalized introduction and reference for researchers who are interested in exploring SFH as bio-imaging probes and will motivate new designs along with instigation of persistent efforts in this hot subject area with great prospects.
1. Introduction
Bio-imaging is an emerging research area defined as the visualization of the biological events in living animals by combining sophisticated bio-imaging probes with modern advanced modalities.1 This visualization of bio-imaging probes in individual cells, tissues, and organs is controlled by the signal produced or detected in response by specific intracellular or extra-cellular molecules. Therefore, higher specificity, stability, and sensitivity of bio-imaging probes have been the main challenge in bio-imaging research.2 To date, numerous bio-imaging tools have been widely used, like fluorescent probes, paramagnetic agents, radio labeled probes and acoustically active nanostructures.3 Although these probes have shown promising results, the application of bio-imaging is still hampered by toxicity, non-specificity, short detention time, and instability.4 Also, the absence of functional groups on small molecules restricts the modification of the bio-imaging probes and then causes a limitation to biological activity and specificity towards the target. Recently, supramolecular fluorescent hydrogel (SFH) based bio-imaging probes for the diagnosis and treatment of diseases have gained great attention owing to their 3D cross-linked structure, excellent biodegradability and biocompatibility, and smart responses to physiological stimuli.5 So the exploration of SFH with the combined advantages of fluorescence and supramolecular hydrogels is highly desirable for bio-imaging applications6 with the sights and insights into both physiological and pathological processes in a real-time and sensitive manner.By carefully designing the gelators alone or with dyes/fluorescent nanomaterials,7 safe, less-invasive and highly sensitive detection as well as rapid sensitivity reaching to the limit of a single molecule and a fast response time of ∼10−8 to 10−10 s by specific SFH can be achieved. Compared with other types of bio-imaging probes like polymer based gelators, SFH have advantages of versatility, bio-compatibility, easy access, low damage, and high stability with ready adaptability to specific molecular events.8 In addition, the fluorescence signal of a molecule can be considerably modified, so that probes on the basis of activation can also be applied9 for the highest spatial resolution of imaging the molecular process at the cellular level. Therefore, the development of rapid, high-throughput and convenient sensing systems based on SFH for detecting disease-related biomarkers is strongly desirable not only for basic science advancement but also for diagnostic applications.10 Various successful examples11,12 have been established to use specific fluorophores non-covalently staining supramolecular self-assemblies to reveal their existence, formation, and degradation.13,14 Considering recent advances in this emerging area, a systematic summary of the bio-imaging properties of SFH is urgently required. Herein, recent developments in the bio-imaging applications of SFH have been summarized.15 Uses of fluorescent hydrogels made by different strategies like hydrogen bonding,16 host–guest recognition,17 metal ligand complexation,18 low molecular weight hydrogels,19 and some others20 in bio-imaging will be introduced and discussed in detail.
This review will also focus on hydrogel systems triggered by enzymes21 and others which are doped with metal NPs or QDs,22 as the control of these networks is particularly interesting for biotechnology-related applications including drug delivery, imaging, biosensors, and tissue engineering (e.g., cell culture, bone regeneration).23 In short, the description of different SFH on the basis of their bio-imaging applications, as shown in Fig. 1 will be explained. Finally, the key challenges and outlooks of SFH in this rapidly developing field for bio-imaging applications will be provided. Along with this, the trends in SFH for the bio-imaging field since 2013 will also be highlighted.
 | ||
| Fig. 1 Overview of representative families of SFH based on different interactions with their applications as bio-imaging tools. | ||
2. Supramolecular fluorescent hydrogels
Supramolecular fluorescent hydrogels combine fluorescence with a self-assembly process resulting in acoustically active nanostructures for imaging. Due to their high biocompatibility and rapid gel-to-sol transition in response to a variety of bio-related stimuli (e.g., enzymatic reactions and biological molecules), SFH with the rational design of nanostructures have great potential for bio-detection uses.24 Before explaining imaging of molecular self-assembly in a cellular environment, some distinctive examples of SFH are presented. There are two types of SFH: one comprises fluorescent hydrogelators (self-assembly),25–32 and the other is formed by fluorescent dyes diffusing into the matrix of the hydrogel (co-assembly).33–472.1. Fluorescent hydrogelator based supramolecular fluorescent hydrogelators
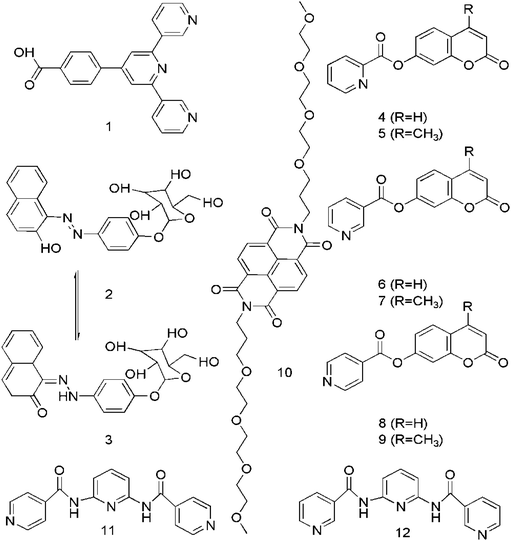
Some hydrogelators have fluorescent groups responsible for imaging properties; for example, Stulz et al. reported the analysis of a terpyridine based supramolecular hydrogel 1 to be used for bio-detection purposes, as presented in Scheme 1, which showed fluorescent characteristics in the gel but not in the sol form; the gel formed in a narrow pH range in aqueous solutions specifically in the presence of sodium ions, and contained between 98% and 99.2% of water.25 Similarly, Shinkai et al. developed a SFH (β-D-glucopyranoside-azonaphthol conjugate, 2 or 3; Scheme 1) in a mixture of water and ethanol (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v). Micro-environmental polarity in the fibrous aggregates of the hydrogelators can be studied by a UV-vis spectral change of the azo-hydrazone tautomerism.26 Polarity of the micro-environment results in azo-hydrazone tautomerism which affects the UV-vis spectra resulting in the estimation of polarity.27,28
20, v/v). Micro-environmental polarity in the fibrous aggregates of the hydrogelators can be studied by a UV-vis spectral change of the azo-hydrazone tautomerism.26 Polarity of the micro-environment results in azo-hydrazone tautomerism which affects the UV-vis spectra resulting in the estimation of polarity.27,28
Coumarin based SFH (4–9) have been designed by Feng et al. (Scheme 1) without conventional gelating motifs in a one-step reaction and applied for imaging purposes. Better visualization of the interaction between substrates and cells could be done by the fluorescent nanofibers self-assembled from these hydrogelators.29 In another work, George et al. reported self-assembly induced pre-associated excimer formation and hence, an enhanced green fluorescence by naphthalene di-imide (NDI) bola-amphiphilic molecule 10 in water, which was applied for imaging purposes.30
Jung et al. demonstrated a strongly fluorescent hydrogel 11 or 12 (Scheme 1), a nanomaterial with intense blue emission in a specified pH region.31 The unique gelation capability of the hydrogel is attributed to the cooperative effects of the π–π stacking and the intermolecular hydrogen bonding interactions. The remarkable fluorescence enhancement is induced by the self-assembled supramolecular nanostructure at pH 7.32 Such blue colored emissive nanomaterials could be of considerable importance in photochemistry.33
2.2. Fluorescent dyes diffusing into the matrix of the hydrogel based supramolecular fluorescent hydrogelators
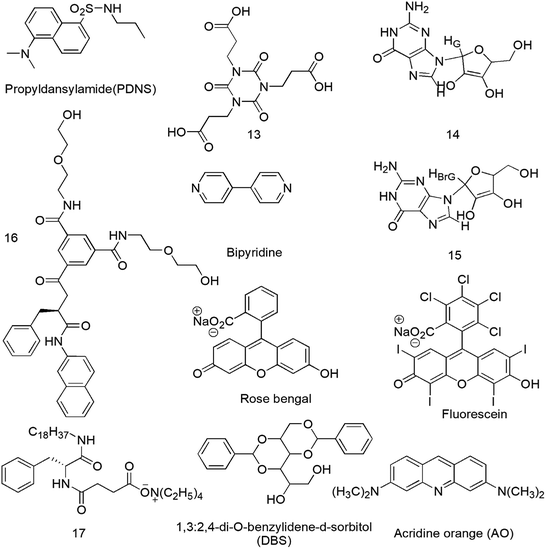
The properties of molecular nanofibrils in a cellular environment can be explored by SFH. Recently, increased interest has been focused on the development of two-component gelling systems in which the presence of two complementary building blocks in solution is required for gel formation to occur.34 The physical properties of the binary gels such as absorption, emission and electronics can be easily controlled, so these gels would be applicable in drug delivery, device systems, optical sensing systems and optoelectronics.35A chromophore or a dye or a molecule with fluorescent properties can be co-gelled with the gelator to incorporate fluorescence. For this purpose, acid–amine interactions are common and Jung et al. reported a cyanurate derivative 13 (Scheme 2) possessing three carboxylic acid groups forming a two-component supramolecular hydrogel for cell imaging in the presence of 4,4-bipyridine by intermolecular hydrogen-bonding interactions between the N atoms of bipyridine and –COOH of 13.36 Recently, fluorescence-spectroscopy-based investigations of a few LMWG gels 14, 15 have shed light on the gel formation processes and on the nature of the environments (hydrophobic, hydrophilic) present within these gels and can be helpful for cell culture study.37 A 1,3,5-cyclohexyltricarboxamide-based gelator 16 comprising two hydrophilic moieties and one hydrophobic substituent containing a naphthalenic group was designed and synthesized (Scheme 2).38 Dansyl is the fluorophore of choice for probing the gel environment because its fluorescence band is strongly solvent dependent and informative for the environment experienced by the fluorophore.39 Highly fluorescent gels with maximum optical properties has been a challenge due to fluorescence quenching associated with the aggregation of molecules.40 So, clay–chromophore hybrid hydrogels were synthesized with aminoclay controlling the spatial distribution of the chromophores to result in fluorescence.41 An efficient light-harvesting method was used between the chromophores anchored to the clay for the controlled and enhanced fluorescence of the resulting hybrids in solution and in the gel state.42 The energy transfer from fluorescent moieties of the supramolecular hydrogel to the employed fluoropore43 and the fluorescent lifetime of 8-anilino-1-naphthalene sulfonic acid (ANS) in supramolecular hydrogels44 are further described. The fluorescence intensity of norfloxacin in the organogel was found to be 10 times stronger than that in the corresponding solutions when this gel was used as a norfloxacin carrier.45 Counting the thermo-reversible property of supramolecular gels, two types of supramolecular hydrogels 17 through the self-assembly of 3-{[(2R)-2-(octadecylamino)-3-phenylpropanoyl]amino}butyrate (TC18PheBu)46 and 1,3:2,4-di-O-benzylidene-D-sorbitol (DBS) along with acridine orange (AO)47 as the guest fluorescent molecule were prepared as shown in Scheme 2 and may be used for thermal responsive biomedical purposes. Added chromophores can affect the mechanical stability of the hydrogels, as most chromophores are hydrophobic and bulky, thus, destabilizing the hydrogel network. Additionally, reduction of luminescence and fluorescence quenching results by the leaking of the fluorescent materials as well as the aggregation of the chromophores due to not having direct chemical bonding with the hydrogel network.48,49
3. Supramolecular fluorescent hydrogelators as bio-imaging probes
Bio-imaging is a process of visualizing specific molecular pathways in vivo, especially the one with a role in disease control. It also facilitates the incorporation of complex biological tools for fast visualization on the molecular basis with further applications in cancer therapy and diagnosis.50 Among the light-emitting materials, white-luminescent solid materials based on supramolecular design have been extensively investigated because the organization of donors and acceptors can be easily modulated by rationally designing the self-assembled supramolecular structures of the nanomaterials, such as hydrogels,51 nanofibers,52 nanoparticles,53 and others.54 In particular, hydrogels, known as soft yet moldable materials, are suitable to construct solid fluorescent light-emitting devices due to the conventional synthetic procedures and preparation methods.55Bio-imaging probes can be divided into three types with respect to the molecular structure, including conventional polymers, supramolecular polymers, and small-molecule based supramolecular fluorescent hydrogels. Out of these, SFH based bio-imaging probes display great potential in cancer diagnosis due to their biocompatibility, 3D cross-linked structure, excellent biodegradability and smart responses to physiological stimuli.56 They have great potential for imaging due to their rapid gel-to-sol transition in response to a variety of bio-related stimuli (e.g., biological molecules and enzymatic reactions) and high biocompatibility. Supramolecular hydrogels that can be easily endocytosed into cells have shown to be favorable biological carriers to load useful bio-imaging agents (e.g., contrast agents, fluorescent dyes, radioactive isotopes, QDs) for in vivo or in vitro bio-imaging.57
Tseng with his group synthesized a novel class of Gd3+ containing supramolecular materials which could serve as MRI agents for diagnosis of cancer metastasis in future.58 A bricks and mortar strategy was used to fabricate calcein based SFH via the aqueous self-assembly of functionalized calcein, folate, and PEG derivative.59 These supramolecular probes possessed good bio-stability, size-dependent fluorescence behavior, and controllable size under physiological conditions, and smart tumor-targeting ability originating from the folate receptor, thereby resulting in exceptional bio-imaging efficiency for Henrietta Lacks (HeLa cells; uterine cell).
Derivatives of fluorophore fluorescein in iso-thiocyanate (FITC) are widely used in bioassays to label proteins and cells. An N-terminal leucine dipeptide 18 is attached to FITC, and this simple conjugate molecule was cyto-compatible and taken up by cells (human dermal and corneal fibroblasts) in contrast to FITC itself as shown in Fig. 2. Above a critical aggregation concentration, the conjugate self-assembles into beta-sheet nanostructures comprising molecular bilayers.60 In recent years, a large number of mechanical, optical SFH based sensors have been actively formed for sensing and detecting varied bio-relevant species, like glycosidases, glycoconjugates, proteins, cancer cells, polyanions, polyamines, and bacteria. SFH using amphiphilic peptide-based hydrogelators61 attached with a fluorophore were fabricated by Xu and co-workers showing potential as fluorescent tools for targeted cancer diagnostics and cell imaging. The stimuli responsive nature of hydrogels can be used to respond to cancer-specific hallmarks. They can act as drug delivery agents for nanoparticle-encapsulated chemotherapeutics, radioisotopes and genes.62 pH dependent drug release in a cancer environment via multidrug chemotherapy has been achieved by incorporating dopamine nanoparticles as a highly effective anticancer agent.63
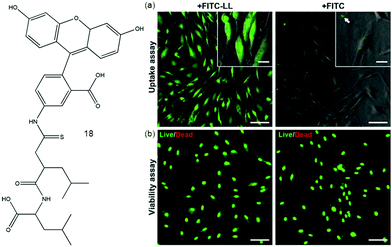 | ||
| Fig. 2 Molecular structure of 18. (a) Internalization and cytocompatibility of FITC-LL in cultures of human fibroblasts. Cells incubated for 24 h with 7.5 × 10−3 wt% of FITC-LL (left) or FITC (right) imaged by fluorescence microscopy to determine the extent of fluorophore internalization (green) or (b) subjected to live/dead cell double staining assay to determine the impact of fluorophore on cell viability. Scale bars 100 μm (panels) or 20 μm (insets). Reproduced with permission.60 Copyright 2015, Elsevier. | ||
One of the most exciting and challenging topics in nanomedicine is the rational design of multifunctional supramolecular probes for both diagnostic and therapeutic purposes.64 Hamachi fabricated a supramolecular hydrogel by co-assembly of a prostate specific antigen (PSA)-cleavable additive with a glycolipid-based stiff and stable hydrogel matrix. The obtained functionalized capsule (SH 19/20) showed the PSA-responsive release of a prostate-specific membrane antigen (PSMA) binding fluorescent substance (Fig. 3), allowing selective targeting of prostate cancer (PCa) cells. Additionally, the released fluorescent substance was internalized and delivered into PCa cells, mediated by the tethered ligand targeting a membrane bound protein PSMA overexpressed on the PCa cell surface.64d Wang et al.65 yielded SFH by a specific peptide–antibiotic interaction by investigating the interfacial self-assembly of an environment-sensitive fluorophore–vancomycin conjugate, which could be used in instantaneous bacterial inhibition and detection.
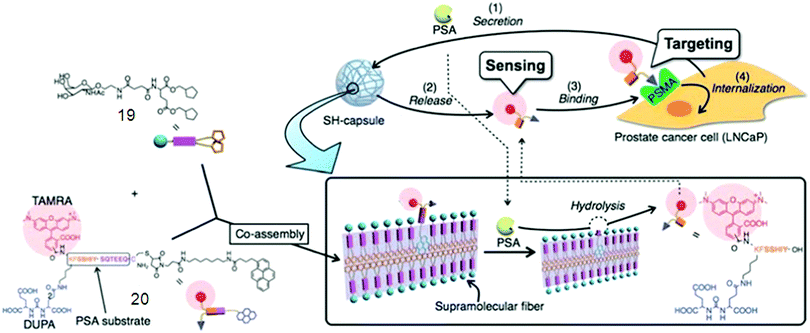 | ||
| Fig. 3 Schematic illustrations of supramolecular hydrogel capsules 19/20. Reproduced with permission.64d Copyright 2010, Royal Society of Chemistry. | ||
Hamachi with his group has done excellent work on the development and design of supramolecular hydrogel-based fluorescent biosensors for detecting various biomarkers with the naked eye. For instance, they reported a polyanion-selective fluorescent supramolecular hybrid sensor consisting of a supramolecular hydrogel, a phosphatase, and aminoethyl modified mesoporous silica particles (NH2-MCM41) loading an anionic fluorescent dye as a probe.66 They also described the hybridization of a supramolecular hydrogel with an anionic layered montmorillonite host adsorbing a cationic fluorescent dye, which gave rise to a fluorocolorimetric sensor for spermine and spermidine in artificial urine, achieving rapid, sensitive and user-friendly naked-eye detection suitable for cancer diagnosis and clinical usage. In addition, bola-amphiphilic glycolipid-based supramolecular hydrogels showing a rapid color change in response to glycosidases could be used to construct a colorimetric sensor array chip for detecting glycosidases with the naked eye. As a unique alternative, a mechanical hydrogel sensor system based on the aptamer with a specific response to targeting proteins was discovered.61,67
Fluorescent hydrogel 21 was prepared by α-amino acids and a fatty acid (azelaic acid) by Tomasini et al. The introduction of fluorescent dansyl moiety into the mixture was used to monitor the internalization process.68 They investigated the spatial distribution of each kind of molecule in a cellular environment based on the successful method to image enzyme-triggered self-assembly of small molecules inside live cells by fluorescent microscopy. Incubation of HeLa cells with each precursor 21a–d (Fig. 4a–d) at the concentration of 500 μM in PBS buffer and comparison of the fluorescent images of these live cells after 30 min of incubation were done.69
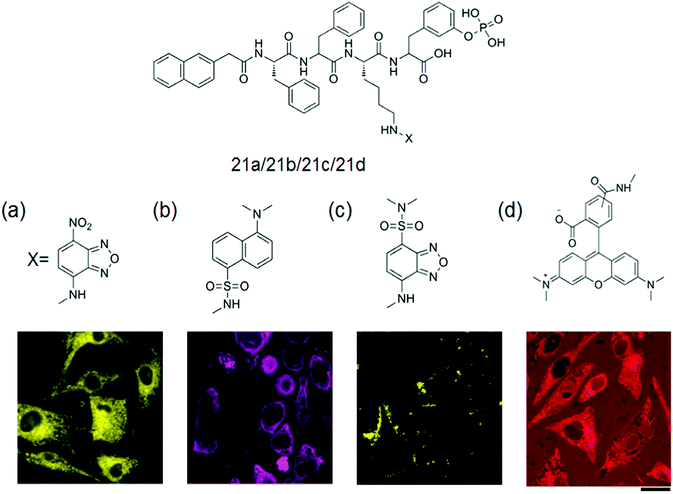 | ||
| Fig. 4 General structure of the gelator 21a–d with fluorescent confocal microscope images of the HeLa cells incubated with 500 μM of (a) 21a, (b) 21b, (c) 21c and (d) 21d in PBS buffer after 30 min of incubation showing the different spatial distribution of fluorescent molecules in a cellular environment. Scale bar represents 25 μm. Reproduced with permission.69 Copyright 2013, American Chemical Society. | ||
A wide variety of supramolecular motifs as shown in Table 1 including host–guest recognition, hydrogen bonding, electrostatic and hydrophobic interactions, and metal–ligand coordination have been used over the last two decades for the development of hydrogels.70
| Intermolecular interactions | Mechanism of self-assembly | Particular groups | Ref. |
|---|---|---|---|
| Host–guest recognition |

|
Cyclodextrins (CDs) cucurbit[n]urils (CB) | 73–76 |
| H-Bonding |
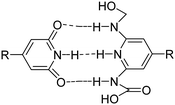
|
Pyridine, carboxylic acid, urea, hydroxyl, nucleobase paring | 77–82 |
| Low molecular weight gelators |
|
Fluorenyl-9-methoxycarbonyl (Fmoc), naphthalene | 83–87 |
| Electrostatic |
|
Glutamic acid and lysine, aspartic acid and arginine | 88–93 |
| Metal–ligand complexes |
|
Peptides with ligand | 94–100 |
| Hydrophobic/π–π |
|
Fluorenyl, naphthyl, pyrenyl, phenyl, chain alkyl, cyclohexane | 101–110 |
| Amphiphilic |
|
L-Glutamic acid, naphthalene diimide (NDI) | 111–123 |
| Enzyme assisted |
|
Peptides and enzyme | 124–143 |
Host–guest recognition based on a wide variety of macrocycles, including pillar[n]arene, cyclodextrin (CD), cucurbit[n]uril (CB[n]), calixarene, and crown ether, is a fascinating and extensively used noncovalent interaction. Particularly, CB[n] and CD-based host–guest couples are biocompatible and nontoxic, and are mainly useful in water, thereby providing a powerful platform to build smart supramolecular hydrogels for many biological applications. Smart pH responsive SFH having applications as drug vehicles for cancer therapy can be constructed by hydrogen bonding.71 However, hydrogen bonding can be easily broken in the case of polar solvents like water. Tough and stiff SFH along with exceptional photoelectric properties in aqueous media can be produced by metal–ligand coordination. Some inert metal ions such as Ru(II), which lead to metallo-supramolecular hydrogel systems, do not show dynamic features on the experimental time-scale. In addition, formation of extremely strong, opaque materials resulted by the strong, multivalent electrostatic interaction between two oppositely charged polyelectrolytes.72
In sharp divergence, the collective use of several orthogonal noncovalent interactions in one system will provide the resulting SFH with enhanced mechanical properties and biostability, as well as good stimuli-responsiveness for bio-imaging use.
3.1. Host–guest complexed supramolecular fluorescent hydrogelators
Supramolecular hydrogels derived by host–guest interactions are of special notice due to their biomedical applications for instance controlled drug release, cell culture, bio/chemo-sensing, and removal of pollutants. For the formation of biocompatible supramolecular hydrogels, cyclodextrins (CD), β-cyclodextrin (β-CD), and cucurbit[n]urils (CB) are the most commonly used host units. The unique complex-forming ability of cyclodextrins (CDs) makes them most suitable candidates for the design and synthesis of self-assembled hydrogels, to be applied in bio-imaging, biomedicine and pharmacotherapy.73In a study, CD was used along with a dye which is made up of 8-hydroxyquinoline to fabricate lithium metal induced gelation.74 The host–guest interaction between β-CD-modified dextran and azobenzene (Azo)-carrying dextran was used for the synthesis of a photo-responsive supramolecular hydrogel which could be used for light-triggered protein release (Fig. 5).74d A host–guest complex is an interesting structure where the ‘host’ is a molecular structure that resembles the shape of a ring, with the central cavity playing an essential role of hosting other ‘threads’ of the guest molecule. Azo, one of the most studied photosensitive units, was used along with β-cyclodextrin (β-CD), for the synthesis of dual stimuli-responsive SFH. UV light irradiation of the hydrogel over 20 min caused a significant decrease in the viscosity of the system, while visible light irradiation over 5 min could recover the viscosity of the system to give the hydrogel.
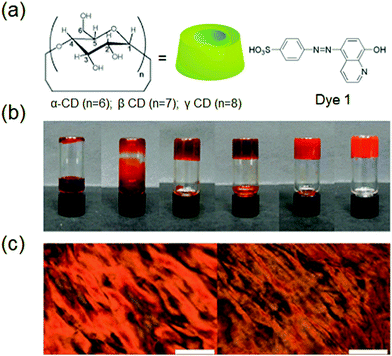 | ||
| Fig. 5 (a) Chemical structures of CD and dye 1, (b) photographic images of the inclusion complexes ([dye 1] and [g-CD] = 60 mM) in the presence of different LiCl concentrations (0, 0.6, 1.2, 30, 60, 360 mM, left-right), and (c) images from a polarized optical microscope of the complex in the absence (left) and presence (right) of LiCl, respectively. Scale bar is 200 mm. Reproduced with permission.74d Copyright 2011, Royal Society of Chemistry. | ||
Supramolecular hydrogels based on host–guest interactions possess exclusive biological properties and tunable functions, for instance, an enhanced photolyzable system is constructed by host–guest recognition between amphiphilic 6-bromoisoquinoline functionalized alkoxyanthracene (AnBq) and cucurbit[7]uril (CB[7]). Additionally, a visible room-temperature phosphorescence (RTP) signal occurs along with the photolysis reaction due to the suppression of intramolecular photoinduced electron transfer.74e
A host–guest interaction mediated hydrogel system coassembled from a phenylalanine derivative gelator (LPF2) and an azo derivative (PPI) was constructed. Azo group induced host–guest interactions with α-cyclodextrin (α-CD) were studied along with the light response by hydrogen bonding interactions between LPF2 and PPI. Lin and co-workers synthesized SFH by a reaction between curcurbit[8]uril (Q[8]) and a chitosan derivative, N-(4-diethylaminobenzyl)chitosan (EBCS). In another work, Appel and co-workers formed a supramolecular hydrogel using cucurbit[8]uril (CB[8]) as host molecules to form reversible cross-links of multivalent Viologen (MV) and 2-naphthoxy (Np) derivatives with high binding constants. This gel formation was observable by the color changes of solution from colorless to bright red upon the addition of CB[8]. Such distinctive gels therefore might have industrial uses which need the controlled viscosity of smart hydrogels.75
DNA host–guest complexes with pH responses were formed with the findings that when the pH was decreased the protonation of the guest diaminopurine naphthalene molecules resulted causing an increased binding strength to the oligothymidine template and an inversion of the helicity of the assembly.76 The single stranded (ss) DNA-templated self-assembly of the host oligothymidine template and the π-conjugated diaminotriazine guests 22 and 23 was studied by David et al. as shown in Fig. 6.
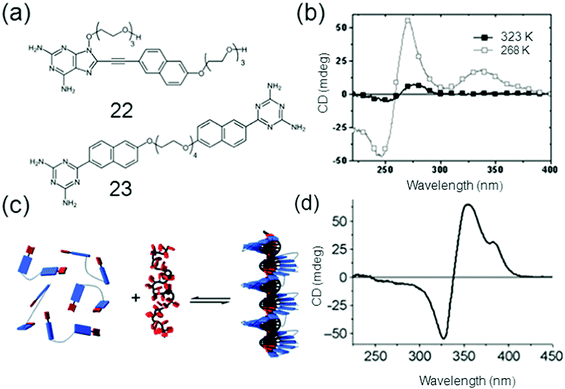 | ||
| Fig. 6 (a) Chemical structures of 22 (Naph DAP) and 23 (GT2); (b) CD spectra of GT2 mixed with dT40 at 50 °C and −10 °C; (c) the self-assembly of 22 and 23 and (d) CD spectrum of NaphDAP; dT40 at −5 °C. Reproduced with permission.76c Copyright 2010, Royal Society of Chemistry. | ||
3.2. Hydrogen bonded supramolecular fluorescent hydrogelators
Hydrogen bonding plays an important role in numerous biomedical applications and is the main driving force for self-assembly of molecules. Particularly, a large class of supramolecular hydrogels with necessary properties, like sensitivity to the environment, almost universal mode of attachment and facile formation processes are formed by hydrogen bonding.77 The molecules will either self-sort to form an aggregated structure or co-assemble in a random manner through self-assembly. Since peptides occur naturally in the human body, making it more suitable for biomedical applications compared to the synthetic materials, biocompatibility of hydrogels can be increased by using peptides as hydrogelators. A new twelve-fold methoxy-triethyleneglycol-jacketed tetraphenoxy-perylene bisimide (MEG-PBI) amphiphile 24 was synthesized which self-assembles into two types of supramolecular aggregates in water: blue coloured strongly coupled J-aggregates consisting of a highly ordered H-bonded triple helix of PBIs and red-coloured aggregates of low order and with weak exciton coupling among the PBIs (Fig. 7).78a Morris et al. have developed a pH-based system that is able to control the self-sorting of naphthalene functionalized dipeptide hydrogelators to form self-assembled networks in water.78 They were able to gradually modify the pH level in the system by hydrolyzing glucono-D-lactone (GDL) to gluconic acid, resulting in hydrogel formation. A novel low-cost fluorometric platform based on sulfur, nitrogen-co-doped graphene quantum dots immersed into nanocellulosic hydrogels was designed and applied in detecting the laccase enzyme. Though most of the methods for detecting laccase are based on their catalytic activity, which is strongly dependent on environmental parameters, a sensitive and selective method based on the fluorescence response of hydrogels containing graphene quantum dots (GQDs), acting as a luminophore towards laccase, was reported as shown in Fig. 7.79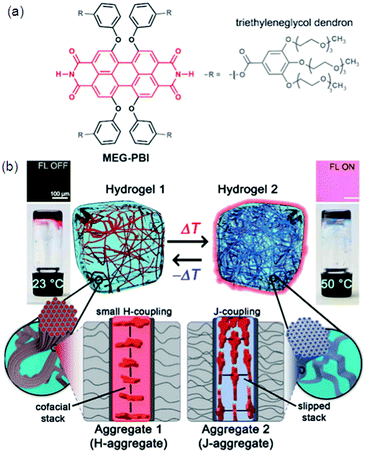 | ||
| Fig. 7 (a) Chemical structure of MEG-PBI. (b) Schematic illustration of the reversible temperature response of the MEG-PBI hydrogel (c 1/4 20 wt%) in the range of 23–50 °C with the tube inversion test: at 23 °C (left) the gel is red and no significant fluorescence (FL) can be detected; upon increasing the temperature to 50 °C (right) the gel turns blue and a fluorescence in the far-red/NIR range (650–750 nm) is observed (lexc 1/4 365–385 nm). The hydrogels are composed of bundled supramolecular fibrous aggregates. The different colour and light emission is due to the formation of different aggregates: at low temperature the PBIs adopt the conventional cofacial arrangement (aggregate 1) leading to a weak H-type excitonic coupling; at higher temperature, a strong J-type excitonic coupling results from the rearrangement of the PBIs in a hydrogen-bonded slipped-stacked architecture (aggregate 2). Reproduced with permission.79 Copyright 2018, Royal Society of Chemistry. | ||
The easily prepared gel matrix stabilizes the fluorescence signal, improves the sensitivity towards laccase and the fluorescence signal of GQDs by avoiding self-quenching. Significant quenching without peak-shifts of GQD fluorescence via energy transfer is believed to be due to noncovalent interactions between the sensor and the analyte. This upfront strategy is able to detect and stabilize laccase, being an added-value for storage and recycling enzymes. Self-assembly can be controlled by different CH⋯O hydrogen bond interactions, so, coumarin-derived isomeric hydrogelators 5, 7 and 9 with different spatial structures were synthesized. Due to intermolecular hydrogen bonds and π–π stacking distance between the gelators, the strongest emission intensity was from 7 assemblies. Gelator 7 should form the strongest intermolecular hydrogen bonds leading to the strongest fluorescent emission due to CH⋯O bond distances and bond angles (2.44 Å and 160° for C2–H2⋯O1 and 2.54 Å and 164° for C11–H11⋯O3).29
The study directly relates the influence of the molecular spatial structure on CH⋯O hydrogen bonds on the overall macroscopic properties of nanofibrous structures.80 Eastoe et al. have used a stilbene-containing photo-surfactant and N,N′-dimethyldodecylamine to synthesize a photo-responsive supramolecular gelator system by refluxing.81 A thiophene derivative was used by Park et al. for the preparation of a highly fluorescent switching co-assembly with a photochromic molecule by efficient intermolecular energy transfer.82 The acid sensitive 2,3-di-n-alkoxyphenazine gelator has been reported by Pozzo and co-workers which displayed a reversible protonation/deprotonation induced fluorescence change.83 Multimodal imaging and bio-sensing can be achieved by biotemplated nanomaterials, for example, QD DNA hydrogels have been prepared having both long term photostability and low toxicity.84
3.3. Low molecular weight supramolecular fluorescent hydrogelators
Low molecular weight gelators (LMWG) have attracted considerable interest in the past few years as supramolecular materials with the application as templates for ion-selective membranes, mineralization, and sensors. Gazit and co-workers discovered that macroscopic characteristics of a gel can be achieved by dissolving peptides at a higher concentration in aqueous solution; 3D spacious volume and stability over a wide temperature plus pH range of the resulting hydrogel were maintained even with less than 1% peptide material.85 A tumor regression analysis of gelators in tumors harvested from mice by performing western blotting for apoptotic markers was done. DOX-gel treatment induces cleavage of caspase as observed in western blot studies, showing activation of the apoptotic pathway causing tumor regression. The TUNEL assay on tumor tissues confirmed the apoptotic mechanism of tumor regression by DOX-gel as shown by a high number of apoptotic TUNEL positive cells illustrating DNA breaks in DOX-gel treated mice in comparison to control and DOX(IV) treated tissues.86 Interesting luminescence, switching and sensing properties may be given by a combination of dyes with LMWG gels. Fluorescent vesicles and chiral hydrogels have been made by electrostatic interactions between positively charged CQDs and negatively charged biosurfactants.86d The PL properties of CQD based hydrogels make them suitable to be applied for imaging purposes. CQD based 25 hydrogels as well as xerogels emit strong blue light as shown by Fig. 8a and b, respectively, which is attributed to their aggregation induced quenching (AIQ) in the solid state. So CQD based hydrogels can act as biocompatible phosphors. Fig. 8c–e also explain a similar kind of PL in the gel or xerogel state as a consequence of AIQ.86d A new class of LMWGs with simple pyridine substituents instead of long alkyl chains or steroidal structures, endowing the gel system with greater potential for optical applications, was prepared by Jung et al.33 The hybridization of a supramolecular hydrogel 26 with a layered inorganic host adsorbing a fluorescent dye resulted in a fluorocolorimetric sensor for spermine and spermidine, important biomarkers for cancer, in artificial urine as shown in Fig. 9.87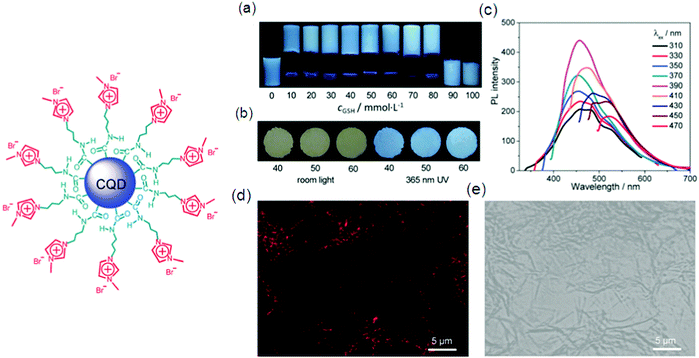 | ||
| Fig. 8 Illustration of the structure of cationic CQDs used in the study. (a) Photos of typical samples in a NaDC/CQDs/GSH/H2O system with varying cGSH (mmol L−1), as indicated; taken under 365 nm UV irradiation. (b) Photos taken under room light and 365 nm UV irradiation of xerogels from typical samples with different cGSH (mmol L−1), as indicated. (c) Wavelength-dependent photoluminescence of a typical hydrogel with cGSH = 50 mmol L−1. (d and e) Confocal fluorescence images of the hydrogel with cGSH = 60 mmol L−1 in dark (d) and bright (e) fields. For all the gels or aqueous solutions, cNaDC = 100 mmol L−1 and cCQDs = 0.5 mg mL−1 Reproduced with permission.86d Copyright 2018, Royal Society of Chemistry. | ||
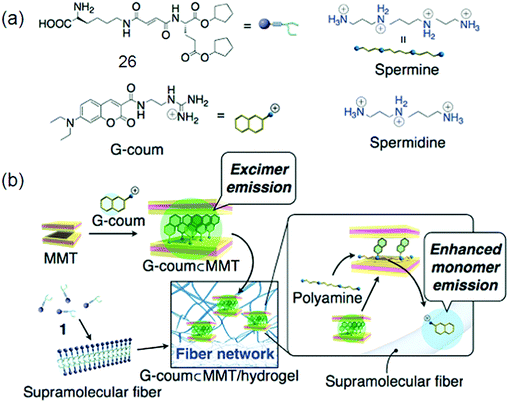 | ||
| Fig. 9 (a) Chemical structures of hydrogelator 26, G-coum, and polyamines; (b) construction and the mechanism of action of the fluorescent dye (G-coum) adsorbed MMT/supramolecular hydrogel 25 hybrid sensory system for polyamines. Reproduced with permission.87 Copyright 2011, American Chemical Society. | ||
A powerful gelator 27 with novel chromophoric but non-fluorescent benzene-1,3,5-tricarboxamide (TOBA) has been made for some aprotic organic solvents. The face-to-face intermolecular H-bonding was proven to be the key motif for inducing strong fluorescence as well as supramolecular aggregation (Fig. 10).89
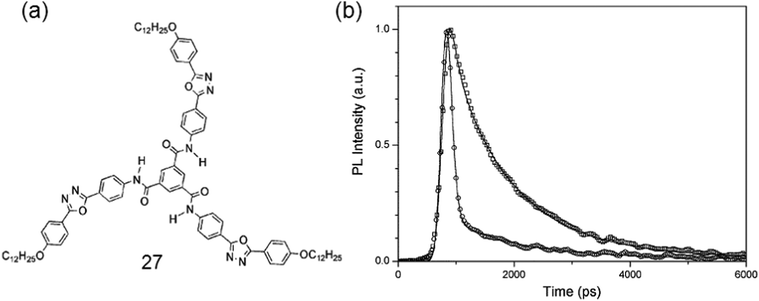 | ||
| Fig. 10 (a) Chemical structure of gelator 27; (b) fluorescence kinetic profiles of the monomer of 26 and H-bonded aggregate states of TOBA. Reproduced with permission.89b Copyright 2004, Royal Society of Chemistry. | ||
Pyrene and fluorene are assumed to be carcinogenic, so, hydrogelators having these moieties lack biocompatibility, which can be enhanced by incorporating a naphthyl group (Nap), a rather common part in clinically accepted drug molecules.90 A salen-linked gelator, composed of a naphthalene based salicylidene aniline group and a sorbitol group, was made with stimuli responsiveness toward temperature, Cu2+, UV light, and OH−.88 LMWGs have also found application in metal ions imaging for example Liu recently utilized PEG based hydrogels as dye-labeling scaffolds for Zn2+ ion imaging of cancer cells.89a
3.4. Ionic and associative interactions in supramolecular fluorescent hydrogelators
Hydrophobic segments like aromatic rings and long alkyl chains can decrease the solubility of the hydrogelators in water. So, introduction of charge(s) into the hydrogelators is essential for increased solubility required for forming a hydrogel. Synthesis and properties of L-lysine alkali-metal salt forming a supramolecular hydrogel were reported by Suzuki et al. He also used a pyridinium bromide salt for the preparation of L-lysine-based hydrogelators.91 The as prepared gelators can gel at very low critical gelation concentration (CGC), i.e., 0.3 wt% at neutral pH. Yang et al. developed two chiral L-phenylalanine-based salts92 as supramolecular hydrogelators.Multicomponent SFH by Fmoc-amino acid derivatives were first reported by Xu et al.93 The resulting hydrogel acts as a reaction medium to mimic a bioluminescence environment with a more than 10-fold enhanced quantum yield of chemiluminescence.94 Banerjee et al. also reported supramolecular hydrogels formed by co-assembling two oppositely charged Fmoc-amino acids with a CGC value of 50 mM (1.8 wt%).95 Despite complex synthesis, multifunctional hydrogels are very interesting, for example pH-sensitive, biodegradable, fluorescent, and self-healing hydrogels (Fig. 11) were manufactured from hyperbranched poly(amido amine) (HPAMAM) and oxidized alginate (OALG).95b OALG (0.05 g mL−1) and HPAMAM (0.15 g mL−1) were used for Gel i, while when 0.10, 0.15 and 0.20 g mL−1 OAL solutions and a HPAMAM solution of the same concentration were used, the obtained hydrogels were named Gel-ii, Gel-iii and Gel-iv, respectively. Shinkai and co-workers have developed different gelators based on 2-anthracenecarboxylic acid (2Ac), attached non-covalently with the gelator counterpart containing a 3,4,5-tris(n-dodecyloxy)-benzoylamide backbone. The photoirradiation of the gel samples produces photocyclodimers having different degrees of stereoselectivity for different systems.96
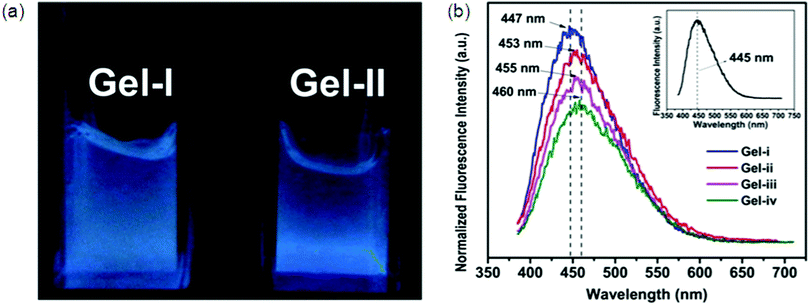 | ||
| Fig. 11 Fluorescent properties of HPAMAM/OALG hydrogels. (a) Fluorescence image of HPAMAM/OALG hydrogels under 365 nm irradiation, and (b) fluorescence spectra of Gel-i and Gel-ii. The inset is the spectrum of neat HPAMAM. The excitation wavelength was 365 nm. In (a), the volume of Gel-ii was slightly lower than that of Gel-i due to the contraction. A higher amount of OALG was used in Gel-ii so that more aldehyde groups participated in the reaction with the amine groups on HPAMAM, leading to a more compact and denser Gel-ii. Reproduced with permission.71i Copyright 2016, Royal Society of Chemistry. | ||
In recent years carbon quantum dots (CQDs) have shown many uses in drug delivery, bio-imaging and bio-sensing. However, the self-assembly of CQDs is still very limited, while still having additional aspects of photoluminescent properties. LMWGs based on short peptide β-Asp FF with excellent affinity towards QDs were prepared and fluorescence spectroscopy was conducted to measure the emission spectra of β-AspFF toluene indicating the strong aggregation of the β-AspFF molecule.97 As compared to the emission colors of free QD520 under 365 nm UV light, encapsulated QD520 in the gel retains the original luminescence colors though slightly weakened. Magnification of the QD520 emission spectra demonstrates that the shape of the absorption peak of encapsulated QDs slightly changed from the original shape. These results suggest that the gel systems did not affect the exceptional photoluminescence (PL) of the encapsulated QDs in the gel. Fluorescence spectroscopy was used to check the emission spectra of β-AspFF toluene gels. β-AspFF toluene gels showed enhanced fluorescence intensity compared with sols which indicated that the aggregation of β-AspFF molecules was strong and the gel system had a high energy content.
3.5. Metal–ligand complexation of supramolecular fluorescent hydrogelators
An intense charge-transfer transition and an enhanced nonlinear optical response can be achieved by the introduction of ligated metal centers into an organic delocalizable framework i.e., a hydrogel. It is quite common to apply metal complexes as gelators for making gels due to their rich optical, electronic, redox, or magnetic properties usually associated with metal centers and the stability of metal complexes in common organic solvents. For example, metal complex-based gelators have shown a wide range of interesting properties like anion binding, color switching, catalysis, responses to ultrasound, redox perturbation, temperature, and fluorescence.98Although the coordination between organic and metal ions is common in nature, the organic molecules used in these complexes for generating hydrogelators are largely centered on several functional groups that serve as the ligands for metal ions. Therefore, these relevant hydrogelators have been classified as follows: (i) ligands containing nitrogen as an electron donor to a metal ion,99 (ii) ligands composed of thiol groups,100 carboxylic groups as the ligands,100a,101 and (iii) others.102 Although some amphiphiles with carboxylic groups themselves self-assemble in water to form nanostructures as the matrixes of hydrogels, organic–inorganic hybrid hydrogels still have attracted considerable interest due to the specific functions conferred by the metal ions103 or inorganic elements. An interesting example is the ruthenium(II) tris(bipyridine) complex, developed by Xu et al. The integration of a tripeptide derivative, a versatile self-assembly motif, with a ruthenium complex affords the first supramolecular metallo-hydrogelator that not only self-assembles in water to form a hydrogel 28 but also exhibits a sol–gel transition upon oxidation of the metal center (Fig. 12).
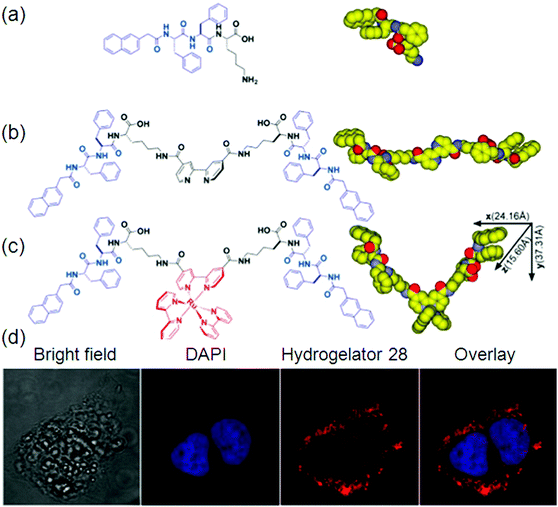 | ||
| Fig. 12 Molecular structures of: (a) self-assembly motif; (b) ligand; (c) metallo-hydrogelator 28; and (d) fluorescence images. Reproduced with permission.103 Copyright 2013, American Chemical Society. | ||
They found that this hydrogel formed by 28 exhibits strong fluorescence upon the irradiation of UV light.24 It is also noteworthy that the long lifetime and photostability of [Ru(bipy)3]2+ will likely find applications in molecular imaging in cells. You and co-workers reported the utilization of simple imidazole-containing ligands as building blocks to form metallogels. Addition of AgNO3 to an aqueous solution of the gelators yielded optically transparent metallogels in a few minutes at room temperature. A series of alkynylplatinum(II) bzimpy complexes (bzimpy = 2,6-(N-alkylbenzimidazol-20-yl)pyridine) have been prepared. The complexes were able to form yellow metallogels in benzene at room temperature. It was found that the emission colors of the metallogel changed from yellow to red upon the gel–sol phase transition at high temperature. Inspired by the molecular structure of Vitamin B12 (VB12), also called cobalamin having metal ions, Wang et al. developed the first example of a hydrogel precursor that is solely responsive to Co2+ under appropriate conditions, which resulted in immediate fluorescence quenching.104
3.6. π–π stacking or hydrophobic interaction based supramolecular fluorescent hydrogelators
Gel formation is mainly derived by interactions such as those between the N atom of pyridine and –NH, and –CH of pyridine, and p–p and CH–p interactions. In particular, fluorescence properties are mainly influenced by the binding strengths of p–p and CH–p interactions in the gel states.105 The existence of π–π interactions within the self-assembled molecules can be easily monitored by fluorescence spectroscopy. Hydrophobic and π–π interactions can be induced by incorporating an aromatic capping group in supramolecular hydrogels. To date, naphthalene (Nap), and 9-fluorenyl-methoxycarbonyl (Fmoc) have been generally applied. Further, other capping groups, including pyrene, anthracene, adamantine, indole, and perylene, have also been used.106 An amide-functionalized phenylethynyl thiophene gelator via hydrophobic interactions has been synthesized by Sun et al.Concentration-dependent fluorescence properties of these gelators confirmed the presence of aggregates.107 This indicates the presence of a strong aromatic interaction between the phenyl side chain moieties of phenylalanine residues in these interacting gelator molecules. The remarkable ability of a charge-transfer (CT) complex prepared from a pyrene-based donor (Py-D) and a naphthalenediimide-based acceptor (NDI-A) led to the formation of a deep-violet in color, transparent hydrogel at room temperature (RT-gel) by slipped stack π–π stacking interactions.108 Non-covalent interactions among pyrene and graphene due to the strong π–π stacking between pyrene and graphene result in the formation of β-sheets between peptides denoted by Py-GAGAGY repeats, facilitating the inter-sheet cross linking among various graphene oxide surfaces (GOS). Further, a strong hydrogel 29 formed by photo-cross linking of the terminal tyrosine motif as shown in Fig. 13.109
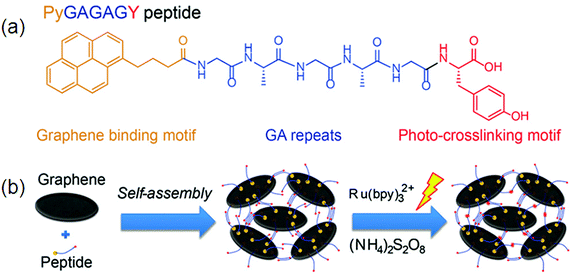 | ||
| Fig. 13 The design of the hybrid hydrogel 30: (a) peptide sequence; (b) hierarchical construction scheme for the hydrogel. Reproduced with permission.109 Copyright 2015, Royal Society of Chemistry. | ||
Peptide graphene hybrid hydrogels for biomedical application were formed by π–π interactions. The hydrogel exhibited low cytotoxicity and can be used for in vivo diagnosis. The introduction of bulky tert-butylphenoxy substituents at the bay positions of the perylene dye exhibited a distorted core and led to a change in aggregation mode from H type to j, which was strongly aggregated due to intermolecular π-stacking and H-bonding.110 Jung et al. reported two fluorescent gels formed by amide-linked tripyridine derivatives 11 and 12, with a para or meta substituent. They demonstrated that both molecules gel in water or water–DMSO and the hydrogelation ability depends mainly on CH–π and π–π stacking or strong intermolecular hydrogen bonding between the amide groups.31 Exploration of SFH with combined effects of fluorescence and the supramolecular hydrogel is highly needed since physiological and pathological processes in the biomedical field can be easily monitored by fluorescence in a real-time and sensitive manner.111 In this regard, work by Discher and co-workers demonstrated that the flexible nanofibers have properties like relative blood circulation time, which make them suitable for bio-imaging use.112In vivo tumor imaging can be done by utilizing precursors and nanofibers. Murine 4T1 cancer cells were intravenously injected into the right axillae of mice for the establishment of a tumor-bearing mouse model and fluorescence imaging was performed by the administration of a nanofiber and a precursor into the 4T1 tumor-bearing mice, respectively. Both the nanofiber and precursor-treated tumor tissues were sliced and the tumor vasculature was immune-stained against platelet/endothelial cell adhesion molecule 1 (PECAM-1) at 0.5 h post-injection.113
Wang et al. reported the use of rhodamine B as a new capping group of self-assembly to provide the hydrophobic and π–π interactions in hydrogel formation. A biocompatible approach of disulfide bond reduction was applied for the synthesis of SFH, inspiring some stimulating research in the formation of nanofibrous hydrogel-based fluorescent probes for a variety of bio-imaging applications.
The fluorescence images of various tissues from 4T1 tumor-bearing mice after treatment with a nanofiber and a precursor 30 (Fig. 14a) are displayed in (Fig. 14b–e) respectively. A strong fluorescence signal is observed in the tumor as shown in Fig. 14b, while a comparatively weak signal is detected in the mouse intestine. In contrast, the precursor bio-distribution in tumor-bearing mice is different from that of the nanofibers as confirmed by obvious fluorescence signals in the tissues of the tumor, intestine and kidneys (Fig. 14c). The nanofiber (Fig. 14d) and precursor (Fig. 14e) distributions in these tissues were also studied at cellular resolution. Much more fluorescent aggregates are observed in the nanofiber-treated tumor slices as compared to the precursor-treated tumor slices. Collectively, the results in Fig. 14d and e agreed very well with those in Fig. 14b and c, revealing that these fluorescent nanofibers can serve as an effective probe for tumor imaging application.114
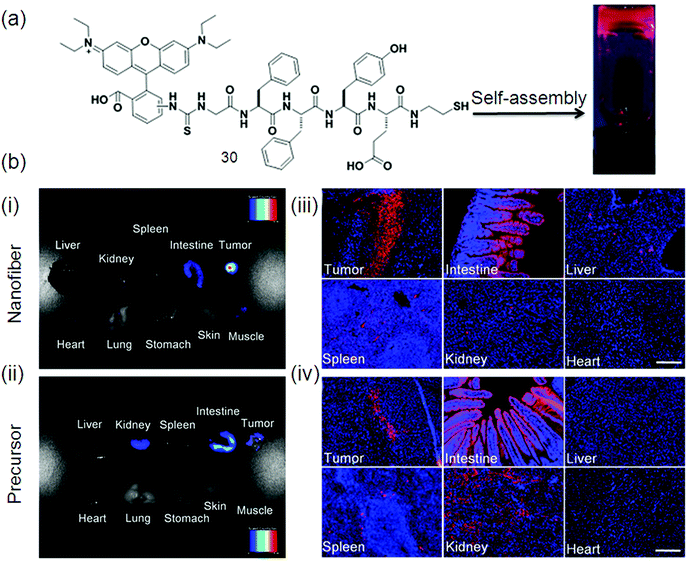 | ||
| Fig. 14 (a) Chemical structure of gelator 31; (b) ex vivo fluorescence imaging of tumor tissue and major organs of mice after intravenous injection of: (i) nanofiber and (ii) precursor. Fluorescence images of tissue slices from mice treated with: (iii) nanofiber and (iv) precursor. The mice were sacrificed at 24 h post-injection. For (iii and iv), the cell nuclei were stained with DAPI. Scale bar represents 200 μm for all the fluorescence images in (iii) and (iv). Reproduced with permission.114 Copyright 2015, Nature. | ||
3.7. Bola-amphiphiles as supramolecular fluorescent hydrogelators
Bola-amphiphiles are a class of hydrogelators composed of two terminal hydrophilic groups linked by a hydrophobic backbone/chain. Variation of the structures and interactions of the head group and the hydrophobic alkyl chains of bola-amphiphiles115 has a great influence on their ability to aggregate and the properties of amphiphile self-assembly for hydrogels. Bola-amphiphiles with two head groups have attracted much attention116 because of the covalent connection of the two head groups cooperating for the self-assembly process, resulting in various morphologies like nanofibers, monolayers, micelles, vesicle, nanoribbons, and nanotubes.117 Morphological changes can be studied by variation of temperature, pH value, photoirradiation, or doping with other components along with the modification of the molecular structure;118 for example, a bola-amphiphile with a L-glucosamide head group and alkyl spacers was prepared by Shimizu and co-workers demonstrating the strong effect of even or odd number of methylene units for self-assembled supramolecular structures of the molecules in water.119 Good molecular building blocks with appropriate functional groups, like a hybrid spacer for the interaction between the spacers and the head groups can be used to control the morphologies of the assembled bola-amphiphiles;120 for example, helical nanotubes formed by bola-amphiphiles having L-glutamic acid as the terminal groups undergo gelation.On the basis of L-valine and L-proline-based peptide bola-amphiphiles, a class of efficient gelators, Miravet et al. designed and developed one bola form amino acid derivative which self-assembles in water to form hydrogels.121 The CGCs for these small molecules range from 4 to 26 mM (from 0.18 to 1.2 wt%). Liu et al. reported bola-amphiphiles with head groups made of L-glutamic acid and a hybrid linker composed of two rigid benzene rings and a butyl segment. They controlled the hierarchical self-assemblies via changing the solution pH; for example, at pH 3, these molecules form a hydrogel in water at a concentration as low as 0.5 wt% (CGC); at pH 12, these hydrogelators then form vesicle-like aggregates.117 Sugiyasu et al. developed metastable and t time-dependent evolution of two-component supramolecular assembly. They found that the systems undergo either self-sorting or co-assembly in time depending on the combination of components122 to form wrinkled surface golf ball-like nanostructures in a 3D fashion using naphthalene diimide (NDI-S) derivatives. The golf ball-like microsphere morphology 31 was obtained by mixing NDI-S in 45% hexane with a CHCl3 solution (Fig. 15); Fig. 15a and b show UV-vis absorption and emission changes of NDI-S (1 × 10−5 M) in CHCl3/hexane (0–50%), respectively.123a Interestingly, addition of pyrene resulted in a time dependent CT mediated morphological transition from a vesicle to fiber and finally gelation at higher concentration. Detailed absorption and FT-IR studies revealed that the morphological transition is driven by the intercalation of electron rich pyrene donors in between the electron-deficient NDI based bola-amphiphiles without disrupting the hydrogen bonding between the hydrazide units of NDI bola-amphiphiles. Detailed time dependent morphological studies using AFM showed that alternatively stacked NDI-pyrene assembly substantially increased the radius of curvature of vesicles, there by leading to intervesicular fusion and rupturing of membranes to form a fibrous structure.123
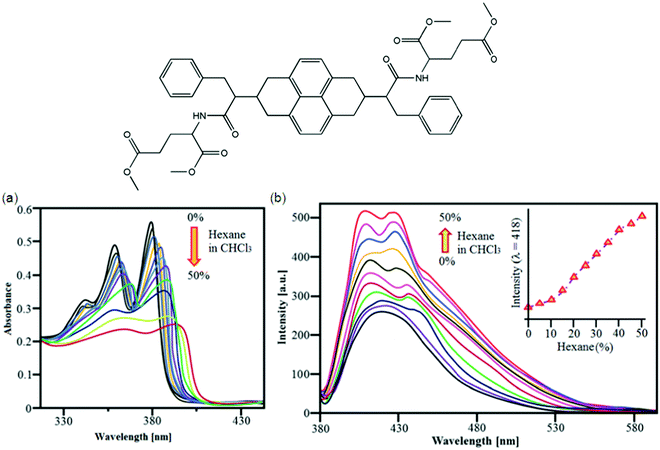 | ||
| Fig. 15 Molecular structure of 31. (a) Solution based self-assembly. (a and b) UV-vis absorption and emission changes of NDI-S (1 × 10−5 M) in CHCl3/hexane (0–50%), respectively. Reproduced with permission123 Copyrights 2016 Royal Society of Chemistry. | ||
Naphthalene diimide (NDI) bola-amphiphilic molecules self-assemble in water to form organic nanoparticles, which exhibit self-assembly induced pre-associated excimer formation and hence enhanced green fluorescence. The NDI based bola-amphiphile 32 forms vesicular assembly under aqueous conditions by using synergistic effects of hydrogen bonding and π–π stacking.124
In recent years, core modified PBIs have been used for the creation of supramolecular assemblies and gels. Cholesterol functionalized PBI gels with tunable optical properties are an interesting case, reported by Shinkai and co-workers.125a Various binary, ternary, and quaternary perylene gels were prepared and subjected to energy transfer studies. Biocompatible organic dyes emitting in the NIR range are highly advantageous in fluorescence imaging techniques. Small peri-guanidine-fused naphthalene monoimide and perylene monoimide chromophores were fabricated. The presented structures possess good water solubility, NIR absorption and emission, and high photostability.
They selectively stain mitochondria with a low background in live and fixed cells after a fast cellular uptake.125 Self-assembly of π-conjugated molecules has attracted a lot of attention for potential use in sensors, as a result, lipid chains or many bola-amphiphiles have been replaced by π-conjugated nanostructures to induce fluorescence. For example, a self-supporting bola-amphiphile hydrogel at low concentrations (1.0 wt%) was designed by Stupp et al., envisioning the applications in simultaneous cell signaling by epitope–receptor interactions in combination with biological epitopes.126
3.8. Enzyme assisted supramolecular fluorescent hydrogelators
Enzyme instructed self-assembly is the best way to explore and evaluate the molecular self-assembly of gelators in many biological processes comprising enzymes. Fate of cells is controlled by multi-step supramolecular assemblies formed inside cells by gelators. There are mainly two sides127 of enzyme-instructed intracellular supramolecular nanofibers: (i) intracellular enzyme catalysis plays the key role and (ii) the supramolecular nanofibers change the viscosity of the cytosol for selective cell death. The in vivo biocompatibility and stability should be evaluated for practical biomedical applications of SFH. From this perspective, Ghanaati's group estimated the supramolecular hydrogel formed from a peptide amphiphile in subcutaneous space.128 Liang's group also considered the biocompatibility of a Fmoc-short peptide-based supramolecular hydrogel in the anterior chamber. In a study, both L- and D-amino acid-based self-assembling nanofibers were checked for in vivo dynamic toxicity and biostability.129 Enzyme based supramolecular hydrogels are widely studied yet a limited number of studies focused on the preparation of SFH via enzymes. Peptide based gelators are important for biological functions along with being substrates for enzymes as they are mostly made up of L-amino acids. Addition of an enzyme to the gelator solution by a process called enzymatic hydrogelation of small peptides may lead to gelation.130Xu et al. reported a straightforward method for studying the enzyme-instructed self-assembly of small molecules inside cells.131 The attachment of a therapeutic agent (e.g., taxol) or a fluorophore (e.g., 4-nitro-2,1,3-benzoxadiazole) to the D-peptide based hydrogelators affords biocompatible hydrogelators, which may find applications in intracellular imaging.132 An enzyme-assisted nanoparticle crosslinking has been reported in this work. The mechanical stability of peptide-based supramolecular hydrogels has been enhanced by more than 3000 times by enzyme assisted nanoparticle crosslinking.133
Pyrene has become a popular moiety to be incorporated into supramolecular hydrogelators due to its aromatic–aromatic interactions which cause emission changes (e.g., the formation of an excimer). So, hydrogelators consisting of a single amino acid and a pyrene group, in a wide range of pH (7.5–14) in the aqueous phase were fabricated.134 A morphological change from helical to tape like occurs in hydrogels by an increase in pH. Phenylalanine is often incorporated into peptides for increasing their ability to self-assemble; for example, Kim et al. reported a biotin-based gelator that displayed extraordinary gelation properties in aqueous media, together with buffer solutions at different pH values.135 A semi-wet and 3D nanofiber network of supramolecular hydrogels can entrap biological substances, without any detrimental influence on the functions of the entrapped constituents.53b,136
Conjugation of L-phenylalanine to biotin will result in a better and stable class of supramolecular hydrogelators. The self-assembly behavior of cholic acid-connected amino acid derivatives was studied,137 and Xu et al. reported an enzyme-instructed self-assembly to form a hydrogel in pericellular space,138 which along with certain drug-resistant cell lines selectively inhibited cancer cells. Specifically, ectophosphatases (e.g., placental alkaline phosphatases (ALPP)) dephosphorylated the precursor made of a small D-peptide to a hydrogelator, which self-assembled with nanofibril formation at a concentration of 280 μM and resulted in a pericellular hydrogel.139 Another substrate for lysyl endo peptidase (LEP) or trypsin (Tryp) was designed by a hydrophilic oligopeptide (pep) chained to an environmentally sensitive fluorophore (DANSen) at the C-terminal of (lysine) Lys. When LEP cleaves the peptide bond between Lys and DANSen (Fig. 16a), the resultant DANSen may move from the aqueous space to the hydrophobic domain due to its strong hydrophobicity, resulting in enhanced fluorescence. Certainly, the emission intensity of pep-1 immobilized in the supramolecular hydrogel clearly increased along with a blue-shift by the addition of LEP; interestingly, this color change can be detected by the naked eye (Fig. 16b).
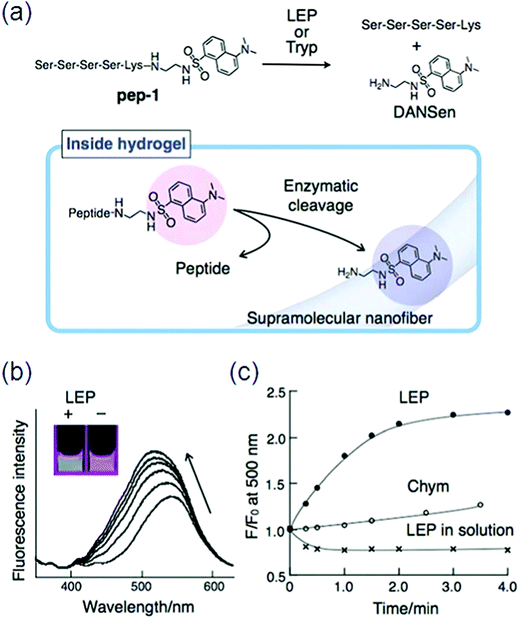 | ||
| Fig. 16 (a) Chemical structure of a peptide substrate (pep-1) and the mechanism of the enzymatic hydrolysis of pep-1 in the hydrogel; (b) fluorescence spectral change of pep-1 embedded in the hydrogel by enzymatic (LEP) hydrolysis and the corresponding emission color change (inset); (c) time course for the change in fluorescence intensity at 500 nm. Reproduced with permission.140 Copyright 2010, Royal Society of Chemistry. | ||
In contrast, the addition of chymotrypsin (Chym) did not induce such a fluorescence change (Fig. 16c).140 Many supramolecular gelators covalently coupled with fluorescent aromatic groups (e.g., pyrene, naphthalene, fluorenyl) have been produced by self-assembly into nanofibers through non-covalent interactions, like hydrogen bonding, electrostatic interactions, p–p stacking, and van der Waals interactions, but quenching problems arose in these fluorescent gelators by aggregation. Moreover, only short wavelength can be emitted through these aromatic moieties.
In order to achieve enhanced biocompatibility and fluorescence efficiency ruthenium complex-based supramolecular hydrogelators have been developed recently, though, the characteristic toxicity of rare earth (e.g., ruthenium) complexes is still unavoidable.141 SFH formed by non-covalent interactions have gained good adaptability to be used as imaging probes being responsive to many stimuli. Multi-fluorescent supramolecular materials are in demand, while, up to now, almost all the SFH have only shown a single fluorescent signal with a single excitation wavelength.142 Supramolecular gels can be made multi-fluorescent by introducing multiple chromophores into the system. These results directly revealed that the present supramolecular hydrogel restores substantial fluidity and therefore provides a unique medium for sensing the enzyme activity. For example, Cao and co-workers obtained a material with high efficiency white-light emission by incorporating fluorescent blue, green, and red chromophores into a conjugated polymer backbone.143 Fluorescence-tunable hydrogels especially emitting white-light were achieved by swelling hydrogels in solutions containing two kinds of dyes.144 So, the synthesis of nano fibrous structures with the properties of easy preparation, strong fluorescence emission, and biocompatibility is becoming a crucial objective145 for aiding cell imaging studies, which has been unachievable up to now.
4. Trends in supramolecular fluorescent hydrogelators (SFH) for bio-imaging since 2013
Bio-imaging by SFH has emerged as a key method to analyze characteristics of cancer cells for effective therapeutics. Tumors have been thought to be initiated by cancer stem cells which have the potential of metastasis and self-renewal.146 Takaishi et al. found that the CD44 positive cells in gastric cancer cell lines have stem-like properties. Poor prognosis is correlated with the CD44 expression level in tumor tissues of gastric cancer patients. So, the identification and elimination of stem-like cancer cells in tumor tissues are raising concerns in positive cancer treatment. Consequently, CD44-specific imaging nanoprobes based on hydrogel complexes have been synthesized with targeted delivery of imaging probes to stem-like cancer cells expressing CD44 using hyaluronic acid (HA). Many HA-modified particles have been used for imaging and treatment of cancers possessing CD44-expressing cells. High-molecular weight HA (over 500 kDa) can be used to make polyplexes due to the polyanionic property and for delivery to CD44 molecules. NIR fluorescence imaging using a NIR-sensitive organic dye (Cy 5.5) was used for in vivo imaging with minimized tissue auto-fluorescence. Thus, a NIR-sensitive supramolecular hydrogel (NIRSH) via a HA molecule was fabricated for the sensitive optical imaging of CD44-expressing stem-like gastric cancer cells (Fig. 17).147 The synthesis of a fluorescent gelator was performed from p-carboxy benzaldehyde and 2-acetyl pyridine in the presence of ammonia. The terpyridine (terpys) units in the as synthesized gelator may make it useful for monitoring ion transport across the cell membrane. Hydrogelators with a width of single molecule can be synthesized by the incorporation of a metal complex into the hydrogelators. Metal complexes are able to modulate the dimensionality of intermolecular interactions, thus elucidating the interactions of the molecular nanofibers with other molecules.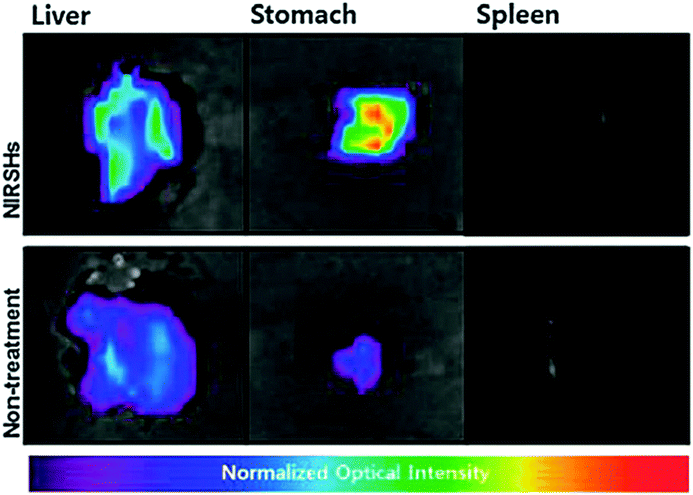 | ||
| Fig. 17 Ex vivo fluorescence images of exercised liver, stomach and spleen of orthotopic and normal mouse models. Reproduced with permission.147 Copyright 2014, Royal Society of Chemistry. | ||
Remarkably, hydrogelators, consisting of the tripeptide motifs, exhibit little affinity to nucleic acids, thus becoming cell compatible at a relatively high concentration. This feature, which agrees well with the origin of the cytotoxicity of polypyridyl ruthenium complexes, may allow them serve as multipurpose hydrogelators and find application in live-cell imaging.103 Cellular microenvironments can also be modulated by photodegradable hydrogels which allow 3D encapsulation of cells. Zhang et al. reported photodegradable hydrogels designed by the self-assembly of short peptides modified with a phototrigger. A biaryl-substituted tetrazole moiety is used as a trigger which upon mild light irradiation undergoes rapid intramolecular photo click ligation to form a highly fluorescent pyrazoline moiety.148
The photomodulation of cellular microenvironments can be demonstrated by cells grown on top of the gel also called stem cell encapsulation. Stem cells encapsulation inside the hydrogels and cells were grown on top of the gel is used to demonstrate the photomodulation of cellular microenvironments. The added fluorescence turn-on response of the gels makes them even more attractive as smart biomaterials for spatially defined modulation of cellular microenvironments. Smart hydrogels that exhibit a function, for instance, in bio-sensing are attracting considerable interest and in this regard, pyrene-based, amino-acid-containing amphiphilic gelators which showed effective gelation properties in binary solvent/water mixtures and also in water were synthesized by Das et al. Such soft materials based on molecular assemblies may find uses in fluorescence sensors.149
A mechanically strong supramolecular hydrogel with up to 98.9% water content was synthesized from molecules with zwitterionic side chains. The hydrogel was thermally reversible, and can bind with DNA, thus may be usable in biological systems.
Yang et al. used the same process for the development of optically transparent and stable hydrogels, which were suitable for three dimensional (3D) cell environments. The fact that the gelator was very effective even at a very low concentration of about 0.1 wt% made it suitable for 3D cell encapsulation and culture of 3T3 cells. Mouse NIH/3T3 fibroblasts were selected and cultured for 24 h on compact nanofibrous films from 728 in order to demonstrate the possibility of using fluorescent nanofibers for visualizing the interaction with cells. At the same time, cell imaging was checked by using calcein-AM to stain the cells (Fig. 18). By fluorescent microscopic channels, the strong emission of the fluorescent nanofiber excitation at a wavelength of 360–370 nm and the stained cell excitation at 470–495 nm, an effective imaging of both cells (green) and nanofibers (blue) was directly accomplished. A better understanding of the interaction between the cells and nanofibers was gained by overlaying the images of fluorescent nanofibers and cells, making such fluorescent nanofibers a promising scaffold to reveal the interaction between substrates and cells.150
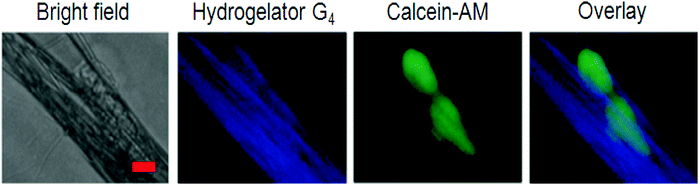 | ||
| Fig. 18 Fluorescence images of NIH-3T3 cells cultured on the nanofibrous films from 7 for 24 h. From left to right: the phase-contrast image, luminescence emission of the nanofibers under UV light (blue), live cells stained using calcein-AM (green), and the overlay image. Reproduced with permission.28 Copyright 2014, Royal Society of Chemistry. | ||
Controllable and mild gelation conditions were used for the synthesis of a salt responsive luminescent hydrogelator of tetraphenylethylene (TPE) via aggregation-induced emission (AIE), making it applicable for tissue engineering and bio-sensing. Interactions between cells and scaffolding materials have been extensively visualized by polymeric fluorescent nanofibers. Fluorescent gelators can be easily made by doping a π-conjugated moiety into the system, for example, quadrupole–quadrupole interactions between aromatic amino acid side chains and the fluorinated N-terminal π-conjugated system have been demonstrated to form supramolecular hydrogels.151 Many π-conjugated systems exhibit fluorescent properties in aqueous solution making it possible to develop self-supporting fluorescent hydrogels. Therefore, a small-molecule hydrogelator has been synthesized that can be used in high-contrast visualization of 3D live cell-material imaging at physiological pH. Due to AIE and with molecular rotation resistance between the phenyl groups, relatively higher fluorescence intensity can be obtained for the hydrogel.152
Biosensors play a vital role in rapid and high-throughput diagnostics or detection in situations where low cost, speed and ease are required, for example, biological markers, ions, gases, enzymes, etc. Selective molecular interactions can lead to a color change of hydrogels making stimulus-responsive smart supramolecular hydrogels. Supramolecular hydrogel-based sensors can work under aqueous conditions, which is of extreme importance because most biological substances remain active only under physiological conditions. Based on the host–guest interaction between a-CD and Tyloxapol, SFH were constructed with stimuli responsiveness towards small molecule additives, pH and temperature making them suitable candidates to be used as sensors.153 Coumarin-derived isomeric supramolecular fluorescent hydrogelators 5, 7 and 9 with different spatial structures were synthesized and the influence of the C–H–O bond on fluorescence was monitored. A study provides the methodology to develop biological targets of supramolecular self-assembly under external stimuli for cancer research. A highly photosensitive multi-functional gelator precursor was rationally designed, which can not only selectively target hepatocellular carcinoma (HCC) cells, but also form intracellular self-assembly of gelators triggered by light, consequently inducing cell death.154 Self-assembling behaviors of small peptides and the subsequent cellular responses can be illustrated by exchanging the positions of phosphorylated serine and tyrosine in the peptide backbone. Unique soft materials for potential biomedical applications can be made by utilizing D-amino acids. HepG2 cells (a type of HCC cell line) with a high ASGP-R expression level and HeLa cells (as control) with little expression of ASGP-R were selected to validate the efficiently targeted cancer cells of multi-functional gelator precursor 33 (Gal-BHMC) (Fig. 19a). Due to the specific interaction between galactose and asialoglycoprotein, the selectively targeted HepG2 cells and effective endocytosis inside cells were directly confirmed by fluorescence images under UV light because of the fluorescence emission properties of 34 Gal-BHMC (Fig. 19b).
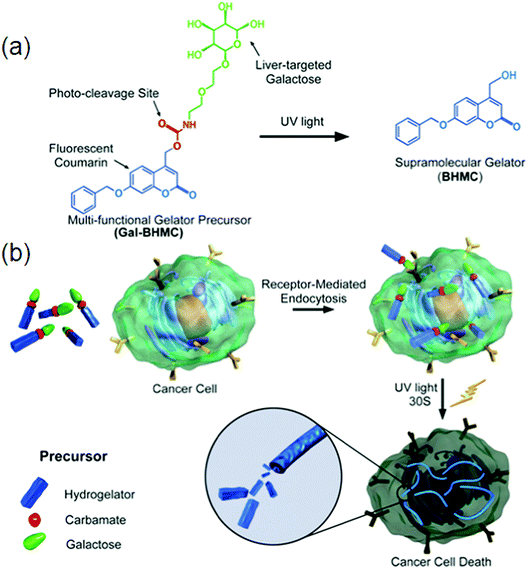 | ||
| Fig. 19 (a) Molecular structure of precursor Gal-BHMC and its conversion to gelator BHMC under UV light irradiation; (b) schematic demonstration of specific targeting of Gal-BHMC onto cancer cells and cell death induced by intracellular self-assembly triggered by light. Reproduced with permission.154 Copyright 2016, Royal Society of Chemistry. | ||
Supramolecular hydrogels based on amino acids and peptides may find promising applications in the fields of tissue engineering and pre-clinical evaluation. Synthetic customization of hydrogelators can be used to tune bioactive behaviors of hydrogels for culturing different cell lines in 3D.70 Fluorescent hydrogels with improved chemical stability, switchable photo-induced emission spectra, and white light fluorescent properties were synthesized by the self-assembly of peptides hyper-crosslinked via coordination bonds. Two self-assembly (SA) motifs, 35 and 36, were synthesized by linking together small molecular SA motifs, Fmoc-FF b and coumarin derivatives a and c through a photo-cleavable bond (Fig. 20).155 All three small SA units a, b, and c form self-assembled nanostructures through strong p–p stacking with the assistance of hydrogen bonding. A potential methodology for in situ observation of cell-related biological events and the critical function of the matrix in 3D biomimetic environments was done by a family of coumarin-based fluorescent hydrogelators. A NIR laser was used to excite the gelators which can be used as a promising biomimetic 3D ECM for visualizing cell–matrix interactions.156 Due to the advantages of low extents of photo-bleaching and photo-damage, two-photon fluorescence microscopy was employed to visualize the cells and nanofibrous structures (Fig. 21a). Cells emitting yellow fluorescence at 585 nm under excitation of 544 nm were also clearly imaged by two-photon fluorescence microscopy (Fig. 21b); blue-emitting nanofibers (emission at 446 nm) under the excitation of NIR light (750 nm) resulted in higher resolution images without light scattering (Fig. 21c) compared with the images upon UV light excitation, confirming clear visualization of the interaction between nanofibers and cells in a 2D environment (Fig. 21d).
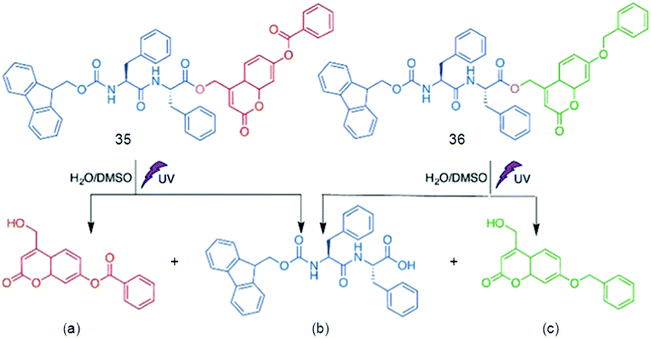 | ||
| Fig. 20 Chemical structures of 40, 41 and their photo-cleavage products (a and b), and (b and c), respectively. Reproduced with permission.155 Copyright 2017, Royal Society of Chemistry. | ||
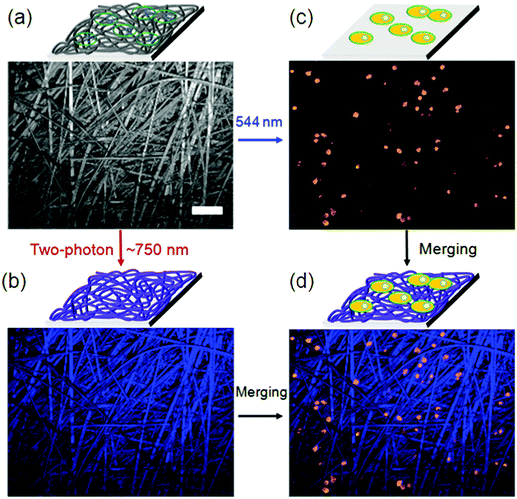 | ||
| Fig. 21 (a) Bright field image of the nanofibers cultured with A549 cells; (b) in situ fluorescence image of blue-emitting nanofibers under excitation of 750 nm; (c) in situ fluorescence image of yellow-emitting A549 cells under excitation of 544 nm; (d) merged cells and nanofibers image from (b) and (c). Scale bar represents 50 mm. Reproduced with permission.155 Copyright 2017, Royal Society of Chemistry. | ||
The result recommended that two-photon-excited nanofibers may be employed to image nanofiber-regulated cell behaviors in a 3D environment under non-invasive NIR light conditions, but that single-photon excitation (UV light) is not suitable. Furthermore, the non-cytotoxicity of coumarin-based gelators was verified by cell counting kit-8 (CCK-8) assays, which supplementarily attest the probability of using such supramolecular hydrogels in 3D bio-imaging.155
Along with that, SFH suffer from limited capability of deep tissue imaging and complicated fabrication routes, so, up conversion fluorescence based SFH are being made with enhanced mechanical properties and deep tissue imaging.157 Brightness and stability of SFH is another challenge on which some recent reports have been published. For in vivo fluorescence based imaging by SFH, NIR light is being tried as a viable alternative for fluorescence-based imaging to minimize scattering and absorption of light by biological molecules.158
Even though considerable advancement has been achieved, the performance of traditional SFH in real-world applications is strictly limited due to the ACQ effect. More recent attention has been focused on the preparation of SFH with AIE properties to be applied in bio-imaging. Huang et al. constructed a bio-inspired SFH by electrostatic interactions between poly(sodium p-styrenesulfonate) and the tetraphenylethene (TPE) derivative containing two quaternary ammonium cations, following the above mentioned concern. In the self-assembly process, AIE fluorescence properties are imparted to this particular SFH by aggregation of TPE, a typical chromophore. Besides, this SFH was responsive to bio-molecules.159 Since the TPE molecules have significant photophysical behavior, Lin and co-workers employed TPE/amino acid (Fig. 22a), i.e., TPE-Ser using Serine (Ser) and TPE-Asp by aspartic acid (Asp), for cell imaging purposes. The biocompatibility of the material used is necessary to be evaluated for the cell imaging application. Colorimetric MTT assay was used to study biocompatibility of TPE-Ser and TPE-Asp by incubating them with 3A6 cell lines for 48 h.159b The percentage viability of 3A6 cells treated with a choice of concentrations of TPE-samples ranging from 10–50 mM is shown in Fig. 22b and c. Both TPE-Ser and TPE-Asp samples showed a reasonable level of viability at 50 mM.159c
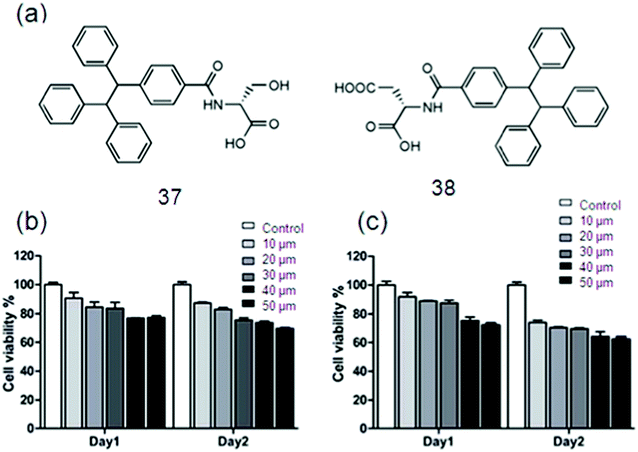 | ||
| Fig. 22 (a) The chemical structures of 42 and 43. Cell viability data of 3A6 cells incubated with 10–50 mM of (b) 42 and (c) 43. Reproduced with permission.159 Copyright 2018, Royal Society of Chemistry. | ||
Transfer of both chirality and energy information plays an important role in biological systems. Circularly polarized light (CPL) has long been used as a factor to determine a product's chirality; recently achiral polymethacrylate with achiral azobenzene has been investigated. The generation, inversion and switching of azobenzene chirality using irradiation with monochromatic incoherent CPL were explained by results.160
Energy transfer is realized in a composite nanohelix system produced by a chiral donor and an achiral acceptor. The achiral acceptor showed both supramolecular chirality and energy transfer amplified circularly polarized luminescence by capturing the chirality and circularly polarized energy from the chiral donor.161 Theoretical simulations are needed to provide new insight into the chirality of CPL-controlled SFH.
5. Conclusions
In this review, an overview of different SFH is presented and their use in bio-imaging i.e., cell imaging, which is certainly driven by their tunable properties, biocompatible chemical components, easy preparation methods, intelligent responses to stimulus, and suitable inner-structures. The ability to introduce multiple and complex cell signaling structures in these hydrogels can stimulate cell imaging.In spite of the major growth of SFH, certain challenges persist to be solved, counting the chirality of the hydrogel matrix, molecular design, mechanical properties, water retention, toxicity, and so on. Since the self-assembly mechanism is not fully revealed the design methodology for these fluorescent gelator structures is still in the primary stage. On a collective note, following are the key issues to be solved for effective use of SFH in bio-imaging; (a) several fluorescent aromatic groups like naphthalenediimide, naphthalene, and pyrene have been incorporated into the hydrogelators to facilitate the self-assembly into nanofibers. However, when π-conjugated fluorophores aggregate, these fluorescent hydrogelators usually suffer from the aggregation-caused quenching (ACQ) problem associated with fluorescence quenching; therefore, this undesired ACQ effect should be minimized in the assemblies. (b) Generally, self-assembled nanofibers are much less than 50 nm in diameter, which makes visualization of cell-scaffolding materials difficult by CLSM because of the diffraction of light by the nanosized material, so if one aims to observe a high-contrast image by CLSM, supramolecular fibers with microsized diameter (>0.5 μm) might be needed. (c) The hydrogel should be formed at the physiological pH for the purpose of 3D cell imaging. (d) To support the mass of a cell, the storage modulus of the gel should be higher than 100 Pa. (e) The gel-to-sol transition temperature should be higher than 37 °C to result in stable gels at physiologically relevant temperature. (f) The gel material should be biocompatible. (g) Scattering and absorption of light by biological matter should be minimized.
Chemical and mechanical stabilities of hydrogel networks are often affected by the introduction of chromophores, as most of the chromophores are hydrophobic and bulky, causing the destabilization of hydrogels. Moreover, leaking of the fluorescent materials plus the aggregation of the chromophores may cause the reduction of luminescence and fluorescence quenching due to lack of direct chemical bonding of the dye molecules with the hydrogel network.162 Hence, generation of bright and stable SFH for bio-imaging remains a significant challenge. The complexation of gelators with metal will minimize the issue of quenching due to the covalent linkage of the ligand with the gelator. Such kind of strategy has two benefits, first, the aggregation and self-quenching of chromophores are prevented due to the fixed position of metal ions in the hydrogel network; second, additional interactions are introduced by the formation of metal–ligand complexes to stabilize the hydrogel network, making the FH even more stable after the incorporation of metal ions.163 Another way of reducing ACQ is by using the AIE phenomenon, which is shown by some organic luminophores; for instance, tetraphenylethene (TPE)164 having freely-rotating groups to consume energy and promote radiation-less decay after excitation in solution. Free rotation of these groups is restricted in the aggregated or crystallized state with higher photoluminescence efficiency (i.e., quantum yield) than the solution phase.
Applications of SFH in the fields of biosensor and tissue repair are limited due to the spontaneous water release from them (syneresis) and limited recyclability. To avoid this critical issue, increasing and tuning the mechanical properties of these hydrogels by appropriately designing gelator structures, followed by the production of hydrogel networks (e.g., crosslinking reaction), is of great significance. Slight mechanical disturbances like agitation may cause the disassembly of the structure and dissolution of SFH, and in turn, limit their further practical application in bio-imaging.
6. Future perspectives
Helical motifs (e.g., DNA or proteins) are very common in many biomolecular systems, where they perform helicity inversion in many physiological processes along with specific biofunctional trans-formations.165 The hybrid co-assembly of fluorophores (fluorescence characteristics) with chiral gelators will result in a highly selective imaging tool for exact view of the spatial organization of components in 3D cells, further pushing forward to new bio-mimetic SFH.166 So, interdisciplinary combination will enable the design and development of next-generation SFH for the purpose of being used in bio-imaging.The dynamic interactions between fluorescent gels and cell culture are to be understood for the mechanism of gelation and cell interaction. The internal microstructures of gelators play a very crucial role in defining the macroscopic physiological properties such as bioactivity of the resulting supramolecular gels. Most of the existing SFH have irregular internal microstructures due to uncontrolled cross-linking behavior driven by multivalent noncovalent interactions. Consequently, it is necessary to rationally select driving forces and self-assembly techniques to form spatially ordered supramolecular hydrogels such as hierarchical self-assembly with well-defined amphiphilic molecular building blocks. Ligand metal complex based gelators for example,61 with single molecule width, can be used to elucidate the interaction between supramolecular nanofibers and proteins in live cells due to the long fluorescence lifetime of [Ru(bipy)3]2+ derivatives.167
Biocompatibility of SFH should also be considered for in vivo and in vitro cell culture.161 The size of supramolecular hydrogels has a significant influence on their biological performances in cells or tissues, like cytotoxicity,168,169 but multivalent supramolecular cross-linking often enables continuous spread of the cross-linked networks, thus leading to the formation of macrohydrogels. The development of a suitable, flexible, and integrated synthetic approach for the control of the hydrogel size has become urgent. For instance, the introduction of competitive solvation groups can simply adjust the equilibrium between the propagation and solvation of the cross-linked network fragments, letting subjective control over the sizes of hydrogels.55 Naturally occurring fluorophores can be a better choice to increase the stability and biocompatibility of SFH, due to their exceptional stability and high quantum yield. One such example having a fluorescent signature is silk cocoon biomaterials. Among them, white silk has been extensively explored in tissue engineering due to its high mechanical strength and biocompatibility.170
For in vivo fluorescence based imaging by supramolecular hydrogelators, another most important challenge is the scattering and absorption of light by biological molecules. Visible light (400–650 nm) is intensely attenuated by different biomolecules, rendering deep tissue imaging all but impossible in this spectral region. In addition, strong auto-fluorescence throughout the visible spectrum is shown by biologically important molecules like collagen, fatty acids, and flavins. Henceforth, near-infrared biological windows from 650 nm to 950 nm (NIR1) and 1000 nm to 1350 nm (NIR2) will be given special attention to be used for in vivo applications due to deeper light penetration to biological systems,147g slow absorption by water and weaker endogenous fluorescence of biomolecules in these spectral regions. Consequently, near-infrared light can be a viable alternative for SFH-based imaging.171
Highly ordered materials are being engineered by using self-assembly processes which are often the consequence of a delicate relationship concerning thermodynamic and kinetic processes in a complex equilibrium landscape.172 So, research on SFH will lead to the integration of molecular science with bioinformatics, and contribute to the use of molecules for better human life by moving from molecules to processes,173 from thermodynamics to kinetics.174 Transfer of both chirality and energy from the molecular to the nanoscale level is crucial for implementation of catalytic activity, and recognition in the life process.175 Hence, functional chiroptical materials can be designed by deep research on chirality and energy i.e. multichannel communications.161 By using fluorescent gelators along with CPL sources at multiple wavelengths, rather than at a single wavelength,160 can result in supramolecular fluorescent assembly with inversion, chiroptical polarization and long term memory to be applied in bio-imaging.176 Vis-IR mediated fluorescence imaging can be achieved via SFH having CPL (with VIS-IR transmission enhancement)177 active groups for cell culture environments so as to avoid the damage from UV light irradiation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Innovation Program of Shanghai Municipal Education Commission (201701070002E00061), the NSFC (51833006, 51573092), and Program for Professors of Special Appointment (Eastern Scholar) at the Shanghai Institutions of Higher Learning.References
- (a) S. Jiang, K. Y. Win, S. Liu, C. P. Teng, Y. Zheng and M. Y. Han, Nanoscale, 2013, 5, 3127 RSC; (b) J. H. Kim, K. Park, H. Y. Nam, S. Lee, K. Kim and I. C. Kwon, Prog. Polym. Sci., 2007, 32, 1031 CrossRef CAS.
- C. Ren, J. Zhang, M. Chen and Z. Yang, Chem. Soc. Rev., 2014, 43, 7257 RSC.
- (a) J. Zhang, C. Ou, Y. Shi, L. Wang, M. Chen and Z. Yang, Chem. Commun., 2014, 50, 12873 RSC; (b) W. C. Chan, D. J. Maxwell, X. Gao, R. E. Bailey, M. Han and S. Nie, Curr. Opin. Biotechnol., 2002, 13, 40 CrossRef CAS PubMed.
- X. D. Xu, B. B. Lin, J. Feng, Y. Wang, S. X. Cheng, X. Z. Zhang and R. X. Zhuo, Macromol. Rapid Commun., 2012, 33, 426 CrossRef CAS PubMed.
- Y. Zhang, B. Zhang, Y. Kuang, Y. Gao, J. Shi, X. X. Zhang and B. Xu, J. Am. Chem. Soc., 2013, 135, 5008 CrossRef CAS PubMed.
- J. Boekhoven and S. I. Stupp, Adv. Mater., 2014, 26, 1642 CrossRef CAS PubMed.
- M. He, J. Li, S. Tan, R. Wang and Y. Zhang, J. Am. Chem. Soc., 2013, 135, 18718 CrossRef CAS PubMed.
- J. Li, Y. Gao, Y. Kuang, J. Shi, X. Du, J. Zhou, H. Wang, Z. Yang and B. Xu, J. Am. Chem. Soc., 2013, 135, 9907 CrossRef CAS PubMed.
- Y. Loo, Y.-C. Wong, E. Z. Cai, C. H. Ang, A. Raju, A. Lakshmanan, A. G. Koh, H. J. Zhou, T.-C. Lim and S. M. Moochhala, Biomaterials, 2014, 35, 4805 CrossRef CAS PubMed.
- J. J. Moon, J. E. Saik, R. A. Poche, J. E. Leslie-Barbick, S.-H. Lee, A. A. Smith, M. E. Dickinson and J. L. West, Biomaterials, 2010, 31, 3840 CrossRef CAS PubMed.
- (a) N. Yan, G. He, H. Zhang, L. Ding and Y. Fang, Langmuir, 2009, 26, 5909 CrossRef PubMed; (b) S. Srinivasan, P. A. Babu, S. Mahesh and A. Ajayaghosh, J. Am. Chem. Soc., 2009, 131, 15122 CrossRef CAS PubMed.
- B. Xing, C. W. Yu, K. H. Chow, P. L. Ho, D. Fu and B. Xu, J. Am. Chem. Soc., 2002, 124, 14846 CrossRef CAS PubMed.
- E. Busseron, Y. Ruff, E. Moulin and N. Giuseppone, Nanoscale, 2013, 5, 7098 RSC.
- Z. Yang, K. Xu, L. Wang, H. Gu, H. Wei, M. Zhang and B. Xu, Chem. Commun., 2005, 4414 RSC.
- H. Shibata, Y. J. Heo, T. Okitsu, Y. Matsunaga, T. Kawanishi and S. Takeuchi, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 17894 CrossRef CAS PubMed.
- Y. J. Heo, H. Shibata, T. Okitsu, T. Kawanishi and S. Takeuchi, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 13399 CrossRef CAS PubMed.
- T. Kawanishi, M. Romey, P. Zhu, M. Holody and S. Shinkai, J. Fluoresc., 2004, 14, 499 CrossRef CAS PubMed.
- X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165 CrossRef CAS PubMed.
- B. W. Rice, M. D. Cable and M. B. Nelson, J. Biomed. Opt., 2001, 6, 432 CrossRef CAS PubMed.
- M. Chen and M. Yin, Prog. Polym. Sci., 2014, 39, 365 CrossRef CAS.
- J. Li and J.-J. Zhu, Analyst, 2013, 138, 2506 RSC.
- X. Zhang, X. Zhang, L. Tao, Z. Chi, J. Xu and Y. Wei, J. Mater. Chem. B, 2014, 2, 4398 RSC.
- R. Dong, Y. Pang, Y. Su and X. Zhu, Biomater. Sci., 2015, 3, 937 RSC.
- T. Nagano, Proc. Jpn. Acad., Ser. B, 2010, 86, 837 CrossRef CAS PubMed.
- A. Griffith, T. J. Bandy, M. Light and E. Stulz, Chem. Commun., 2013, 49, 731 RSC.
- M. Asai, K. Sugiyasu, N. Fujita and S. Shinkai, Chem. Lett., 2004, 33, 120 CrossRef CAS.
- M. Amaike, H. Kobayashi and S. Shinkai, Chem. Lett., 2001, 620 CrossRef CAS.
- M. Amaike, H. Kobayashi, K. Sakurai and S. Shinkai, Supramol. Chem., 2002, 14, 245 CrossRef CAS.
- W. Ji, G. Liu, M. Xu, X. Dou and C. Feng, Chem. Commun., 2014, 50, 15545 RSC.
- M. Kumar and S. J. George, Nanoscale, 2011, 3, 2130 RSC.
- T. H. Kim, J. Seo, S. J. Lee, S. S. Lee, J. Kim and J. H. Jung, Chem. Mater., 2007, 19, 5815 CrossRef CAS.
- J. Ahn, S. Park, J. H. Lee, S. H. Jung, S. J. Moon and J. H. Jung, Chem. Commun., 2013, 49, 2109 RSC.
- K. J. Channon, G. L. Devlin, S. W. Magennis, C. E. Finlayson, A. K. Tickler, C. Silva and C. E. MacPhee, J. Am. Chem. Soc., 2008, 130, 5487 CrossRef CAS PubMed.
- K. Fan, H. Kong, X. Wang, X. Yang and J. Song, RSC Adv., 2016, 6, 80934 RSC.
- A. Friggeri, O. Gronwald, K. J. Van Bommel, S. Shinkai and D. N. Reinhoudt, J. Am. Chem. Soc., 2002, 124, 10754 CrossRef CAS PubMed.
- S. H. Jung, H. Lee, S. Park and J. H. Jung, New J. Chem., 2012, 36, 1957 RSC.
- R. N. Das, Y. P. Kumar, S. Pagoti, A. J. Patil and J. Dash, Chem. – Eur. J., 2012, 18, 6008 CrossRef CAS PubMed.
- M. Montalti, L. S. Dolci, L. Prodi, N. Zaccheroni, M. C. Stuart, K. J. van Bommel and A. Friggeri, Langmuir, 2006, 22, 2299 CrossRef CAS PubMed.
- R. Nishiyabu, H. Kobayashi and Y. Kubo, RSC Adv., 2012, 2, 6555 RSC.
- S. S. Babu, V. K. Praveen, S. Prasanthkumar and A. Ajayaghosh, Chem. – Eur. J., 2008, 14, 9577 CrossRef CAS PubMed.
- K. Haraguchi, Curr. Opin. Solid State Mater. Sci., 2007, 11, 47 CrossRef CAS.
- K. V. Rao, K. Datta, M. Eswaramoorthy and S. J. George, Angew. Chem., 2011, 123, 1211 CrossRef.
- A. Del Guerzo, A. G. Olive, J. Reichwagen, H. Hopf and J. P. Desvergne, J. Am. Chem. Soc., 2005, 127, 17984 CrossRef CAS PubMed.
- S. Mukhopadhyay, G. Krishnamoorthy and U. Maitra, J. Phys. Chem. B, 2003, 107, 2189 CrossRef CAS.
- S. H. Seo and J. Y. Chang, Chem. Mater., 2005, 17, 3249 CrossRef CAS.
- X. Fu, N. Wang, S. Zhang, H. Wang and Y. Yang, J. Colloid Interface Sci., 2007, 315, 376 CrossRef CAS PubMed.
- H. Wang, W. Zhang, X. Dong and Y. Yang, Talanta, 2009, 77, 1864 CrossRef CAS PubMed.
- R. Haldar, K. V. Rao, S. J. George and T. K. Maji, Chem. – Eur. J., 2012, 18, 5848 CrossRef CAS PubMed.
- T. Ozawa, H. Yoshimura and S. B. Kim, Anal. Chem., 2012, 85, 590 CrossRef PubMed.
- R. Abbel, R. van der Weegen, W. Pisula, M. Surin, P. Leclère, R. Lazzaroni, E. e. W. Meijer and A. P. Schenning, Chem. – Eur. J., 2009, 15, 9737 CrossRef CAS PubMed.
- X. Yang, R. Lu, P. Xue, B. Li, D. Xu, T. Xu and Y. Zhao, Langmuir, 2008, 24, 13730 CrossRef CAS PubMed.
- B. Liu, H. Nie, X. Zhou, S. Hu, D. Luo, D. Gao, J. Zou, M. Xu, L. Wang and Z. Zhao, Adv. Funct. Mater., 2016, 26, 776 CrossRef CAS.
- S. Zhang, Nat. Biotechnol., 2003, 21, 1171 CrossRef CAS PubMed.
- M. Du, W. Song, Y. Cui, Y. Yang and J. Li, J. Mater. Chem., 2011, 21, 2228 RSC.
- (a) X. Liu, D. Wu, H. Wang and Q. Wang, Adv. Mater., 2014, 26, 4370 CrossRef CAS PubMed; (b) C. Ren, H. Wang, D. Mao, X. Zhang, Q. Fengzhao, Y. Shi, D. Ding, D. Kong, L. Wang and Z. Yang, Angew. Chem., Int. Ed., 2015, 54, 4823 CrossRef CAS PubMed; (c) H. Wang, Z. Luo, Y. Wang, T. He, C. Yang, C. Ren, L. Ma, C. Gong, X. Li and Z. Yang, Adv. Funct. Mater., 2016, 26, 1822 CrossRef CAS; (d) Q. Wei, M. Xu, C. Liao, Q. Wu, M. Liu, Y. Zhang, C. Wu, L. Cheng and Q. Wang, Chem. Sci., 2016, 7, 2748 RSC; (e) Y. Zhang, R. Zhou, J. Shi, N. Zhou, I. R. Epstein and B. Xu, J. Phys. Chem. B, 2013, 117, 6566 CrossRef CAS PubMed.
- Q. Zhu, F. Qiu, B. Zhu and X. Zhu, RSC Adv., 2013, 3, 2071 RSC.
- H. Wang, S. Wang, H. Su, K. J. Chen, A. L. Armijo, W. Y. Lin, Y. Wang, J. Sun, K. i. Kamei and J. Czernin, Angew. Chem., Int. Ed., 2009, 48, 4344 CrossRef CAS PubMed.
- K. J. Chen, S. M. Wolahan, H. Wang, C. H. Hsu, H. W. Chang, A. Durazo, L. P. Hwang, M. A. Garcia, Z. K. Jiang and L. Wu, Biomaterials, 2011, 32, 2160 CrossRef CAS PubMed.
- R. Dong, H. Chen, D. Wang, Y. Zhuang, L. Zhu, Y. Su, D. Yan and X. Zhu, ACS Macro Lett., 2012, 1, 1208 CrossRef CAS.
- (a) I. Böhm, K. Isenbügel, H. Ritter, R. Branscheid and U. Kolb, Angew. Chem., Int. Ed., 2011, 50, 7407 CrossRef PubMed; (b) S. Ueda, H. Masutani, H. Nakamura, T. Tanaka, M. Ueno and J. Yodoi, Antioxid. Redox Signaling, 2002, 4, 405 CrossRef CAS PubMed; (c) S. Kirkham, I. W. Hamley, A. M. Smith, R. M. Gouveia, C. J. Connon, M. Reza and J. Ruokolainen, Colloids Surf., B, 2016, 137, 104 CrossRef CAS PubMed.
- (a) R. Dong, H. Chen, D. Wang, Y. Zhuang, L. Zhu, Y. Su, D. Yan and X. Zhu, ACS Macro Lett., 2012, 1, 1208 CrossRef CAS; (b) Y. Gao, J. Shi, D. Yuan and B. Xu, Nat. Commun., 2012, 3, 1033 CrossRef PubMed.
- (a) A. GhavamiNejad, M. SamariKhalaj, L. E. Aguilar, C. H. Park and C. S. Kim, Sci. Rep., 2016, 6, 33594 CrossRef CAS PubMed; (b) M. Sepantafar, R. Maheronnaghsh, H. Mohammadi, F. Radmanesh, M. M. Hasani-sadrabadi, M. Ebrahimi and H. Baharvand, Trends Biotechnol., 2017, 35, 1074 CrossRef CAS PubMed; (c) J. Basso, A. Miranda, S. Nunes, T. Cova, J. Sousa, C. Vitorino and A. Pais, Gels, 2018, 4, 62 CrossRef.
- S. Senapati, A. K. Mahanta, S. Kumar and P. Maiti, Signal Transduction Targeted Ther., 2018, 3, 7 CrossRef PubMed.
- (a) M. Ikeda, T. Yoshii, T. Matsui, T. Tanida, H. Komatsu and I. Hamachi, J. Am. Chem. Soc., 2011, 133, 1670 CrossRef CAS PubMed; (b) R. Ochi, K. Kurotani, M. Ikeda, S. Kiyonaka and I. Hamachi, Chem. Commun., 2013, 49, 2115 RSC; (c) A. Wada, S.-i. Tamaru, M. Ikeda and I. Hamachi, J. Am. Chem. Soc., 2009, 131, 5321 CrossRef CAS PubMed; (d) M. Ikeda, R. Ochi, A. Wada and I. Hamachi, Chem. Sci., 2010, 1, 491 RSC.
- C. Ren, H. Wang, X. Zhang, D. Ding, L. Wang and Z. Yang, Chem. Commun., 2014, 50, 3473 RSC.
- M. Ikeda, R. Ochi, A. Wada and I. Hamachi, Chem. Sci., 2010, 1, 491 RSC.
- (a) V. Jayawarna, M. Ali, T. A. Jowitt, A. F. Miller, A. Saiani, J. E. Gough and R. V. Ulijn, Adv. Mater., 2006, 18, 611 CrossRef CAS; (b) W. Bai, N. A. Gariano and D. A. Spivak, J. Am. Chem. Soc., 2013, 135, 6977 CrossRef CAS PubMed.
- N. Castellucci, G. Sartor, N. Calonghi, C. Parolin, G. Falini and C. Tomasini, Beilstein J. Org. Chem., 2013, 9, 417 CrossRef CAS PubMed.
- (a) Y. Zhang, Z. Yang, F. Yuan, H. Gu, P. Gao and B. Xu, J. Am. Chem. Soc., 2004, 126, 15028 CrossRef CAS PubMed; (b) Y. Gao, J. Shi, D. Yuan and B. Xu, Nat. Commun., 2012, 3, 1033 CrossRef PubMed; (c) Y. Gao, Y. Kuang, X. Du, J. Zhou, P. Chandran, F. Horkay and B. Xu, Langmuir, 2013, 29, 15191 CrossRef CAS PubMed.
- (a) J. Zhang and P. X. Ma, Adv. Drug Delivery Rev., 2013, 65, 1215 CrossRef CAS PubMed; (b) Z. Yang, H. Gu, D. Fu, P. Gao, J. K. Lam and B. Xu, Adv. Mater., 2004, 16, 1440 CrossRef CAS; (c) K. J. van Bommel, C. van der Pol, I. Muizebelt, A. Friggeri, A. Heeres, A. Meetsma, B. L. Feringa and J. van Esch, Angew. Chem., Int. Ed., 2004, 43, 1663 CrossRef CAS PubMed; (d) X. Q. Dou and C. L. Feng, Adv. Mater., 2017, 29, 1604062 CrossRef PubMed.
- (a) F. Zhao, M. L. Ma and B. Xu, Chem. Soc. Rev., 2009, 38, 883 RSC; (b) H. Wang and S. C. Heilshorn, Adv. Mater., 2015, 27, 3717 CrossRef CAS PubMed; (c) L. Sacconi, Pure Appl. Chem., 1971, 27, 161 CAS; (d) H. McEwen, E. Y. Du, J. P. Mata, P. Thordarson and A. D. Martin, J. Mater. Chem. B, 2017, 5, 9412 RSC; (e) J. Li, NPG Asia Mater., 2010, 2, 112 CrossRef; (f) V. Jayawarna, S. M. Richardson, A. R. Hirst, N. W. Hodson, A. Saiani, J. E. Gough and R. V. Ulijn, Acta Biomater., 2009, 5, 934 CrossRef CAS PubMed; (g) Y. Hong, M. D. Pritzker, R. L. Legge and P. Chen, Colloids Surf., B, 2005, 46, 152 CrossRef CAS PubMed; (h) X. Q. Dou, P. Li, S. Lu, X. Tian, Y. Tang, J. D. Mercer-Chalmers, C. L. Feng and D. Zhang, J. Mol. Liq., 2013, 180, 129 CrossRef CAS.
- (a) H. Su, Y. Wang, C. F. Anderson, J. M. Koo, H. Wang and H. Cui, Chin. J. Polym. Sci., 2017, 35, 1194 CrossRef CAS; (b) J. J. Panda, A. Mishra, A. Basu and V. S. Chauhan, Biomacromolecules, 2008, 9, 2244 CrossRef CAS PubMed; (c) A. Mujeeb, A. F. Miller, A. Saiani and J. E. Gough, Acta Biomater., 2013, 9, 4609 CrossRef CAS PubMed; (d) V. A. Kumar, N. L. Taylor, S. Shi, N. C. Wickremasinghe, R. N. D'Souza and J. D. Hartgerink, Biomaterials, 2015, 52, 71 CrossRef CAS PubMed; (e) M. K. Kang, J. S. Colombo, R. N. D’Souza and J. D. Hartgerink, Biomacromolecules, 2014, 15, 2004 CrossRef CAS PubMed; (f) M. Kabiri, I. Bushnak, M. T. McDermot and L. D. Unsworth, Biomacromolecules, 2013, 14, 3943 CrossRef CAS PubMed; (g) E. L. Bakota, Y. Wang, F. R. Danesh and J. D. Hartgerink, Biomacromolecules, 2011, 12, 1651 CrossRef CAS PubMed; (h) A. Baeissa, N. Dave, B. D. Smith and J. Liu, ACS Appl. Mater. Interfaces, 2010, 2, 3594 CrossRef CAS PubMed.
- (a) W. Deng, H. Yamaguchi, Y. Takashima and A. Harada, Chem. – Asian J., 2008, 3, 687 CrossRef CAS PubMed; (b) M. Guo, M. Jiang, S. Pispas, W. Yu and C. Zhou, Macromolecules, 2008, 41, 9744 CrossRef CAS; (c) W. Ha, J. Yu, X. y. Song, J. Chen and Y. p. Shi, ACS Appl. Mater. Interfaces, 2014, 6, 10623 CrossRef CAS PubMed; (d) W. Ha, J. Yu, X. y. Song, Z. j. Zhang, Y. q. Liu and Y. p. Shi, J. Mater. Chem. B, 2013, 1, 5532 RSC; (e) J. Li, X. Li, Z. Zhou, X. Ni and K. W. Leong, Macromolecules, 2001, 34, 7236 CrossRef CAS; (f) J. Liu, G. Chen, M. Guo and M. Jiang, Macromolecules, 2010, 43, 8086 CrossRef CAS; (g) J. Liu, G. Chen and M. Jiang, Macromolecules, 2011, 44, 7682 CrossRef CAS; (h) C. Tang, A. M. Smith, R. F. Collins, R. V. Ulijn and A. Saiani, Langmuir, 2009, 25, 9447 CrossRef CAS PubMed; (i) S. Yagai and A. Kitamura, Chem. Soc. Rev., 2008, 37, 1520 RSC; (j) I. Yoshimura, Y. Miyahara, N. Kasagi, H. Yamane, A. Ojida and I. Hamachi, J. Am. Chem. Soc., 2004, 126, 12204 CrossRef CAS PubMed.
- (a) E. A. Appel, F. Biedermann, U. Rauwald, S. T. Jones, J. M. Zayed and O. A. Scherman, J. Am. Chem. Soc., 2010, 132, 14251 CrossRef CAS PubMed; (b) E. A. Appel, X. J. Loh, S. T. Jones, C. A. Dreiss and O. A. Scherman, Biomaterials, 2012, 33, 4646 CrossRef CAS PubMed; (c) L. Chen, X. Zhao, Y. Lin, Z. Su and Q. Wang, Polym. Chem., 2014, 5, 6754 RSC; (d) P. S. Jong, J. Sooyeon, C. W. Dong, K. P. Jae, K. Ketack, P. Eun-Kyoung and S. Ki-Won, Chem. Commun., 2011, 47, 4736 RSC; (e) D. González-Rodríguez and A. P. Schenning, Chem. Mater., 2010, 23, 310 CrossRef; (f) L. Teng and M. Xiang, Dyes Pigm., 2018, 148, 306 CrossRef.
- (a) G. F. Liu, W. Ji, W. L. Wang and C. L. Feng, ACS Appl. Mater. Interfaces, 2014, 7, 301 CrossRef PubMed; (b) K. Peng, I. Tomatsu and A. Kros, Chem. Commun., 2010, 46, 4094 RSC.
- (a) M. Balaz, S. Tannir and K. Varga, Coord. Chem. Rev., 2017, 349, 66 CrossRef CAS; (b) L. J. Prins, D. N. Reinhoudt and P. Timmerman, Angew. Chem., Int. Ed., 2001, 40, 2382 CrossRef CAS; (c) P. G. A. Janssen, N. J. M. Brankaert, X. Vila and A. P. H. J. Schenning, Soft Matter, 2010, 6, 1494 RSC; (d) H. Jung, J. S. Park, J. Yeom, N. Selvapalam, K. M. Park, K. Oh, J. A. Yang, K. H. Park, S. K. Hahn and K. Kim, Biomacromolecule, 2014, 15, 707 CrossRef CAS PubMed.
- (a) C. Bao, R. Lu, M. Jin, P. Xue, C. Tan, G. Liu and Y. Zhao, Org. Biomol. Chem., 2005, 3, 2508 RSC; (b) D. Bardelang, M. Zaman, I. L. Moudrakovski, S. Pawsey, J. C. Margeson, D. Wang, X. Wu, J. A. Ripmeester, C. I. Ratcliffe and K. Yu, Adv. Mater., 2008, 20, 4517 CrossRef CAS; (c) K. L. Morris, L. Chen, J. Raeburn, O. R. Sellick, P. Cotanda, A. Paul, P. C. Griffiths, S. M. King, R. K. O’Reilly and L. C. Serpell, Nat. Commun., 2013, 4, 1480 CrossRef PubMed; (d) A. Wu, F. Lu, P. Sun, X. Qiao, X. Gao and L. Zheng, Langmuir, 2017, 33, 13982 CrossRef CAS PubMed.
- A. Cayuela, M. Soriano, C. Carrillo-Carrion and M. Valcarcel, Chem. Commun., 2016, 52, 1311 RSC.
- (a) G. Clavier, J. Mater. Chem., 1998, 8, 2575 RSC; (b) C. Ruiz-Palomero, S. Benítez-Martínez, M. L. Soriano and M. Valcárcel, Anal. Chim. Acta, 2017, 974, 93 CrossRef CAS PubMed; (c) C. L. Wong, C. T. Poon and V. W. W. Yam, Organometallics, 2017, 36, 2661 CrossRef CAS.
- W. Ji, G. Liu, Z. Li and C. Feng, ACS Appl. Mater. Interfaces, 2016, 8, 5188 CrossRef CAS PubMed.
- J. Eastoe, M. Sánchez-Dominguez, P. Wyatt and R. K. Heenan, Chem. Commun., 2004, 2608 RSC.
- J. W. Chung, S. J. Yoon, S. J. Lim, B. K. An and S. Y. Park, Angew. Chem., Int. Ed., 2009, 48, 7030 CrossRef CAS PubMed.
- G. Clavier, J. Pozzo, H. Bouas-Laurent, C. Liere, C. Roux and C. Sanchez, J. Mater. Chem., 2000, 10, 1725 RSC.
- L. Zhang, S. R. Jean, S. Ahmed, P. M. Aldridge, X. Li, F. Fan and S. O. Kelley, Nat. Commun., 2017, 8, 381 CrossRef PubMed.
- (a) J. A. Foster, R. M. Edkins, G. J. Cameron, N. Colgin, K. Fucke, S. Ridgeway, A. G. Crawford, T. B. Marder, A. Beeby and S. L. Cobb, Chem. – Eur. J., 2014, 20, 279 CrossRef CAS PubMed; (b) N. Fujita, M. Asai, T. Yamashita and S. Shinkai, J. Mater. Chem., 2004, 14, 2106–2114 RSC; (c) N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821 RSC; (d) F. Würthner, B. Hanke, M. Lysetska, G. Lambright and G. S. Harms, Org. Lett., 2005, 7, 967 CrossRef PubMed.
- (a) B. Adhikari, J. Nanda and A. Banerjee, Chem. – Eur. J., 2011, 17, 11488 CrossRef CAS PubMed; (b) A. Mahler, M. Reches, M. Rechter, S. Cohen and E. Gazit, Adv. Mater., 2006, 18, 1365 CrossRef CAS; (c) M. Singh, S. Kundu, V. Sreekanth, R. K. Motiani, S. Sengupta, A. Srivastava and A. Bajaj, Nanoscale, 2014, 21, 12849 RSC; (d) X. Sun, G. Li, Y. Yin, Y. Zhang and H. Li, Soft Matter, 2018, 14, 6983 RSC.
- (a) P. Xue, T. He, H. Wu, H. Xie, R. Shen, F. Yue, J. Wang and Y. Zhang, Supramol. Chem., 2017, 29, 627 CrossRef CAS; (b) M. Ikeda, T. Yoshii, T. Matsui, T. Tanida, H. Komatsu and I. Hamachi, J. Am. Chem. Soc., 2011, 133, 1670 CrossRef CAS PubMed.
- L. Z. Zhao, C. H. Zhou, J. Wang, D. S. Tong, W. H. Yu and H. Wang, Soft Matter, 2015, 11, 9229 RSC.
- (a) T. Liu and S. Liu, Anal. Chem., 2011, 83, 2775 CrossRef CAS PubMed; (b) N. Shi, G. Yin, M. Han and Z. Xu, Colloids Surf., B, 2008, 66, 84 CrossRef CAS PubMed; (c) S. Y. Ryu, S. Kim, J. Seo, Y. W. Kim, O. H. Kwon, D. J. Jang and S. Y. Park, Chem. Commun., 2004, 70 RSC.
- (a) E. Calatrava-Pérez, S. A. Bright, S. Achermann, C. Moylan, M. O. Senge, E. B. Veale, D. C. Williams, T. Gunnlaugsson and E. M. Scanlan, Chem. Commun., 2016, 52, 13086 RSC; (b) R. B. Elmes, M. Erby, S. A. Bright, D. C. Williams and T. Gunnlaugsson, Chem. Commun., 2012, 48, 2588 RSC; (c) S. Roy, A. Dasgupta and P. K. Das, Langmuir, 2007, 23, 11769 CrossRef CAS PubMed.
- (a) S. Dutta, A. Shome, S. Debnath and P. K. Das, Soft Matter, 2009, 5, 1607 RSC; (b) P. Gao, L. Z. Liu, C. L. Zhan, Y. B. Zhou and M. H. Liu, Acta Chim. Sin., 2004, 62, 895 CAS; (c) M. Suzuki, S. Owa, M. Yumoto, M. Kimura, H. Shirai and K. Hanabusa, Tetrahedron Lett., 2004, 45, 5399 CrossRef CAS; (d) M. Suzuki, M. Yumoto, M. Kimura, H. Shirai and K. Hanabusa, Tetrahedron Lett., 2004, 45, 2947 CrossRef CAS; (e) M. Suzuki, M. Yumoto, M. Kimura, H. Shirai and K. Hanabusa, Chem. Lett., 2004, 33, 1496 CrossRef CAS.
- S. Cao, X. Fu, N. Wang, H. Wang and Y. Yang, Int. J. Pharm., 2008, 357, 95 CrossRef CAS PubMed.
- (a) Q. Wang, L. Li and B. Xu, Chem. – Eur. J., 2009, 15, 3168 CrossRef CAS PubMed; (b) Q. Wang, Z. Yang, M. Ma, C. K. Chang and B. Xu, Chem. – Eur. J., 2008, 14, 5073 CrossRef CAS PubMed; (c) Z. Yang, L. Wang, J. Wang, P. Gao and B. Xu, J. Mater. Chem., 2010, 20, 2128 RSC.
- A. Dawn, T. Shiraki, S. Haraguchi, H. Sato, K. Sada and S. Shinkai, Chem. – Eur. J., 2010, 16, 3676 CrossRef CAS PubMed.
- B. Adhikari, J. Nanda and A. Banerjee, Soft Matter, 2011, 7, 8913 RSC.
- A. Dawn, N. Fujita, S. Haraguchi, K. Sada, S. i. Tamaru and S. Shinkai, Org. Biomol. Chem., 2009, 7, 4378 RSC.
- X. Peng, H. Ting, W. Huiqiong, X. Hongtao, S. Rujuan, Y. Fan, W. Jide and Z. Yi, Supramol. Chem., 2017, 29, 627 CrossRef.
- (a) A. Gasnier, G. Royal and P. Terech, Langmuir, 2009, 25, 8751 CrossRef CAS PubMed; (b) A. Kishimura, T. Yamashita and T. Aida, J. Am. Chem. Soc., 2005, 127, 179 CrossRef CAS PubMed; (c) D. Li, Q. Zhang, X. Wang, S. Li, H. Zhou, J. Wu and Y. Tian, Dyes Pigm., 2015, 120, 175 CrossRef CAS; (d) A. Y. Y. Tam, K. M. C. Wong and V. W.-W. Yam, J. Am. Chem. Soc., 2009, 131, 6253 CrossRef CAS PubMed; (e) J. H. van Esch and B. L. Feringa, Angew. Chem., Int. Ed., 2000, 39, 2263 CrossRef CAS.
- G. O. Lloyd and J. W. Steed, Nat. Chem., 2009, 1, 437 CrossRef CAS PubMed.
- (a) O. Baranova, S. Khizhnyak and P. Pakhomov, J. Struct. Chem., 2014, 55, 169 CrossRef CAS; (b) P. Casuso, P. Carrasco, I. Loinaz, H. J. Grande and I. Odriozola, Org. Biomol. Chem., 2010, 8, 5455 RSC; (c) I. Odriozola, P. Casuso, I. Loinaz, G. Cabañero and H. J. Grande, Org. Biomol. Chem., 2011, 9, 5059 RSC; (d) J.-S. Shen, D. H. Li, Q. G. Cai and Y. B. Jiang, J. Mater. Chem., 2009, 19, 6219 RSC; (e) P. Yadav and A. Ballabh, RSC Adv., 2014, 4, 563 RSC.
- (a) S. Bhowmik, S. Banerjee and U. Maitra, Chem. Commun., 2010, 46, 8642 RSC; (b) L. C. Cai, Y. J. Wang, J. Li and J. B. Huang, Acta Phys.-Chim. Sin., 2012, 28, 2298 CAS; (c) A. Chakrabarty, U. Maitra and A. D. Das, J. Mater. Chem., 2012, 22, 18268 RSC; (d) F. F. da Silva, F. L. de Menezes, L. L. da Luz and S. Alves, New J. Chem., 2014, 38, 893 RSC; (e) P. Duan, N. Yanai, H. Nagatomi and N. Kimizuka, J. Am. Chem. Soc., 2015, 137, 1887 CrossRef CAS PubMed; (f) Y. Qiao, Y. Lin, Z. Yang, H. Chen, S. Zhang, Y. Yan and J. Huang, J. Phys. Chem. B, 2010, 114, 11725 CrossRef CAS PubMed; (g) Y. Qiao, Y. Lin, S. Zhang and J. Huang, Chem. – Eur. J., 2011, 17, 5180 CrossRef CAS PubMed; (h) J.-S. Shen, G. J. Mao, Y. H. Zhou, Y. B. Jiang and H. W. Zhang, Dalton Trans., 2010, 39, 7054 RSC; (i) M. Suzuki, M. Yumoto, H. Shirai and K. Hanabusa, Org. Biomol. Chem., 2005, 3, 3073 RSC; (j) H. Wang, X. Li, F. Fang and Y. Yang, Dalton Trans., 2010, 39, 7294 RSC; (k) Y. Zheng, Y. Li, C. Tan and Q. Wang, Photochem. Photobiol., 2011, 87, 641 CrossRef CAS PubMed.
- (a) M. Carraro, A. Sartorel, G. Scorrano, C. Maccato, M. H. Dickman, U. Kortz and M. Bonchio, Angew. Chem., Int. Ed., 2008, 47, 7275 CrossRef CAS PubMed; (b) S. Comby, E. M. Surender, O. Kotova, L. K. Truman, J. K. Molloy and T. Gunnlaugsson, Inorg. Chem., 2013, 53, 1867 CrossRef PubMed; (c) D. Dupin, R. Gavara, J. Llorca, J. C. Lima and L. Rodríguez, Chem. Commun., 2013, 49, 72 RSC; (d) Z. He, H. Ai, B. Li and L. Wu, Chin. Sci. Bull., 2012, 57, 4304 CrossRef CAS; (e) S. A. Joshi and N. D. Kulkarni, Chem. Commun., 2009, 2341 RSC; (f) X. Ma, D. Yu, N. Tang and J. Wu, Dalton Trans., 2014, 43, 9856 RSC; (g) A. J. Moro, B. Rome, E. Aguiló, J. Arcau, R. Puttreddy, K. Rissanen, J. C. Lima and L. Rodríguez, Org. Biomol. Chem., 2015, 13, 2026 RSC; (h) X. S. Xiao, W. Lu and C. M. Che, Chem. Sci., 2014, 5, 2482 RSC; (i) P. Xue, B. Yao, J. Sun, Z. Zhang and R. Lu, Chem. Commun., 2014, 50, 10284 RSC.
- (a) S. J. Bradberry, A. J. Savyasachi, R. D. Peacock and T. Gunnlaugsson, Faraday Discuss., 2015, 185, 413 RSC; (b) Y. Zhang, B. Zhang, Y. Kuang, Y. Gao, J. Shi, X. X. Zhang and B. Xu, J. Am. Chem. Soc., 2013, 135, 5008 CrossRef CAS PubMed.
- (a) A. Baral, S. Roy, A. Dehsorkhi, I. W. Hamley, S. Mohapatra, S. Ghosh and A. Banerjee, Langmuir, 2014, 30, 929 CrossRef CAS PubMed; (b) W. Lu, Y. C. Law, J. Han, S. S. Y. Chui, D. L. Ma, N. Zhu and C. M. Che, Chem. – Asian J., 2008, 3, 59 CrossRef CAS PubMed; (c) X. Wang, T. He, L. Yang, H. Wu, R. Zhang, Z. Zhang, R. Shen, J. Xiang, Y. Zhang and C. Wei, Nanoscale, 2016, 8, 6479 RSC.
- S. Y. Qin, Y. F. Chu, L. Tao, S.-S. Xu, Z. Y. Li, R. X. Zhuo and X. Z. Zhang, Soft Matter, 2011, 7, 8635 RSC.
- (a) Y. Gao, Y. Kuang, Z. F. Guo, Z. Guo, I. J. Krauss and B. Xu, J. Am. Chem. Soc., 2009, 131, 13576 CrossRef CAS PubMed; (b) Y. Kuang, J. Shi, J. Li, D. Yuan, K. A. Alberti, Q. Xu and B. Xu, Angew. Chem., Int. Ed., 2014, 53, 8104 CrossRef CAS PubMed; (c) A. J. Lampkins, O. Abdul-Rahim, H. Li and R. K. Castellano, Org. Lett., 2005, 7, 4471 CrossRef CAS PubMed; (d) Q. Lin, P. P. Mao, Y. Q. Fan, P. P. Jia, J. Liu, Y. M. Zhang, H. Yao and T. B. Wei, Soft Matter, 2017, 13, 7360 RSC; (e) A. D. Martin, A. B. Robinson, A. F. Mason, J. P. Wojciechowski and P. Thordarson, Chem. Commun., 2014, 50, 15541 RSC; (f) A. S. Weingarten, R. V. Kazantsev, L. C. Palmer, M. McClendon, A. R. Koltonow, A. P. Samuel, D. J. Kiebala, M. R. Wasielewski and S. I. Stupp, Nat. Chem., 2014, 6, 964 CrossRef CAS PubMed; (g) C. Yang, D. Li, Z. Liu, G. Hong, J. Zhang, D. Kong and Z. Yang, J. Phys. Chem. B, 2011, 116, 633 CrossRef PubMed; (h) Y. Zhang, H. Gu, Z. Yang and B. Xu, J. Am. Chem. Soc., 2003, 125, 13680 CrossRef CAS PubMed.
- C. C. Tsou and S. S. Sun, Org. Lett., 2006, 8, 387 CrossRef CAS PubMed.
- (a) S. Bhattacharjee, B. Maiti and S. Bhattacharya, Nanoscale, 2016, 8, 11224 RSC; (b) S. Ghosh, X. Q. Li, V. Stepanenko and F. Würthner, Chem. – Eur. J., 2008, 14, 11343 CrossRef CAS PubMed; (c) M. Krysmann, V. Castelletto and I. W. Hamley, Soft Matter, 2007, 3, 1401 RSC.
- J. Wu, A. Chen, M. Qin, R. Huang, G. Zhang, B. Xue, J. Wei, Y. Li, Y. Cao and W. Wang, Nanoscale, 2015, 7, 1655 RSC.
- (a) D. Ding, D. Mao, K. Li, X. Wang, W. Qin, R. Liu, D. S. Chiam, N. Tomczak, Z. Yang and B. Z. Tang, ACS Nano, 2014, 8, 12620 CrossRef CAS PubMed; (b) V. R. Kondepati, H. M. Heise and J. Backhaus, Anal. Bioanal. Chem., 2008, 390, 125 CrossRef CAS PubMed; (c) X. Q. Li, X. Zhang, S. Ghosh and F. Würthner, Chem. – Eur. J., 2008, 14, 8074 CrossRef CAS PubMed.
- H. Wang, J. Liu, A. Han, N. Xiao, Z. Xue, G. Wang, J. Long, D. Kong, B. Liu and Z. Yang, ACS Nano, 2014, 8, 1475 CrossRef CAS PubMed.
- Y. Geng, P. Dalhaimer, S. Cai, R. Tsai, M. Tewari, T. Minko and D. E. Discher, Nat. Nanotechnol., 2007, 2, 249 CrossRef CAS PubMed.
- D. Ding, Z. Zhu, R. Li, X. Li, W. Wu, X. Jiang and B. Liu, ACS Nano, 2011, 5, 2520 CrossRef CAS PubMed.
- H. Wang, D. Mao, Y. Wang, K. Wang, X. Yi, D. Kong, Z. Yang, Q. Liu and D. Ding, Sci. Rep., 2015, 5, 16680 CrossRef CAS PubMed.
- M. J. Rosen and J. T. Kunjappu, Surfactants and Interfacial Phenomena, 4th edn, 2004, vol. 39 Search PubMed.
- M. De Rosa and A. Gambacorta, Prog. Lipid Res., 1988, 27, 153 CrossRef CAS PubMed.
- (a) S. Franceschi, N. de Viguerie, M. Riviere and A. Lattes, New J. Chem., 1999, 23, 447 RSC; (b) L. Frkanec, M. Jokić, J. Makarević, K. Wolsperger and M. Žinić, J. Am. Chem. Soc., 2002, 124, 9716 CrossRef CAS PubMed; (c) T. Wang, J. Jiang, Y. Liu, Z. Li and M. Liu, Langmuir, 2010, 26, 18694 CrossRef CAS PubMed.
- (a) R. Iwaura, F. J. Hoeben, M. Masuda, A. P. Schenning, E. Meijer and T. Shimizu, J. Am. Chem. Soc., 2006, 128, 13298 CrossRef CAS PubMed; (b) T. Shimizu, Angew. Chem., Int. Ed., 2006, 45, 4601 CrossRef PubMed; (c) M. Kogiso, S. Ohnishi, K. Yase, M. Masuda and T. Shimizu, Langmuir, 1998, 14, 4978 CrossRef CAS; (d) T. Lu, F. Han, Z. Li, J. Huang and H. Fu, Langmuir, 2006, 22, 2045 CrossRef CAS PubMed; (e) X. Wang, Y. Shen, Y. Pan and Y. Liang, Langmuir, 2001, 17, 3162 CrossRef CAS; (f) Y. Yan, W. Xiong, X. Li, T. Lu, J. Huang, Z. Li and H. Fu, J. Phys. Chem. B, 2007, 111, 2225 CrossRef CAS PubMed.
- (a) N. Kameta, M. Masuda, H. Minamikawa and T. Shimizu, Langmuir, 2007, 23, 4634 CrossRef CAS PubMed; (b) T. Shimizu and M. Masuda, J. Am. Chem. Soc., 1997, 119, 2812 CrossRef CAS.
- (a) S. Bai, N. Javid, P. W. Frederix, S. Fleming, C. Pappas and R. V. Ulijn, Langmuir, 2014, 30, 7576 CrossRef CAS PubMed; (b) P. Gao and M. Liu, Langmuir, 2006, 22, 6727 CrossRef CAS PubMed; (c) C. Kaes, A. Katz and M. W. Hosseini, Chem. Rev., 2000, 100, 3553 CrossRef CAS PubMed; (d) I. Maity, D. B. Rasale and A. K. Das, RSC Adv., 2013, 3, 6395 RSC; (e) T. Wang, Y. Li and M. Liu, Soft Matter, 2009, 5, 1066 RSC; (f) J. Rubio, I. Alfonso, M. I. Burguete and S. V. Luis, Chem. Commun., 2012, 48, 2210 RSC; (g) L. Travaglini, A. D’Annibale, M. C. di Gregorio, K. Schillén, U. Olsson, S. Sennato, N. V. Pavel and L. Galantini, J. Phys. Chem. B, 2013, 117, 9248 CrossRef CAS PubMed.
- (a) V. J. Nebot, S. Díaz-Oltra, J. Smets, S. Fernández Prieto, J. F. Miravet and B. Escuder, Chem. – Eur. J., 2014, 20, 5762 CrossRef CAS PubMed; (b) V. J. Nebot, B. Escuder, J. F. Miravet, J. Smets and S. Fernández-Prieto, Langmuir, 2013, 29, 9544 CrossRef CAS PubMed; (c) B. n. Nieto-Ortega, V. J. Nebot, J. F. Miravet, B. Escuder, J. T. L. p. Navarrete, J. Casado and F. J. Ramírez, J. Phys. Chem. Lett., 2012, 3, 2120 CrossRef CAS PubMed.
- T. Fukui, M. Takeuchi and K. Sugiyasu, Sci. Rep., 2017, 7, 2425 CrossRef PubMed.
- (a) S. P. Goskulwad, D. D. La, R. S. Bhosale, M. Al Kobaisi, S. V. Bhosale and S. V. Bhosale, RSC Adv., 2016, 6, 39392 RSC; (b) T. Weil, T. Vosch, J. Hofkens, K. Peneva and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 9068 CrossRef CAS PubMed.
- M. R. Molla and S. Ghosh, Chem. – Eur. J., 2012, 18, 9860 CrossRef CAS PubMed.
- (a) A. M. Sanders, T. J. Dawidczyk, H. E. Katz and J. D. Tovar, ACS Macro Lett., 2012, 1, 1326 CrossRef CAS; (b) K. Sugiyasu, S. i. Kawano, N. Fujita and S. Shinkai, Chem. Mater., 2008, 20, 2863 CrossRef CAS; (c) B. D. Wall, S. R. Diegelmann, S. Zhang, T. J. Dawidczyk, W. L. Wilson, H. E. Katz, H. Q. Mao and J. D. Tovar, Adv. Mater., 2011, 23, 5009 CrossRef CAS PubMed; (d) S. Kaloyanova, Y. Zagranyarski, S. Ritz, M. Hanulova, K. Koynov, A. Vonderheit and K. Peneva, J. Am. Chem. Soc., 2016, 138, 2881 CrossRef CAS PubMed.
- D. A. Stone, L. Hsu and S. I. Stupp, Soft Matter, 2009, 5, 1990 RSC.
- R. J. Williams, T. E. Hall, V. Glattauer, J. White, P. J. Pasic, A. B. Sorensen, L. Waddington, K. M. McLean, P. D. Currie and P. G. Hartley, Biomaterials, 2011, 32, 5304 CrossRef CAS PubMed.
- S. Ghanaati, M. J. Webber, R. E. Unger, C. Orth, J. F. Hulvat, S. E. Kiehna, M. Barbeck, A. Rasic, S. I. Stupp and C. J. Kirkpatrick, Biomaterials, 2009, 30, 6202 CrossRef CAS PubMed.
- (a) L. Liang, X. D. Xu, C. S. Chen, J. H. Fang, F. G. Jiang, X. Z. Zhang and R. X. Zhuo, J. Biomed. Mater. Res., Part B, 2010, 93, 324 CrossRef PubMed; (b) C. Yang, L. Chu, Y. Zhang, Y. Shi, J. Liu, Q. Liu, S. Fan, Z. Yang, D. Ding and D. Kong, ACS Appl. Mater. Interfaces, 2015, 7, 2735 CrossRef CAS PubMed.
- S. Toledano, R. J. Williams, V. Jayawarna and R. V. Ulijn, J. Am. Chem. Soc., 2006, 128, 1070 CrossRef CAS PubMed.
- (a) D. Iveković, S. Milardović and B. Grabarić, Biosens. Bioelectron., 2004, 20, 872 CrossRef PubMed; (b) C. S. Kumar, Kirk-Othmer Encyclopedia of Chemical Technology, 2007 Search PubMed; (c) H. Liu, Z. Lv, K. Ding, X. Liu, L. Yuan, H. Chen and X. Li, J. Mater. Chem. B, 2013, 1, 5550 RSC; (d) Y. Murakami and M. Maeda, Macromolecules, 2005, 38, 1535 CrossRef CAS; (e) Z. Yang, G. Liang and B. Xu, Acc. Chem. Res., 2008, 41, 315 CrossRef CAS PubMed.
- (a) A. R. Hirst, S. Roy, M. Arora, A. K. Das, N. Hodson, P. Murray, S. Marshall, N. Javid, J. Sefcik and J. Boekhoven, Nat. Chem., 2010, 2, 1089 CrossRef CAS PubMed; (b) J. Li, Y. Kuang, Y. Gao, X. Du, J. Shi and B. Xu, J. Am. Chem. Soc., 2012, 135, 542 CrossRef PubMed; (c) J. Marcus, Chem. Commun., 2012, 48, 8404 RSC; (d) Z. A. Schnepp, R. Gonzalez-McQuire and S. Mann, Adv. Mater., 2006, 18, 1869 CrossRef CAS; (e) Z. Yang, P. L. Ho, G. Liang, K. H. Chow, Q. Wang, Y. Cao, Z. Guo and B. Xu, J. Am. Chem. Soc., 2007, 129, 266 CrossRef CAS PubMed; (f) Z. Yang, G. Liang, Z. Guo, Z. Guo and B. Xu, Angew. Chem., Int. Ed., 2007, 46, 8216 CrossRef CAS PubMed; (g) Z. Yang, K. Xu, Z. Guo, Z. Guo and B. Xu, Adv. Mater., 2007, 19, 3152 CrossRef CAS; (h) J. Zhou, X. Du, Y. Gao, J. Shi and B. Xu, J. Am. Chem. Soc., 2014, 136, 2970 CrossRef CAS PubMed; (i) Y. Gao, J. Shi, D. Yuan and B. Xu, Nat. Commun., 2012, 3, 1033 CrossRef PubMed.
- (a) L. Lv, H. Liu, X. Chen and Z. Yang, Colloids Surf., B, 2013, 108, 352 CrossRef CAS PubMed; (b) B. Ding, Y. Li, M. Qin, Y. Ding, Y. Cao and W. Wang, Soft Matter, 2013, 9, 4672 RSC; (c) Y. Li, Y. Ding, M. Qin, Y. Cao and W. Wang, Chem. Commun., 2013, 49, 8653 RSC.
- (a) G. T. Crisp and J. Gore, Synth. Commun., 1997, 27, 2203 CrossRef CAS; (b) P. Graceffa and S. Lehrer, J. Biol. Chem., 1980, 255, 11296 CAS; (c) M. Martari and R. D. Sanderson, S. Afr. J. Chem., 2008, 61, 47 CAS.
- J. H. Kim, S. Y. Lim, D. H. Nam, J. Ryu, S. H. Ku and C. B. Park, Biosens. Bioelectron., 2011, 26, 1860 CrossRef CAS PubMed.
- (a) S. Bhuniya, S. M. Park and B. H. Kim, Org. Lett., 2005, 7, 1741 CrossRef CAS PubMed; (b) N. Kameta, M. Masuda, G. Mizuno, N. Morii and T. Shimizu, Small, 2008, 4, 561 CrossRef CAS PubMed; (c) Y. Li, Y. Ding, M. Qin, Y. Cao and W. Wang, Chem. Commun., 2013, 49, 8653 RSC; (d) A. Ojida, Y. Mito-Oka, M. a. Inoue and I. Hamachi, J. Am. Chem. Soc., 2002, 124, 6256 CrossRef CAS PubMed; (e) R. A. Pires, Y. M. Abul-Haija, D. S. Costa, R. Novoa-Carballal, R. L. Reis, R. V. Ulijn and I. Pashkuleva, J. Am. Chem. Soc., 2015, 137, 576 CrossRef CAS PubMed; (f) J. Shi, X. Du, D. Yuan, J. Zhou, N. Zhou, Y. Huang and B. Xu, Biomacromolecules, 2014, 15, 3559 CrossRef CAS PubMed; (g) P. K. Vemula, J. E. Kohler, A. Blass, M. Williams, C. Xu, L. Chen, S. R. Jadhav, G. John, D. I. Soybel and J. M. Karp, Sci. Rep., 2014, 4, 4466 CrossRef PubMed.
- S. i. Tamaru, S. Kiyonaka and I. Hamachi, Chem. – Eur. J., 2005, 11, 7294 CrossRef CAS PubMed.
- Y. Kuang and B. Xu, Angew. Chem., Int. Ed., 2013, 52, 6944 CrossRef CAS PubMed.
- (a) F. Huang, H. Wu and Y. Cao, Chem. Soc. Rev., 2010, 39, 2500 RSC; (b) J. Li, Y. Kuang, J. Shi, J. Zhou, J. E. Medina, R. Zhou, D. Yuan, C. Yang, H. Wang and Z. Yang, Angew. Chem., 2015, 127, 13505 CrossRef.
- (a) Y. Li, T. Park, J. K. Quansah and S. C. Zimmerman, J. Am. Chem. Soc., 2011, 133, 17118 CrossRef CAS PubMed; (b) S. Kiyonaka, K. Sada, I. Yoshimura, S. Shinkai, N. Kato and I. Hamachi, Nat. Mater., 2004, 3, 58 CrossRef CAS PubMed.
- (a) N. Isobe, D.-S. Lee, Y. J. Kwon, S. Kimura, S. Kuga, M. Wada and U. J. Kim, Cellulose, 2011, 18, 1251 CrossRef CAS; (b) S. Kupfer, L. Zedler, J. Guthmuller, S. Bode, M. D. Hager, U. S. Schubert, J. Popp, S. Gräfe and B. Dietzek, Phys. Chem. Chem. Phys., 2014, 16, 12422 RSC.
- (a) J. R. Kumpfer and S. J. Rowan, J. Am. Chem. Soc., 2011, 133, 12866 CrossRef CAS PubMed; (b) X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed.
- L. Ying, C. L. Ho, H. Wu, Y. Cao and W. Y. Wong, Adv. Mater., 2014, 26, 2459 CrossRef CAS PubMed.
- (a) R. Abbel, C. Grenier, M. Pouderoijen, J. Stouwdam and E. Philippe, J. Am. Chem. Soc., 2009, 131, 3 CrossRef PubMed; (b) Q. Zhao, Y. Chen, S. H. Li and Y. Liu, Chem. Commun., 2018, 54, 200 RSC.
- J. Luo, X. Li, Q. Hou, J. Peng, W. Yang and Y. Cao, Adv. Mater., 2007, 19, 1113 CrossRef CAS.
- (a) R. Besançon, S. Valsesia-Wittmann, A. Puisieux, C. C. de Fromentel and V. Maguer-Satta, Curr. Med. Chem., 2009, 16, 394 CrossRef; (b) M. F. Clarke, J. E. Dick, P. B. Dirks, C. J. Eaves, C. H. Jamieson, D. L. Jones, J. Visvader, I. L. Weissman and G. M. Wahl, Cancer Res., 2006, 66, 9339 CrossRef CAS PubMed; (c) J. D. Hartgerink, E. Beniash and S. I. Stupp, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5133 CrossRef CAS PubMed; (d) Y. Kim, K. M. Joo, J. Jin and D. H. Nam, Int. J. Stem Cells, 2009, 2, 109 CrossRef CAS PubMed.
- (a) K. Ghaffarzadehgan, M. Jafarzadeh, H. R. Raziee, H. R. Sima, E. Esmaili-Shandiz, H. Hosseinnezhad, A. Taghizadeh-Kermani, O. Moaven and M. Bahrani, World J. Gastroenterol., 2008, 14, 6376 CrossRef PubMed; (b) M. Kurisawa, J. E. Chung, Y. Y. Yang, S. J. Gao and H. Uyama, Chem. Commun., 2005, 4312 RSC; (c) E. K. Lim, H. O. Kim, E. Jang, J. Park, K. Lee, J. S. Suh, Y. M. Huh and S. Haam, Biomaterials, 2011, 32, 7941 CrossRef CAS PubMed; (d) J. C. Miller and J. H. Thrall, J. Am. Coll. Radiol., 2004, 1, 4 CrossRef PubMed; (e) J. Park, M. Ku, E. Kim, Y. Park, Y. Hong, S. Haam, J.-H. Cheong, E. S. Park, J.-S. Suh and Y.-M. Huh, Integr. Biol., 2013, 5, 669 RSC; (f) E. Elmalem, F. Biedermann, M. R. Scherer, A. Koutsioubas, C. Toprakcioglu, G. Biffi and W. T. Huck, Chem. Commun., 2014, 50, 8930 RSC; (g) G. Deng, S. Li, Z. Sun, W. Li, L. Zhou, J. Zhang and L. Cai, Theranostics, 2018, 8, 4116 CrossRef PubMed.
- C. Zhang, C. Liu, X. Xue, X. Zhang, S. Huo, Y. Jiang, W. Q. Chen, G. Zou and X. J. Liang, ACS Appl. Mater. Interfaces, 2013, 6, 757 CrossRef PubMed.
- S. Mukherjee, T. Kar and P. Kumar Das, Chem. – Asian J., 2014, 9, 2798 CrossRef CAS PubMed.
- S. Takaishi, T. Okumura, S. Tu, S. S. Wang, W. Shibata, R. Vigneshwaran, S. A. Gordon, Y. Shimada and T. C. Wang, Stem Cells, 2009, 27, 1006 CrossRef CAS PubMed.
- (a) E. R. Draper, M. Wallace, R. Schweins, R. J. Poole and D. J. Adams, Langmuir, 2017, 33, 2387 CrossRef CAS PubMed; (b) Y. Fu, W. Xu, Q. He and J. Cheng, Sci. China: Chem., 2016, 59, 3 CrossRef CAS; (c) D. Grafahrend, K. H. Heffels, M. V. Beer, P. Gasteier, M. Möller, G. Boehm, P. D. Dalton and J. Groll, Nat. Mater., 2011, 10, 67 CrossRef CAS PubMed; (d) S. M. Hsu, Y. C. Lin, J. W. Chang, Y. H. Liu and H. C. Lin, Angew. Chem., Int. Ed., 2014, 53, 1921 CrossRef CAS PubMed; (e) S.-M. Hsu, J. W. Chang, F. Y. Wu, Y. C. Lin, T. S. Lai, H. Cheng and H. C. Lin, RSC Adv., 2015, 5, 32431 RSC; (f) M. Ikeda, T. Tanida, T. Yoshii and I. Hamachi, Adv. Mater., 2011, 23, 2819 CrossRef CAS PubMed; (g) R. Krini, C. W. Ha, P. Prabhakaran, H. E. Mard, D. Y. Yang, R. Zentel and K. S. Lee, Macromol. Rapid Commun., 2015, 36, 1108 CrossRef CAS PubMed; (h) Y. Kuang, X. Du, J. Zhou and B. Xu, Adv. Healthcare Mater., 2014, 3, 1217 CrossRef CAS PubMed; (i) D. M. Ryan, T. M. Doran and B. L. Nilsson, Langmuir, 2011, 27, 11145 CrossRef CAS PubMed; (j) V. A. Schulte, K. Hahn, A. Dhanasingh, K. H. Heffels and J. Groll, Biofabrication, 2014, 6, 024106 CrossRef PubMed; (k) Y. Shi, J. Wang, H. Wang, Y. Hu, X. Chen and Z. Yang, PLoS One, 2014, 9, 106968 CrossRef PubMed; (l) A. M. Smith, R. J. Williams, C. Tang, P. Coppo, R. F. Collins, M. L. Turner, A. Saiani and R. V. Ulijn, Adv. Mater., 2008, 20, 37 CrossRef CAS.
- (a) L. H. Hsu, S. M. Hsu, F. Y. Wu, Y. H. Liu, S. R. Nelli, M. Y. Yeh and H. C. Lin, RSC Adv., 2015, 5, 20410 RSC; (b) J. Li, R. Kooger, M. He, X. Xiao, L. Zheng and Y. Zhang, Chem. Commun., 2014, 50, 3722 RSC.
- (a) Y. Choi, S. H. Jung, A. Lee, M. L. Seo and J. H. Jung, Bull. Korean Chem. Soc., 2015, 36, 1725 CrossRef CAS; (b) J. Shen, J. Pang, T. Kalwarczyk, R. Hołyst, X. Xin, G. Xu, X. Luan and Y. Yang, J. Mater. Chem. C, 2015, 3, 8104 RSC.
- W. Ji, G. Liu, F. Wang, Z. Zhu and C. Feng, Chem. Commun., 2016, 52, 12574 RSC.
- (a) C. W. Chu and B. J. Ravoo, Chem. Commun., 2017, 53, 12450 RSC; (b) W. Ji, S. Zhang, G. A. Filonenko, G. Li, T. Sasaki, C. Feng and Y. Zhang, Chem. Commun., 2017, 53, 4702 RSC; (c) J. Zhou, X. Du, J. Wang, N. Yamagata and B. Xu, Front. Chem. Sci. Eng., 2017, 11, 509 CrossRef CAS PubMed.
- W. Ji, L. Li, O. Eniola-Adefeso, Y. Wang, C. Liu and C. Feng, J. Mater. Chem. B, 2017, 5, 7790 RSC.
- Y. Dong, M. Lin, G. Jin, Y. I. Park, M. Qiu, Y. Zhao, H. Yang, A. Li and T. J. Lu, Nanotechnology, 2017, 28, 175702 CrossRef PubMed.
- C. Kallepitis, Nat. Commun., 2017, 8, 14843 CrossRef CAS PubMed.
- (a) W. Hu, J. Xiaofan, L. Yang, L. Zhengtao, T. Guping and H. Feihe, J. Mater. Chem. B, 2018, 6, 2728 RSC; (b) K. Reichenbacher, H. I. Suss and J. Hulliger, Chem. Soc. Rev., 2005, 34, 22 RSC; (c) C. Nien-Tzu, C. D. Rajan, S. Nai-Chia, L. Yen-Hsu, L. Yen-Chu, L. Jhong-Hua and L. Hsin-Chieh, RSC Adv., 2018, 8, 20922 RSC.
- L. Wang, L. Yin, W. Zhang, X. Zhu and M. Fujiki, J. Am. Chem. Soc., 2017, 139, 13218 CrossRef CAS PubMed.
- D. Yang, P. Duan, L. Zhang and M. Liu, Nat. Commun., 2017, 8, 15727 CrossRef CAS PubMed.
- L. D. Koh, Y. Cheng, C. P. Teng, Y. W. Khin, X. J. Loh, S. Y. Tee, M. Low, E. Ye, H. D. Yu and Y. W. Zhang, Prog. Polym. Sci., 2015, 46, 86 CrossRef CAS.
- Y. Xia, B. Xue, M. Qin, Y. Cao, Y. Li and W. Wang, Sci. Rep., 2017, 7, 9691 CrossRef PubMed.
- (a) Y. Hong, J. W. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC; (b) K. Li, W. Qin, D. Ding, N. Tomczak, J. Geng, R. Liu, J. Liu, X. Zhang, H. Liu and B. Liu, Sci. Rep., 2013, 3, 1150 CrossRef PubMed.
- R. Nishiyabu, C. Aimé, R. Gondo, T. Noguchi and N. Kimizuka, Angew. Chem., Int. Ed., 2009, 48, 9465 CrossRef CAS PubMed.
- J. Seo, J. W. Chung, E. H. Jo and S. Y. Park, Chem. Commun., 2008, 2794 RSC.
- (a) T. Rabilloud, J. M. Strub, S. Luche, J. L. Girardet, A. van Dorsselaer and J. Lunardi, Proteome, 2000, 1, 1 CrossRef; (b) G. Li, T. Sasaki, S. Asahina, M. C. Roy, T. Mochizuki, K. Koizumi and Y. Zhang, Chem, 2017, 2, 283 CrossRef CAS; (c) E. Du, X. Hu, S. Roy, P. Wang, K. Deasy, T. Mochizuki and Y. Zhang, Chem. Commun., 2017, 53, 6033 RSC.
- N. A. Peppas, Biomedical applications of hydrogels handbook, Springer Science & Business Media, 2010 Search PubMed.
- C. Jiang, B. Liu, M. Y. Han and Z. Zhang, Small Methods, 2018, 1700379 CrossRef.
- (a) T. S. Kusurkar, I. Tandon, N. K. Sethy, K. Bhargava, S. Sarkar, S. K. Singh and M. Das, Sci. Rep., 2013, 3, 3290 CrossRef PubMed; (b) N. C. Tansil, Y. Li, C. P. Teng, S. Zhang, K. Y. Win, X. Chen, X. Y. Liu and M. Y. Han, Adv. Mater., 2011, 23, 1463 CrossRef CAS PubMed.
- (a) A. Croce and G. Bottiroli, Eur. J. Histochem., 2014, 58 Search PubMed; (b) M. Monici, Biotechnol. Annu. Rev., 2005, 11, 227 CAS; (c) P. Reineck and B. C. Gibson, Adv. Opt. Mater., 2017, 5 Search PubMed.
- (a) F. L. De La Puente and J. F. Nierengarten, Fullerenes: Principles and Applications, 2nd edn, 2011, ISBN-13: 978-0-85404-551-8 RSC; (b) T. Gunnlaugsson, Nat. Chem., 2016, 8, 6 CrossRef CAS PubMed.
- T. Bánsági, V. K. Vanag and I. R. Epstein, Science, 2011, 331, 1309 CrossRef PubMed.
- (a) I. R. Epstein, Nature, 1995, 374, 321 CrossRef CAS PubMed; (b) I. R. Epstein, V. K. Vanag, A. C. Balazs, O. Kuksenok, P. Dayal and A. Bhattacharya, Acc. Chem. Res., 2011, 45, 2160 CrossRef PubMed; (c) Y. Hu, R. Lin, K. Patel, A. G. Cheetham, C. Kan and H. Cui, Coord. Chem. Rev., 2016, 320, 2 CrossRef; (d) X. Yan, P. Zhu and J. Li, Chem. Soc. Rev., 2010, 39, 1877 RSC.
- G. A. Hembury, V. V. Borovkov and Y. Inoue, Chem. Rev., 2008, 108, 1 CrossRef CAS PubMed.
- J. D. van der Laan, J. B. Wright, S. A. Kemme and D. A. Scrymgeour, Appl. Opt., 2018, 57, 5464 CrossRef CAS PubMed.
- X. Zeng, J. Chu, W. Cao, W. Kang and R. Zhang, Appl. Opt., 2018, 57, 6817 CrossRef PubMed.
Footnote |
| † Co-first authors. |
| This journal is © The Royal Society of Chemistry 2019 |