Open Access Article
Open Access ArticleA novel method for the synthesis of 1,2-benzisoxazoline-3-one and its application to hypochlorite recognition†
Yutao
Yang‡
ab,
Fangjun
Huo‡
b,
Caixia
Yin
*a,
Ming
Xu
c,
Ying
Hu
d,
Jianbin
Chao
b,
Yongbin
Zhang
b,
Timothy E.
Glass
*c and
Juyoung
Yoon
*d
aInstitute of Molecular Science, Key Laboratory of Materials for Energy Conversion and Storage of Shanxi Province, Shanxi University, Taiyuan 030-006, P. R. China. E-mail: yincx@sxu.edu.cn
bResearch Institute of Applied Chemistry, Shanxi University, Taiyuan, 030006, China
cDepartment of Chemistry, University of Missouri 601 South College Avenue, Columbia, Missouri 65211, USA. E-mail: glasst@missouri.edu
dDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul, 120-750, Korea. E-mail: jyoon@ewha.ac.kr
First published on 5th July 2016
Abstract
The reaction of salicylhydroxamic acid with hypochlorite produces 1,2-benzisoxazoline-3-one, a heterocycle that contains a fluorophore. As a result, this reaction was used as the basis for a new, selective and sensitive fluorescence system for the recognition of hypochlorite. The effectiveness of the method was demonstrated by its use to detect hypochlorite in a disinfectant solution as well as to image hypochlorite in cells.
In modern-day organic chemistry, a growing trend exists to develop green and sustainable synthetic processes.1,2 The preparation of heterocyclic compounds has remained an important issue in synthetic chemistry because these substances play important roles in coordination chemistry, biochemistry, and pharmaceutical and materials science.3 Many biologically active natural products are heterocycles that are used in the agrochemical, pharmaceutical and flavorant industries.4,5 For example, 1,2-benzisoxazole derivatives have been widely utilized as anti-cancer, anti-microbial and anti-thrombotic agents.6 As a result, the observation that 1,2-benzisoxazoline-3-ones can be prepared from hydroxamic acids, using either thionyl chloride at low temperature, or 1,1′-carbonyldiimidazole (CDI) at high temperature, is relevant.7 The design and discovery of new methods to synthesize heterocyclic compounds, like 1,2-benzisoxazoline-3-ones, remain important.
Reactive oxygen species (ROS) are involved in a number of biological functions.8 Among ROS, the hypochlorite ion (ClO−) has gained importance because of the biological roles it plays.9 The hypochlorite ion is produced in living systems mainly by myeloperoxidase (MPO) catalyzed peroxidation of chloride ions.10 However, because of its high and nonselective reactivity, abnormal production of hypochlorite can lead to various diseases including cardiovascular ailments,11 arthritis,12 neuronal degeneration13 and cancer.14 As a consequence, methods for sensitive and selective detection of the hypochlorite ion are highly significant. To date, a variety of fluorescent probes for ClO− sensing, which are designed based on HOCl induced oxidation reactions, have been described.15 However, fluorescent probes that display a fluorescence enhancement (turn-on) response and that function in 100% aqueous media are scarce. In addition, tedious synthetic procedures for these probes can have serious disadvantages. In the current study, we report a simple, rapid and economical method to sense ClO−.
The method uncovered to prepare 1,2-benzisoxazoline-3-one (2), shown in Scheme 1, is both simple and efficient. Specifically, treatment of a methanol solution of salicylhydroxamic acid (1) at ambient temperature with excess sodium hypochlorite leads to rapid (30 s) formation of 2. This heterocycle is isolated in 82% yield following implementation of a simple extraction procedure. Compound 2 was confirmed by 1H-NMR, 13C-NMR and ESI-MS.
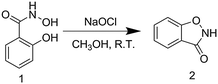 | ||
| Scheme 1 Synthesis of 1,2-benzisoxazoline-3-one (2) from salicylhydroxamic acid (1) using hypochlorite. | ||
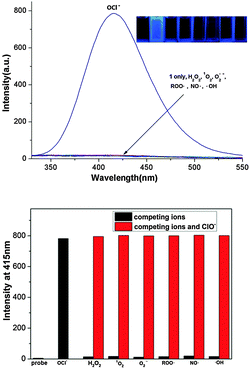
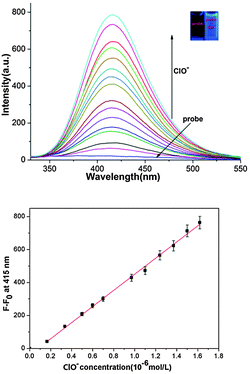
Because 1,2-benzisoxazoline-3-one is fluorescent and the oxidation reaction producing it is both rapid and efficient, we envisaged that the process would serve as the basis for a new method for HOCl detection and imaging the physiological generation of this ROS. In Fig. 1 emission spectra are displayed which show the fluorescence changes that occur when ClO− (1.0 equiv.) and other ROS and RNS (10 equiv., H2O2, 1O2, ˙O2−, ROO˙, NO˙ and ˙OH) are added to aqueous HEPES (10 mM, pH 7.4) solutions of 1. The spectra demonstrate that only ClO− causes production of 2 in association with a large increase in the intensity of the fluorescence band with a maximum at 415 nm (300 nm excitation). UV-Vis spectroscopic monitoring of the reaction between 1 and ClO− shows that an increase in absorption occurs at 265 nm as the reaction proceeds (Fig. S3, ESI†) and fluorescence titration data show that a ClO− concentration dependent fluorescence enhancement occurs at 415 nm (Fig. 2a).
Analysis of the fluorescence titration profile of the reaction of 1 (2.5 μM) with ClO− demonstrates that the limit of detection of the system for ClO− is 49 nM (Fig. 2b). As shown in Fig. S5 (ESI†), probe 1 shows fluorescence enhancement at pH in the range of 6–12 upon the addition of HOCl.
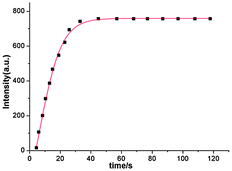
Time-dependent changes in the fluorescence spectra, brought about by the addition of 10 equiv. of ClO− to a solution of 1, were monitored. The results (Fig. 3) show that reaction to form 2 is completed within 30 s, indicating that the probe reacts rapidly with this ROS under the conditions employed. This fast response suggests that the process can be possibly employed directly for the quantitative detection of ClO−.
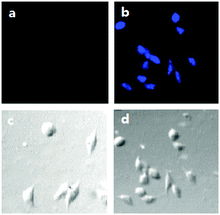
The ability of 1 to be used to visualize ClO− within living cells was also evaluated. For this purpose, laser confocal fluorescence imaging was carried using a Leica TCS SP5 laser scanning microscope, with an optical window in the blue channel (400–450 nm) as a signal output. As the images given in Fig. 4a show, HepG2 cells incubated with the 2.5 μM probe for 30 min at 37 °C do not fluoresce when selectively excited at 300 nm. In contrast, HepG2 cells do display blue fluorescence after they are incubated with 2 μM of 1 for 30 min at 37 °C and then treated with 5 μM ClO− (Fig. 4b). The results of these whole cell experiments demonstrate that the new probe is cell permeable and that it can be used to visualize ClO− within living cells.
To investigate the applicability of the new probe for monitoring ClO− in real samples, the levels of this ROS in a common disinfectant were examined (Table 1). Aliquots of a commercial bleach solution, without pretreatment, were added to a solution of 1 in buffer. By comparing the observed fluorescence intensities to those of a standard curve (Fig. S4, ESI†), the concentration of ClO− in the bleach sample was determined to be 105 ± 3 mM (Table S1, ESI†).
In summary, in this study we uncovered a simple, rapid and economical method for synthesis of the interesting heterocycle, 1,2-benzisoxazoline-3-one, using ClO− promoted oxidation of salicylhydroxamic acid at room temperature for 30 s. Compared with traditional protocols used to prepare this substance,7 the new method is more environmentally benign. In addition, we demonstrated that this process can be used as a fluorescent probe for ClO−. The application of this method to the synthesis of other heterocyclic compounds and to the development of new fluorescent probes with longer wavelength emission characteristics is under current investigation.
The work was supported by the National Natural Science Foundation of China (No. 21472118), the Taiyuan Technology star special (No. 12024703), the Program for the Top Young and Middle-aged Innovative Talents of Higher Learning Institutions of Shanxi (2013802), the Talents Support Program of Shanxi Province (2014401), the Shanxi Province Outstanding Youth Fund (No. 2014021002) and the National Science Foundation (CHE-1507119). This research was also supported by a grant from the National Creative Research Initiative programs of the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (No. 2012R1A3A2048814).
Notes and references
- (a) L. G. Voskressensky, A. A. Festa and A. V. Varlamov, Tetrahedron, 2014, 70, 551–572 CrossRef CAS; (b) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed; (c) F. F. Wei, Y. Lu, S. He, L. C. Zhao and X. S. Zeng, Anal. Methods, 2012, 4, 616 RSC.
- V. Polshettiwar and R. S. Verma, Curr. Opin. Drug Discovery Dev., 2007, 10, 723 CAS.
- G. C. Rodrigues, D. F. Feijó, M. T. Bozza, P. Pan, D. Vullo, S. Parkkila, C. T. Supuran, C. Capasso, A. P. Aguiar and A. B. Ver-melho, J. Med. Chem., 2014, 57, 298 CrossRef CAS PubMed.
- R. Properzi and E. Marcantoni, Chem. Soc. Rev., 2014, 43, 779 RSC.
- I. Flament and C. Chevalier, Chem. Ind., 1988, 18, 592 Search PubMed.
- (a) M. Pinho and M. V. D. Teresa, Curr. Org. Chem., 2005, 9, 925 CrossRef; (b) M. Jain and C. H. Kwon, J. Med. Chem., 2003, 46, 5428 CrossRef CAS PubMed.
- W. Liu, F. Lau, K. Liu, H. B. Wood, G. Zhou, Y. Chen, Y. Li, T. E. Akiyama, G. Castriota, M. Einstein, C. Wang, M. E. McCann, T. W. Doebber, M. Wu, C. H. Chang, L. McNamara, B. McKeever, R. T. Mosley, J. P. Berger and P. T. Meinke, J. Med. Chem., 2011, 54, 8541 CrossRef PubMed.
- (a) X. Chen, F. Wang, J. Y. Hyun, T. Wei, J. Qiang, X. Ren, I. Shin and J. Yoon, Chem. Soc. Rev., 2016, 45, 2976 RSC; (b) X. Chen, X. Tian, I. Shin and J. Yoon, Chem. Soc. Rev., 2011, 40, 4783 RSC; (c) X. H. Li, G. X. Zhang, H. M. Ma, D. Q. Zhang, J. Li and D. B. Zhu, J. Am. Chem. Soc., 2004, 126, 11543 CrossRef CAS PubMed.
- (a) X. H. Cheng, H. Z. Jia, T. Long, J. Feng, J. G. Qin and Z. Li, Chem. Commun., 2011, 47, 11978 RSC; (b) S. Khati, R. Musa and J. Vaya, Bioorg. Med. Chem., 2007, 15, 3661 CrossRef PubMed.
- Y. K. Yang, H. J. Cho, J. Lee, I. Shin and J. Tae, Org. Lett., 2009, 11, 859 CrossRef CAS PubMed.
- S. Sugiyama, K. Kugiyama, M. Aikawa, S. Nakamura, H. Ogawa and P. Libby, Arterioscler., Thromb., Vasc. Biol., 2004, 24, 1309 CrossRef CAS PubMed.
- M. J. Steinbeck, L. J. Nesti, P. F. Sharkey and J. J. Parvizi, J. Orthop. Res., 2007, 25, 1128 CrossRef CAS PubMed.
- D. I. Pattison and M. J. Davies, Chem. Res. Toxicol., 2001, 14, 1453 CrossRef CAS PubMed.
- D. I. Pattison and M. J. Davies, Biochemistry, 2006, 45, 8152 CrossRef CAS PubMed.
- (a) Q. L. Xu, C. H. Heo, G. Kim, H. W. Lee, H. M. Kim and J. Yoon, Angew. Chem., Int. Ed., 2015, 54, 4890 CrossRef CAS PubMed; (b) Q. L. Xu, S. Lee, K. M. Lee, W. J. Lee and J. Yoon, J. Am. Chem. Soc., 2013, 135, 9944 CrossRef CAS PubMed; (c) L. Yuan, L. Wang, B. K. Agrawalla, S. J. Park, H. Zhu, B. Sivaraman, J. Peng, Q. H. Xu and Y. T. Chang, J. Am. Chem. Soc., 2015, 137, 5930 CrossRef CAS PubMed; (d) L. Yuan, W. Y. Lin and H. Chen, Biomaterials, 2013, 34, 9566 CrossRef CAS PubMed; (e) Q. A. Best, N. Sattenapally, D. J. Dyer, C. N. Scott and M. E. McCarroll, J. Am. Chem. Soc., 2013, 135, 13365 CrossRef CAS PubMed; (f) Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 5680 CrossRef CAS PubMed; (g) Z. Lou, P. Li, Q. Pan and K. Han, Chem. Commun., 2013, 49, 2445 RSC; (h) J. J. Hu, N. K. Wong, Q. Gu, X. Bai, S. Ye and D. Yang, Org. Lett., 2014, 16, 3544 CrossRef CAS PubMed; (i) H. Zhu, J. Fan, J. Wang, H. Mu and X. J. Peng, J. Am. Chem. Soc., 2014, 136, 12820 CrossRef CAS PubMed; (j) X. Chen, K. A. Lee, E. M. Ha, K. M. Lee, Y. Y. Seo, H. K. Choi, H. N. Kim, M. J. Kim, C. S. Cho, S. Y. Lee, W. J. Lee and J. Yoon, Chem. Commun., 2011, 47, 4373 RSC; (k) W. Y. Lin, L. L. Long and B. B. Chen, Chem. – Eur. J., 2009, 15, 2305 CrossRef CAS PubMed; (l) J. Zhou, L. H. Li, W. Shi, X. H. Gao, X. H. Li and H. M. Ma, Chem. Sci., 2015, 6, 4884 RSC; (m) S. Kenmoku, Y. Urano and H. Kojima, J. Am. Chem. Soc., 2007, 129, 7313 CrossRef CAS PubMed; (n) N. Zhao, Y. H. Wu, R. M. Wang, L. X. Shi and Z. N. Chen, Analyst, 2011, 136, 2277 RSC; (o) F. J. Huo, J. J. Zhang, Y. T. Yang, J. B. Chao, C. X. Yin, Y. B. Zhang and T. G. Chen, Sens. Actuators, B, 2012, 44, 166 Search PubMed; (p) J. Kim and Y. Kim, Analyst, 2014, 139, 2986 RSC; (q) J. L. Fan, H. Y. Mu, H. Zhu, J. Y. Wang and X. J. Peng, Analyst, 2015, 140, 4594 RSC; (r) W. Zhang, W. Liu, P. Li, J. Q. Kang, J. Y. Wang, H. Wang and B. Tang, Chem. Commun., 2015, 51, 10150 RSC; (s) X. Chen, K. A. Lee, X. Ren, J. C. Ryu, G. Kim, J. H. Ryu, W. J. Lee and J. Yoon, Nat. Protoc., 2016, 11, 1219 CrossRef CAS PubMed; (t) Q. Xu, C. H. Heo, J. A. Kim, H. S. Lee, Y. Hu, D. Kim, K. M. K. Swamy, G. Kim, S. J. Nam, H. M. Kim and J. Yoon, Anal. Chem., 2016, 88, 6615 CrossRef CAS PubMed; (u) S. Goswami, K. Aich, S. Das, B. Pakhira, K. Ghoshal, C. K. Quah, M. Bhattacharyya, H. K. Fun and S. Sarkar, Chem. – Asian J., 2015, 10, 694 CrossRef CAS PubMed; (v) S. Goswami, S. Das, K. Aich, P. K. Nandi, K. Ghoshal, C. K. Quah, M. Bhattacharyya, H. K. Fun and H. A. Abdel-Aziz, RSC Adv., 2014, 4, 24881 RSC; (w) S. Goswami, A. K. Das, A. Manna, A. K. Maity, P. Saha, C. K. Quah, H. K. Fun and H. A. Abdel-Aziz, Anal. Chem., 2014, 86, 6315 CrossRef CAS PubMed; (x) S. Goswami, A. Manna, S. Paul, C. K. Quah and H. Fun, Chem. Commun., 2013, 49, 11656 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tb01392a |
| ‡ Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |