A facile and one-step ethanol-thermal synthesis of MoS2 quantum dots for two-photon fluorescence imaging†
Wei
Gu
a,
Yinghan
Yan
a,
Xuni
Cao
b,
Cuiling
Zhang
*a,
Caiping
Ding
a and
Yuezhong
Xian
*a
aDepartment of Chemistry, School of Chemistry and Molecular Engineering, East China Normal University, 500 Dongchan Road, Shanghai 200241, China. E-mail: clzhang@chem.ecnu.edu.cn; yzxian@chem.ecnu.edu,cn
bSchool of Biotechnology, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China
First published on 23rd November 2015
Abstract
Two-photon fluorescent (TPF) molybdenum disulfide quantum dots (MoS2 QDs) were synthesized through a facile and one-step solvothermal approach. The MoS2 QDs exhibit small size and high stability. Because of their low toxicity and TPF ability, the MoS2 QDs are successfully applied in two-photon fluorescence bio-imaging.
Introduction
With the development of two-photon microscopy, two-photon fluorescence imaging has become a powerful tool for research in biological areas because of its unique advantages, such as low tissue autofluorescence, large penetration depth, and reduced photobleaching.1–4 Fluorescent probes with two-photon excitation have been widely applied in two-photon fluorescence (TPF) imaging,5,6 and these can simultaneously absorb two less-energetic photons to reach the excited state of fluorophores. The TPF probes for cellular imaging should possess large two-photon absorption cross sections, low toxicity and low photobleaching. Nowadays, great success has been achieved on the synthesis of TPF probes, including organic dyes, semiconductor quantum dots, rare earth ion doped nanoparticles and carbon-based materials (carbon dots and graphene quantum dots (GQDs)). However, the poor photobleaching and small two-photon absorption cross section of organic dyes7 and the unavoidable toxicity of the heavy metals of semiconductor quantum dots8 overwhelmingly limit their positive applications for cellular imaging. Although carbon dots and GQDs do not have these problems, and have been extensively used as TPF probes,9,10 searching for stable and non-toxic alternatives obtained by a facile synthesis route is still an intense challenge.Recently, as a kind of typical layered transition-metal dichalcogenide, molybdenum disulfide (MoS2) has drawn great attention, for it can be easily exfoliated from molybdenite compounds. The crystal structure consists of covalently bonded S–Mo–S single layers interacting by van der Waals forces.11,12 Compared with MoS2 nanosheets, MoS2 QDs have stronger quantum confinement and edge effects, which will make them show unique and extra electrical/optical properties. So far, MoS2 QDs have been used in fluorescent sensors,13 hydrogen evolution reactions14 and bioimaging.15 General methods for the synthesis of MoS2 QDs can be classified into two types: bottom-up and top-down. For the former, MoS2 QDs were synthesized through a hydrothermal route by using molybdate salt and thiol-containing small molecules as precursors.13,16 The final product was obtained by a time-consuming dialysis process. For the latter, MoS2 QDs were prepared by a variety of methods, such as mechanical and chemical exfoliation,14,17–19 the electrochemically induced Fenton reaction,20 and the thermal ablation method21. For instance, Shaijumon's group reported the preparation of MoS2 QDs using a liquid exfoliation technique involving bath sonication followed by ultrasound probe sonication of MoS2 flakes.14 Li and co-workers demonstrated that the electrochemically induced Fenton reaction could be used to generate QDs by etching MoS2 nanosheets.20 Recently, Wu et al. prepared MoS2 QDs through thermal ablation of MoS2 nanosheets in N,N-dimethylformamide (DMF) which has a high boiling point.21 Despite the great success of the synthesis of MoS2 QDs, it is urgent to develop a simple approach for the preparation of MoS2 QDs with stable fluorescence.
Herein, we report the design and synthesis of MoS2 QDs for TPF cellular imaging. The MoS2 QDs were prepared by a facile, environmentally friendly, top-down, ethanol-thermal route from bulk MoS2. These QDs exhibit stable TPF, high dispersibility, small size, non-toxicity and good biocompatibility. Furthermore, TPF imaging for the cellular nucleus is successfully realized using MoS2 QDs as probes. We believe that the MoS2 QDs are a kind of promising probe for applications in in vitro and in vivo TPF bio-imaging.
Results and discussion
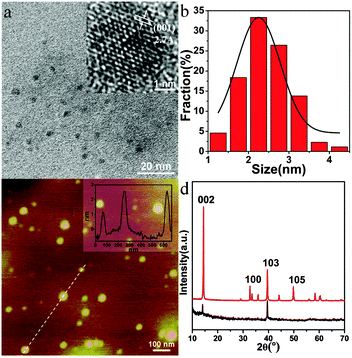
MoS2 QDs were prepared by the combination of ultrasonication and ethanol-thermal treatment. The as-prepared MoS2 QDs exhibit bright blue fluorescence. Uniform MoS2 QDs without aggregation are observed in the TEM image (Fig. 1a). The lateral size distribution of MoS2 QDs ranged from 1.2 to 4.2 nm, and the average size is about 2.9 nm. Their suitable size distribution ensures the possibility to enter the cellular nucleus. Because of the low boiling point of ethanol, the as-prepared MoS2 QDs do not agglomerate after removing the solvent by vacuum distillation and re-dispersing in water. The highly crystalline structure of the QDs with a hexagonal lattice is visualized in the high-resolution TEM (HRTEM) image (Fig. 1b) and the lattice fringe spacing is 0.27 nm derived from the (100) lattice of MoS2.22,23 As shown in Fig. 1c, the thickness of the MoS2 QDs varies from 1.4 to 2.8 nm, which is comparable with the interlayer space of few-layer MoS2 nanosheets.24,25The XRD pattern of the MoS2 QDs is then investigated, and the bulk MoS2 is used as a reference. It shows a strong diffraction peak at 2θ = 14.41° and three lower peaks at 2θ = 32.71°, 39.56°, and 49.82° for bulk MoS2 (Fig. 1d, red line). These peaks were attributed to the (002), (100), (103) and (105) planes of MoS2, respectively. For the MoS2 QDs (Fig. 1d, black line), only two peaks can be detected at 2θ = 14.37° (002) and 39.57° (103), and the signal of the (002) plane obviously decreased with the disappearance of most of the other peaks, indicating the formation of mono- or few-layered MoS2 QDs. Besides, the Raman spectra of the MoS2 QDs exhibited two typical phonon modes of the in-plane vibration of Mo and S atoms (E12g) and the out-of-plane vibration of S atoms (A1g) at 379.2 cm−1 and 403.9 cm−1, respectively, with a frequency difference of 24.3 cm−1 (Fig. S1, ESI†).6,26 According to the “frequency difference-thickness relation” of exfoliated MoS2 nanosheets, their frequency difference could correspond to that of few-layered MoS2 QDs,24 which is consistent with the AFM measurement. To explore the changes in chemical composition, the XPS of the QDs are characterized. As shown in Fig. S2a and b (ESI†), Mo 3d3/2, Mo 3d5/2, S 2s, S 2p1/2, and S 2p3/2 peaks are observed at 232.9, 229.7, 226.9, 163.8, and 162.6 eV, respectively, which belong to the dominant 2H MoS2 phase of the as-prepared MoS2 QDs.27,28 The characteristic peaks arising from Mo 3d3/2 and Mo 3d5/2 corresponded to the +4 oxidation state of Mo, and the S 2p3/2 at 162.6 eV is ascribed to the −2 oxidation state of S.29 The low intensity peak at 236.0 eV may result from slight oxidation of Mo during solvothermal reaction. These results demonstrate that the MoS2 QDs are successfully prepared.
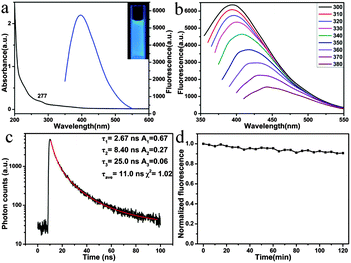
The UV-vis spectrum of the MoS2 QDs shows a shoulder peak at about 277 nm (black line in Fig. 2a), which could be attributed to the blue-shifted convoluted Z, C, and D excitonic peaks.23,30 Under the irradiation of 365 nm, the MoS2 QD solution displays a strong blue fluorescence (blue line in Fig. 2a and the inset in Fig. 2a). Fig. 2b exhibits the fluorescence emission spectra of the MoS2 QDs at excitation wavelengths ranging from 300 to 380 nm. It can be seen that the emission peak shows a red shift (from ca. 400 nm to 460 nm) as the excitation shifts towards longer wavelengths. Excitation-dependent fluorescence properties have been widely reported, such as in GQDs,31 carbon dots,32 and MoS2 QDs.20,21. Although the exact origin of the excitation-dependent photoluminescence of these QDs is still under debate, it might be attributed to the polydispersity or the surface state of the MoS2 QDs.33 The fluorescence intensity decreases remarkably with the increase of the excitation wavelength, which is consistent with MoS2 QDs or fluorescent MoS2 nanoflakes prepared by other methods.13,20,34 In addition, the fluorescence quantum yield was measured using quinine sulfate as the standard, and it was about 3.1%, which was comparable to that reported for MoS2 QDs and GQDs.13,35,36 The fluorescence lifetime of MoS2 (Fig. 2c) was τave = 11.0 ns, suggesting that the MoS2 QDs were suitable for biological and optoelectronic applications.37
The stability of fluorophores is crucially important for bioimaging application. Significantly, the MoS2 QDs display excellent photostability. The QDs retain almost the original fluorescence intensity after irradiation for 2 h (Fig. 2d). The effect of pH on the performance of QDs was further investigated. As shown in Fig. S3a (ESI†), the as-prepared MoS2 QDs exhibit higher fluorescence intensity under acidic conditions than under alkaline conditions. As the pH was switched repeatedly between 2 and 12, the fluorescence intensity could be varied reversibly (Fig. S3b, ESI†). Compared with conventional semiconductor quantum dots, the results indicate that the pH hardly affects the fluorescence properties of the MoS2 QDs.
The TPF properties of the MoS2 QDs were further evaluated. As shown in Fig. 3a, the as-prepared MoS2 QDs show the maximum TPF emission at 428 nm with the excitation wavelength at 690 nm. From TPF imaging of MoS2 QDs (Fig. 3b), it can be seen that the MoS2 QDs display blue fluorescence under excitation of 690 nm. Recently, Song's group reported the synthesis of water-soluble monolayer MoS2 QDs through a bottom-up route with N-acetyl-L-cysteine (NAC) as a capping reagent,16 and the QDs show upconversion fluorescence due to the two successive energy transfer processes from the capping reagent NAC to MoS2 QDs accompanied with the depletion of the intermediate excited state by the upconversion process. With regard to our strategy, MoS2 QDs were obtained through a top-down route without any surface ligand; therefore, the TPF mechanism might be that two photons arrive at the QDs simultaneously and combine their energies to promote MoS2 in the ground state to an excited state, and then proceed along the normal fluorescence-emission pathway. As it has been well-recognized, the two-photon action cross section is an essential attribute for two-photon excited materials. By using rhodamine B as the reference, the two-photon absorption cross-section of the MoS2 QDs is estimated to be 9500 ± 500 GM (Goeppert-Mayer unit, with 1 GM = 10−50 cm4 s per photon), which is much larger than that of organic dyes and compatible to that of semiconductor quantum dots and graphitic-C3N4 quantum dots.38,39
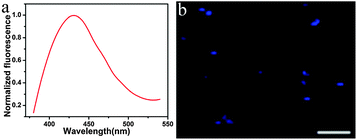 | ||
| Fig. 3 (a) Two-photon fluorescence of the MoS2 QDs (Ex: 690 nm) and (b) TPF image of solid MoS2 QDs (Ex: 690 nm, scale bar: 5 μm). | ||
Toxicity assessment is also of critical importance for the biological application. In this work, the MTT assay was used to evaluate the cytotoxicity of MoS2 QDs using MDA-MB-468 cells as a model. As shown in Fig. 4, low concentrations of MoS2 QDs have little toxicity toward these cells. Moreover, the cells maintained about 80% viability (as high as 150 μg mL−1) after incubation with QDs. In addition, the cells incubated with different concentrations of MoS2 QDs were stained with Hoechst 33342 and PI (Fig. S4, ESI†). It is well known that Hoechst 33342 can penetrate the cell membranes and label the cell nucleus with blue fluorescence, while PI is commonly used for identifying dead cells. It can be seen that the cell viability is very good even when the concentration of MoS2 QDs is up to 150 μg mL−1. Almost no dead cells are found because the living cells can't be stained by PI. These data indicate that the MoS2 QDs might be promising probes in cellular imaging.
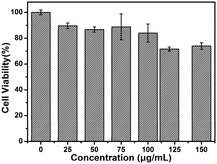 | ||
| Fig. 4 Cell viability by the MTT assay of MDA-MB-468 cells (the concentrations of MoS2 QDs are 0, 25, 50, 75, 100, 120, 150 μg mL−1, respectively). | ||
To demonstrate the MoS2 QD utility for TPF bioimaging, we further employ QDs for in vitro cell imaging. Fig. 5 and Fig. S5 (ESI†) show the TPF images of MDA-MB-468 cells incubated with MoS2 QDs for different durations. It can be observed that weak fluorescence appears in the cells within 10 min. The fluorescence intensity is enhanced gradually with the incubation time, and remains constant after 2 h. To confirm the distribution of MoS2 QDs in the cells, the TPF images were further investigated by culturing MDA-MB-468 and HeLa cells with QDs (50 μg mL−1) for 2 h. It can be observed in Fig. S6 (ESI†) that the MoS2 QDs are able to label the cell nucleus of MDA-MB-468 cells and HeLa cells, which is similar to that of graphitic-C3N4 QDs reported by Xie et al.39 There may be some specific interactions between MoS2 QDs and DNA (main components of chromatin). As shown in Fig. S7 (ESI†), with the addition of DNA, the fluorescence intensity of the solution of MoS2 QDs increases. Although the mechanism is not clear, it might be associated with its charge-transfer excited states that are sensitive to the external environment. Liu et al. reported water-soluble nanodots with an average diameter of 3.3 ± 0.5 nm for TPF imaging of cellular nuclei.40 They find enhanced fluorescence with the addition of DNA, and ascribe the phenomenon to the significant increment in the hydrophobicity of nanodots upon interaction with DNA. As a control, scarcely any fluorescence could be found in the cells without the MoS2 QDs (Fig. S8, ESI†). What's more, the fluorescence intensity of the MoS2 QDs within cells remains almost unchanged after irradiation under 690 nm for 2 h (Fig. S9, ESI†). The results imply that the QDs have great potential in applications of the TPF bioimaging.
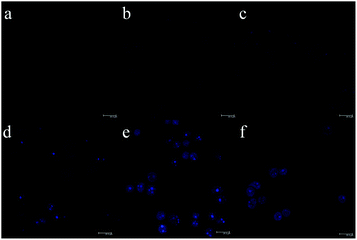 | ||
| Fig. 5 TPF images of MDA-MB-468 cells incubated with MoS2 QDs (50 μg mL−1) for 0 min to 3 h (a: 0 min, b: 10 min, c: 30 min, d: 1 h, e: 2 h, and f: 3 h). | ||
Conclusions
In summary, a facile, top-down, one-step ethanol-thermal route has been developed to synthesize MoS2 QDs with TPF behaviour. The QDs exhibit small size, high dispersibility, low cytotoxicity and excellent photostability. Most importantly, the as-prepared MoS2 QDs are well suited for staining cellular nuclei, and are adaptable for the further study of TPF bioimaging. Such MoS2 QDs are promising probes for applications in biological and deep-tissue imaging.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (21175046) and the Shanghai Natural Science Foundation (15ZR1411600).Notes and references
- W. Denk, J. H. Strickler and W. W. Webb, Science, 1990, 248, 73–76 CAS.
- F. Helmchen and W. Denk, Nat. Methods, 2005, 2, 932–940 CrossRef CAS PubMed.
- H. Kim, B. R. Kim, J. H. Hong, J.-S. Park, K. J. Lee and B. R. Cho, Angew. Chem., Int. Ed., 2007, 46, 7445–7448 CrossRef CAS PubMed.
- H. J. Kim, J. H. Han, M. K. Kim, C. S. Lim, H. Kim and B. R. Cho, Angew. Chem., Int. Ed., 2010, 49, 6786–6789 CrossRef CAS PubMed.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S.-Y. Xie and Y.-P. Sun, J. Am. Chem. Soc., 2009, 129, 11318–11319 CrossRef PubMed.
- S. Balendhran, J. Z. Ou, M. Bhaskaran, S. Sriram, S. Ippolito, Z. Vasic, E. Kats, S. Bhargava, S. Zhuiykovd and K. Kalantar-zadeh, Nanoscale, 2012, 4, 461–466 RSC.
- C. Xu and W. W. Webb, J. Opt. Soc. Am. B, 1996, 13, 481–491 CrossRef CAS.
- K. B. Male, B. Lachance, S. Hrapovic, G. Sunahara and J. H. T. Luong, Anal. Chem., 2008, 80, 5487–5493 CrossRef CAS PubMed.
- B. Kong, A. Zhu, C. Ding, X. Zhao, B. Li and Y. Tian, Adv. Mater., 2012, 24, 5844–5848 CrossRef CAS PubMed.
- Q. Liu, B. Guo, Z. Rao, B. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS PubMed.
- A. Molina-Sánchez and L. Wirtz, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 1–8 CrossRef.
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- Y. Wang and Y. Ni, Anal. Chem., 2014, 86, 7463–7470 CrossRef CAS PubMed.
- D. Gopalakrishnan, D. Damien and M. M. Shaijumon, ACS Nano, 2014, 8, 5297–5303 CrossRef CAS PubMed.
- J. Ou, A. F. Chrimes, Y. Wang, S. Tang, M. S. Strano and K. Kalantar-zadeh, Nano Lett., 2014, 14, 857–863 CrossRef CAS PubMed.
- H. Huang, C. Du, H. Shi, X. Feng, J. Li, Y. Tan and W. Song, Part. Part. Syst. Charact., 2015, 32, 72–79 CrossRef CAS.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. Chen and M. Chhowalla, Nano Lett., 2011, 11, 5111–5116 CrossRef CAS PubMed.
- H. D. Ha, D. J. Han, J. S. Choi, M. Park and T. S. Seo, Small, 2014, 10, 3858–3862 CrossRef CAS PubMed.
- X. Zhang, Z. Lai, Z. Liu, C. Tan, Y. Huang, B. Li, M. Zhao, L. Xie, W. Huang and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 1–5 CrossRef CAS.
- B. Li, L. Chen, H. Zou, J. Lei, H. Luo and N. Li, Nanoscale, 2014, 6, 9831–9838 RSC.
- S. Xu, D. Li and P. Wu, Adv. Funct. Mater., 2015, 25, 1127–1136 CrossRef CAS.
- Y. Liu, H. Nan, X. Wu, W. Pan, W. Wang, J. Bai, W. Zhao, L. Sun, X. Wang and Z. Ni, ACS Nano, 2013, 7, 4202–4209 CrossRef CAS PubMed.
- Y. C. Wang, J. Z. Ou, S. Balendhran, A. F. Chrimes, M. Mortazavi, D. D. Yao, M. R. Field, K. Latham, V. Bansal, J. R. Friend, S. Zhuiykov, N. V. Medhekar, M. S. Strano and K. Kalantar-zadeh, ACS Nano, 2013, 7, 10083–10093 CrossRef CAS PubMed.
- L. Changgu, Y. Hugen, L. E. Brus, T. F. Heinz, J. Hone and S. Ryu, ACS Nano, 2010, 4, 2695–2700 CrossRef PubMed.
- Q. Ji, Y. Zhang, T. Gao, Y. Zhang, D. Ma, M. Liu, Y. Chen, X. Qiao, P.-H. Tan, M. Kan, J. Feng, Q. Sun and Z. Liu, Nano Lett., 2013, 13, 3870–3877 CrossRef CAS PubMed.
- H. Li, Q. Zhang, C. C. R. Yap, B. K. Tay, T. H. T. Edwin, A. Olivier and D. Baillargeat, Adv. Funct. Mater., 2012, 22, 1385–1390 CrossRef CAS.
- N. H. Turner and A. M. Single, Surf. Interface Anal., 1990, 15, 215–222 CrossRef CAS.
- V. O. Koroteev, L. G. Bulusheva, I. P. Asanov, E. V. Shlyakhova, D. V. Vyalikh and A. V. Okotrub, J. Phys. Chem. C, 2011, 115, 21199–21204 CAS.
- J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 17881–17888 CrossRef CAS PubMed.
- J. Wilcoxon and G. Samara, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 7299–7302 CrossRef CAS.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS PubMed.
- H. Nie, M. Li, Q. Li, S. Liang, Y. Tan, L. Sheng, W. Shi and S. X.-A. Zhang, Chem. Mater., 2014, 26, 3104–3112 CrossRef CAS.
- D. Gopalakrishnan, D. Damien, B. Li, H. Gullappalli, V. K. Pillai, P. M. Ajayan and M. M. Shaijumon, Chem. Commun., 2015, 51, 6293–6296 RSC.
- V. Stengl and J. Henych, Nanoscale, 2013, 5, 3387–3394 RSC.
- R. Liu, D. Wu, X. Feng and K. Mullen, J. Am. Chem. Soc., 2011, 133, 15221–15223 CrossRef CAS PubMed.
- D. Wang, L. Wang, X. Dong, Z. Shi and J. Jin, Carbon, 2012, 50, 2147–2154 CrossRef CAS.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844–849 CrossRef CAS PubMed.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Nat. Methods, 2008, 5, 763–775 CrossRef CAS PubMed.
- X. Zhang, H. Wang, H. Wang, Q. Zhang, J. Xie, Y. Tian, J. Wang and Y. Xie, Adv. Mater., 2014, 26, 4438–4443 CrossRef CAS PubMed.
- K. Y. Pu, K. Li, X. Zhang and B. Liu, Adv. Mater., 2010, 22, 4186–4189 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Raman spectrum, XPS spectra, pH-dependent fluorescence spectrum, Hoechst 33342/PI staining, TPF images of MDA-MB-468 incubated for different times, TPF images of MDA-MB-468 and HeLa cells, fluorescence spectrum of MoS2 QDs in the presence of DNA, control experiment for TPF imaging, photostability of MoS2 QDs within cells. See DOI: 10.1039/c5tb01839k |
| This journal is © The Royal Society of Chemistry 2016 |