Research on the post-chain extension of aqueous polyurethane dispersions
Xiaomeng Peng,
Wusheng Wang*,
Naiyu Ma,
Wenhe Guo,
Xuekang Yu and
Xin Chen
College of Chemistry and Chemical Engineering of Anhui University, Hefei 230601, China
First published on 10th March 2016
Abstract
The post-chain extension efficiency of aqueous polyurethane dispersions (APUDs) was in inverse proportion to the amount of residual amine (NH2) groups, which could be measured by the color reaction with ninhydrin. In this research, the absorbance of chromogenic groups which appeared at 550–570 nm was investigated using ultraviolet-visible spectrophotometry. The results showed that there existed a higher chain extension efficiency at both high and low dispersion temperatures when water was used as the chain extender. The post-chain extension efficiency was affected by the “cage effect” of the inner particles of the APUDs. When ethylene diamine was used as the chain extender, the chain extension efficiency of APUDs increased with the decreasing of the particle size at low temperature, whereas the chain extension efficiency increased with the increase of particle size at the higher temperature. This unexpected phenomenon may be caused by the interaction of the “cage effect” and the interface reaction.
1. Introduction
Aqueous polyurethane dispersions (APUDs) have found widespread applications in coatings, leathers, foams, elastomers, fibers and adhesives in the past 40 years because of their unique properties such as versatility, high performance and low environmental pollution.1–4 A prepolymer dispersion process has been widely used to synthesise high performance APUDs for industrial applications.5 For the prepolymer dispersion process, post-chain extension was considered to be the key factor to obtaining APUDs with a higher molecular weight.6,7 The post-chain extension of the aqueous polyurethane prepolymer is very complex. A NCO-terminated prepolymer will react with water and amine chain extender. Although numerous reports8–13 have been devoted to investigating APUDs, the reaction mechanism of post-chain extension is still ambiguous.Previous studies mainly focused on the effect of post-chain extension on the properties of APUDs. Li et al.14 studied the corresponding post-chain extension behavior of renewable waterborne polyurethane dispersions (WBPUDs) using ethylene diamine (EDA), adipic dihydrazide (ADH) and water as chain extenders. The results showed that EDA extended the NCO-terminated polyurethane dispersion (PUD) prepolymer independently of the moment of its addition at both 30 °C and 50 °C; however, ADH only took place after removing the 2-butanone. Furthermore, the water chain extension reached a good balance at 50 °C, where the PUD had a high molecular weight, a small particle size and a narrow distribution. Lei et al.15 studied the effects of using the amine chain extender EDA, diethylene triamine, triethylene tetramine on the properties of WBPUDs. The results revealed that the post-chain extension mainly occurred on the surface of the WBPU particle, and the actual degree of maximum post-chain extension was 60%. Jhon et al.5 investigated the amount of residual NCO groups of the PUD prepolymer on the surface of particle. They found that both the total surface areas and the amounts of optimal chain extender would keep increasing with the decreasing of polyurethane particle size. It is believed, from previous reports devoted to the post-chain extension of APUDs, that the residual NCO groups react with the amine post-chain extender on the particle surface, but there have been no reports of research which have considered the competitive reactions of water and the polyamine chain extender.
Theoretically, in addition to the residual polyamine chain extender, there will also exist residual amine (NH2) groups after the post-chain extension of APUDs from the reaction between NCO groups and water. If the concentration of the residual NH2 groups of APUDs can be accurately measured, the reaction mechanism of post-chain extension can be traced and determined systematically. However, it is difficult to measure the residual NH2 groups using nuclear magnetic resonance, infrared spectroscopy and chemical titration because of the active hydrogen in the NH2 groups and the concentration of the residual NH2 groups is close to zero.
In this research, a series of APUDs were synthesized with different post-chain extension temperatures and concentrations of hydrophilic groups, and the post-chain extension of the APUDs was investigated with a more accurate method. The amount of residual NH2 groups was determined quantitatively using the color reaction of NH2 groups with ninhydrin, and the absorbance of the chromogenic groups was measured using ultraviolet-visible spectrophotometry (UV-vis). To a certain extent, the absorbance was corrected by the amount of residual NH2 groups present. The effect of particle size and post-chain extension temperature on the post-chain extension efficiency of APUDs are also discussed.
2. Experimental
2.1 Materials
Hexamethylene diisocyanate (HDI) was supplied by the Wanhua Chemical Group. Polytetramethylene glycol (PTMG) (Mn = 1000) was purchased from Qingdao Yutian Chemical. 2,2-Dimethylol propionic acid (DMPA) was obtained from Shanghai Nuotai Chemical Company. Diethylene glycol (DEG), 2-methyl-1,3-propanediol (MPD), triethylamine (TEA), EDA, organic bismuth, acetone, glacial acetic acid and ethanol were all supplied by SCR Chemicals. Ninhydrin was obtained from Tianjin Guangfu Chemical. Prior to use, the HDI, DEG and MPD were purified by distillation under vacuum.2.2 Synthesis of the aqueous polyurethane dispersions
A 500 ml three-necked round flask equipped with a reflux condenser, mercury thermometer and stirrer was used as reactor. The PTMG was introduced into the flask and stirred for 0.5 h at 90 °C. After cooling the reaction mixture to 60 °C, HDI was added and the reaction proceeded for 2 h at 85 °C. Then DMPA, DEG, MPD and acetone were added and the reaction mixture was subsequently stirred for 1 h at 85 °C. Then the temperature was cooled to 70 °C, organic bismuth catalysts and acetone were added to the flask and reacted for 3 h at 65 °C. Then an appropriate volume of acetone was added to the flask to adjust the viscosity. TEA was then added to the reactor and the reaction proceeded for 5 min with stirring to neutralize the carboxyl groups in the prepolymer. Then the waterborne polyurethane prepolymer was cooled to different temperatures (0 °C, 10 °C, 20 °C, 30 °C, 40 °C and 50 °C). Then the prepolymer was transferred to an emulsor and distilled water of the same temperature as the prepolymer was added under vigorous mechanical stirring. Finally, a series of APUDs with different emulsifying temperatures were obtained after the EDA chain extender was added to the aqueous polyurethane prepolymer emulsion.2.3 Characterization
The average particle size of the APUDs was measured using a Zetasizer Nano ZS90 laser particle sizer (Malvern Instruments, UK). All the samples were diluted with distilled water at a constant concentration of 0.1 wt%.The relative amount of residual NH2 groups in the APUDs was measured using UV-vis spectrophotometry with a UV-3600 (Shimadzu, Japan).
3. Results and discussion
3.1 Residual NH2 measurement using a ninhydrin color reaction
Configuring precisely the different concentrations of butane diamine solution, the corresponding volume of ninhydrin was added to make the butane diamine solution colored. Then the absorption peak intensity of the butane diamine solutions were measured using UV-vis, and there existed a single absorption peak in the range of 550–570 nm. The working curve diagram is shown in Fig. 2, with absorption peak intensity and the concentration of NH2 groups as the y and x axes, respectively.
3.2 Water chain extension research
The reaction between the NCO groups and water is usually divided into two steps. In the first step, the NCO groups reacted with water to form carbamic acid, which was normally unstable and then resolved into amine and carbon dioxide. After that, the amine would react with another isocyanate to form disubstituted urea, as shown in eqn (1).
 | (1) |
Generally, the reactivity of the water with isocyanate was somewhat slower than that of the amine. The NH2-terminated prepolymer would react with the other prepolymer to cause a post-chain extension reaction of the APUD. The post-chain extension efficiency of the APUD increased as the temperature increased. In this research, it has been found that the relationship between post-chain efficiency and dispersion temperature was complex.
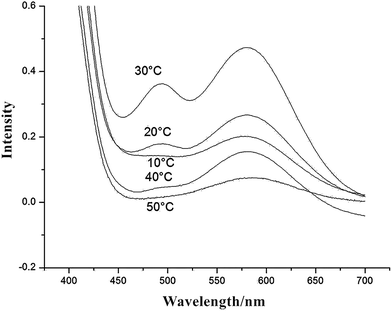
Fig. 3 shows that the UV-vis absorption intensity of the residual NH2 groups of APUDs with different dispersion temperatures which were prepared without the diamine chain extender. The absorption intensity was proportional to the residual NH2 groups' concentration. It could be seen that for all of the APUDs samples with mass residual NH2 groups, there existed weaker absorption peak under both higher temperature (40–50 °C) and lower temperature (10–20 °C) dispersion. Simultaneously the strongest absorption peak appeared at 30 °C.
These unexpected results can be explained by a reasonable inference that the prepolymer molecule reacts with water to form a NH2-terminated prepolymer which will be restricted by another prepolymer molecular chain. This phenomenon is defined as the “cage effect”. The “cage effect” will result in the NH2-terminated prepolymer molecular chain rarely reacting with another NCO-terminated prepolymer, in contrast to the easily formation of NH2 groups from the NCO-terminated prepolymer reacting with water because the water molecules diffuse freely in the APUD micelles, as shown as Fig. 4.
The influence of dispersion temperature on water chain extension efficiency can be easily clarified. The NH2-terminated prepolymer chain reaction with another NCO-terminated prepolymer chain is related to the viscosity of the APUD system. When the system viscosity increases, the “cage effect” is enhanced, and the probability of collision of the NH2 groups and NCO groups in another prepolymer molecular chain decreases. The viscosity of the APUD system is associated with the temperature, and the higher the temperature is, the lower the viscosity of the system will be. Therefore, when the dispersion temperature decreases to 10 °C, the reaction probability between water and the NCO groups dramatically decreases, which leads to the residual NCO groups concentration increasing, and the probability of collision of the NH2 groups and NCO groups in another prepolymer molecular chain also increases, thus the chain extension efficiency is enhanced. When the dispersion temperature is more than 30 °C, the “cage effect” is weakened, and the probability of collision between NH2 groups and NCO groups increases as a consequence of the increasing APUD molecular motion ability and water reactivity, and so the water chain extension efficiency increases. In the middle temperature interval, the reaction rates between water and NCO groups to form NH2 groups is fast although the “cage effect” is still prominent. The collision probability between NH2 groups and NCO groups is smaller, and the interaction of the water's reactivity and the “cage effect” decreases the water chain extension efficiency.
3.3 EDA chain extension research
Fig. 5 shows that the influence of the average particle size on EDA chain extension efficiency at different post-chain extension temperatures. As shown in Fig. 5(a)–(d), when the post-chain extension temperature was lower than 40 °C, the smaller particle size would result in less residual NH2 groups and higher chain extension efficiency. A similar conclusion was also reported by Wu et al.18 When the post-chain extension temperature was higher than 30 °C, the results were different from the general conclusion: the larger the particle size, the less residual NH2 groups there were, and the higher the chain extension efficiency. | ||
| Fig. 5 The influence of average particle size on residual NH2 group with different temperatures: (a) 0 °C; (b) 10 °C; (c) 20 °C; (d) 30 °C; (e) 40 °C; (f) 50 °C. | ||
The unexpected results were caused by the combined action of interface reaction and the “cage effect”. The interface reaction is a dominant factor when the post-chain extension temperature is lower than 40 °C. Inside the particle, the decreased particle size will result in the increased water swelling ratio. The probability of a reaction between water and the NCO groups inside the APUD particle increases, the residual NH2 groups inside particle increase because the “cage effect” is stronger at low temperature, and the post-chain extension reaction taking place inside the particle is decreased. On the surface of the particle, with a decrease in particle size, the total specific surface area of the APUD particle increases, and the surface post-chain extension efficiency increases because the interface reaction increases. The combined action of the “cage effect” and interface reaction causes the higher post-chain extension efficiency, when the particle size is smaller at low post-chain extension temperatures, as shown in Fig. 5(a)–(d). The reaction mechanism is shown in Fig. 6.
When the post-chain extension temperature is greater than 30 °C, the “cage effect” is a dominant factor. With the particle size increasing, the total specific surface area of the APUD particle decreases, and the surface post-chain extension efficiency decreases because the interface reaction decreases. In addition, the water swelling ratio decreases as the particle size increases, and the reaction probability between water and the NCO groups decreases, the residual NCO groups concentration increases, the probability of collision of the NH2 groups and NCO groups in another prepolymer molecular chain increases because of the “cage effect” and decreases at a high temperature. The combined action results in the higher post-chain extension efficiency, when the particle size is larger at high post-chain extension temperature. The reaction model of the interface reaction and the “cage effect” is shown in Fig. 4. The reaction mechanism is shown Fig. 7.
4. Conclusion
In this work, the post-chain extension efficiency of APUDs using water and EDA as chain extension agent was investigated using UV-vis spectrophotometry. The residual NH2 groups' concentration was measured using its color reaction with ninhydrin. The water chain extension research indicated that a relatively high chain extension efficiency could be achieved with either higher temperature (50 °C and 40 °C) or lower temperature (10 °C). The reason for this could be explained by the “cage effect”. In addition, the EDA post-chain extension with different post-chain extension temperatures was also investigated, and the results showed that the post-chain extension of APUDs was influenced by the combined action of the interface reaction and the “cage effect”. The interface reaction was a dominant factor when the post-chain temperature was below 40 °C, whereas the “cage effect” was a key factor when the post-chain temperature was higher than 30 °C. The combined results showed that APUDs with a smaller particle size had a better post-chain extension efficiency at the low post-chain extension temperature, whereas APUDs with a larger particle size had a better post-chain extension efficiency at the higher post-chain extension temperature. The results of this study will be significant for obtaining a higher performance in the industrial production of APUD.Acknowledgements
The research was supported by the Ministry of Science and Technology of the People's Republic of China (863 Project, No. 2015AA033903).References
- M. M. Rahman and W.-K. Lee, J. Appl. Polym. Sci., 2009, 114, 3767–3773 CrossRef CAS.
- M. M. Rahman, H.-D. Kim and W.-K. Lee, J. Adhes. Sci. Technol., 2009, 23, 177–193 CrossRef CAS.
- M.-S. Yen, P.-Y. Tsai and P.-D. Hong, Colloids Surf., A, 2006, 279, 1–9 CrossRef CAS.
- H. Du, Y. Zhao, Q. Li, J. Wang, M. Kang, X. Wang and H. Xiang, J. Appl. Polym. Sci., 2008, 110, 1396–1402 CrossRef CAS.
- Y. K. Jhon, I. W. Cheong and J. H. Kim, Colloids Surf., A, 2001, 179, 71–78 CrossRef CAS.
- A. K. Nanda, D. A. Wicks, S. A. Madbouly and J. U. Otaigbe, J. Appl. Polym. Sci., 2005, 98, 2514–2520 CrossRef CAS.
- Y. Wang, C. Ruan, J. Sun, M. Zhang, Y. Wu and K. Peng, Polym. Degrad. Stab., 2011, 96, 1687–1694 CrossRef CAS.
- R. A. Brown, R. G. Coogan, D. G. Fortier, M. S. Reeve and J. D. Rega, Prog. Org. Coat., 2005, 52, 73–84 CrossRef CAS.
- A. Dong, G. Hou and D. Sun, J. Colloid Interface Sci., 2003, 266, 276–281 CrossRef CAS PubMed.
- S. M. S. Mohaghegh, M. Barikani and A. A. Entezami, Colloids Surf., A, 2006, 276, 95–99 CrossRef CAS.
- P. Florian, K. K. Jena, S. Allauddin, R. Narayan and K. V. S. N. Raju, Ind. Eng. Chem. Res., 2010, 49, 4517–4527 CrossRef CAS.
- K.-L. Noble, Prog. Org. Coat., 1997, 32, 131–136 CrossRef CAS.
- J. Y. Jang, Y. K. Jhon, I. W. Cheong and J. H. Kim, Colloids Surf., A, 2002, 196, 135–143 CrossRef CAS.
- Y. Li, B. A. J. Noordover, R. A. T. M. van Benthem and C. E. Koning, Eur. Polym. J., 2014, 52, 12–22 CrossRef CAS.
- L. Lei, L. Zhong, X. Lin, Y. Li and Z. Xia, Chem. Eng. J., 2014, 253, 518–525 CrossRef CAS.
- C. B. Bottom, S. S. Hanna and D. J. Siehr, Biochem. Educ., 1978, 6, 4–5 CrossRef CAS.
- M. Friedman, J. Agric. Food Chem., 2004, 52, 385–406 CrossRef CAS PubMed.
- G. Wu, J. Li, C. Chai, Z. Ge, J. Lin and Y. Luo, RSC Adv., 2015, 5, 97710–97719 RSC.
| This journal is © The Royal Society of Chemistry 2016 |