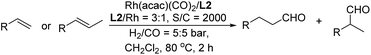New tetraphosphite ligands for regioselective linear hydroformylation of terminal and internal olefins†
Zongpeng Zhang‡
,
Caiyou Chen‡,
Qian Wang,
Zhengyu Han,
Xiu-Qin Dong* and
Xumu Zhang*
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei 430072, P. R. China. E-mail: xumu@whu.edu.cn; xiuqindong@whu.edu.cn
First published on 28th January 2016
Abstract
We successfully developed new tetraphosphite ligands L1–L5 and applied them to the rhodium-catalyzed hydroformylation of terminal and internal olefins. High catalytic reactivities and excellent regioselectivities for linear aldehydes were obtained in the rhodium-catalyzed hydroformylation of simple olefins (l/b ratio up to 90, 98.9% linear selectivity, 99.2% conversion) using the tetraphosphite ligand L2. And the tetraphosphite ligand L2 also displayed moderate to good linear regioselectivities for challenging substrates styrene and internal olefin 2-octene.
Introduction
Since its discovery by Otto Roelen in 1938,1 the hydroformylation reaction is one of the most efficient routes for the functionalization of olefins to approach aldehydes now.2 It has been developed into one of the most important homogeneous catalytic processes with rhodium-based catalysts in the field of industrial chemistry.3 The corresponding aldehyde products are very important compounds and valuable intermediates for the synthesis of various chemicals, such as alcohols, amines and esters etc.4 They are widely applied to construct blocks for pharmaceuticals, agrochemicals, commodities and fine chemicals.5Ligand is one of the most significant factors to access high activity and selectivity of hydroformylation reaction catalyzed by the rhodium-based catalysts. Therefore, much attention has been paid to designing efficient and privileged ligands for the formation of industrially important aldehydes. A variety of excellent bisphosphorous ligands have been successfully developed for Rh-catalyzed hydroformylation reactions in the past decades, such as bisbi,6 biphephos,7 naphos,8 xantphos,9 calix4 arene-based bisphosphites,10 pyrrole-based bisphos-phoramidites,11 and self-assembled bisphosphanes.12 In addition, some new tetraphosphorus ligands were developed in our lab.13 These ligands owned outstanding catalytic properties for their unique four identical coordination modes.13a,b Importantly, due to much higher local phosphine concentration around the metal center, the tetraphosphorus ligands afforded better chelating ability and thus exhibited much better regioselectivities compared with the corresponding bisphosphorus ligands. Latter we also successfully developed new triphosphorus ligands.14 Similar to the tetraphosphorus ligands, the triphosphorus ligands also have better chelating ability with two identical coordination modes with rhodium and exhibited better regioselectivities compared with the corresponding bisphosphorus ligands. Although great efforts have been made to develop new ligands for linear hydroformylation, new ligands are still highly desirable to further resolve the problems of catalytic efficiency and selectivity.
Based on our long standing interest of tetraphosphorus ligands in hydroformylation,13 our efforts were devoted to further developing new phosphorus ligands with excellent performance. Extensive research shown that the phosphines were typical-donors ligands and phosphites were strong-acceptors ligands. The phosphite ligands can facilitate the CO dissociation from the metal centers in the catalytic species. Therefore, it is helpful to greatly improve the reactivity by using the phosphite ligands in Rh-catalyzed hydroformylation reaction. We believe that the new tetraphosphite ligands L1–L5 with four identical coordination modes with rhodium will show good reactivities and regioselectivities in the linear hydroformylation (Fig. 1). Importantly, ligands L1–L5 are very concise and can be facilely synthesized. Herein, we present the synthetic route of new tetraphosphite ligands L1–L5, and the application in Rh-catalyzed hydroformylation reaction of simple and unfunctionalized olefins, providing the desired products in high conversions with moderate to excellent regioselectivities.
Results and discussion
The new tetraphosphite ligands L1–L5 were efficiently synthesized from readily available starting materials (Scheme 1).15 The ligand backbone 2,6,2′,6′-tetramethoxybenzene 1 was smoothly deprotected with BBr3,15a formed the key intermediate [1,1′-biphenyl]-2,2′,6,6′-tetraol 2. The condensation of compound 2 with the preformed phosphorochloridite in the presence of the triethylamine as hydrogen chloride scavenger provided the desired tetraphosphite ligands L1–L5 in good yields.15cWith the tetraphosphite ligands L1–L5 in hand, we began our studies by evaluating them in the linear hydroformylation of 1-octene as the model substrate with the catalyst generated in situ by mixing Rh(acac)(CO)2 and ligands L1–L5 in toluene. As shown in Table 1, ligands L1–L5 displayed high reactivities and excellent regioselectivities (Table 1, entries 1–5). Almost all of the reactions finished within 2 h. To our delight, the ligand L2 was revealed as the best ligand in terms of regioselectivity (ratio of l/b up to 31, Table 1, entry 2). Ligands screening results demonstrated that the substituents on the biphenyl ring played a key role in determining the regioselectivity.
| Entry | L | Conv.b (%) | l/bc | Lineard (%) | Iso.e (%) | TONf |
|---|---|---|---|---|---|---|
| a S/C = 2000, [Rh] = 0.2 μM, toluene as solvent, 1-octene as the substrate, decane as internal standard, L1–L5 as the ligand.b Conversion of 1-octene was determined on the basis of GC analysis.c Linear/branched ratio was determined on the basis of GC analysis.d Percentage of linear aldehyde.e Percentage of the isomerized alkene.f Turn over number (TON) was determined on the basis of the alkene conversion by GC analysis. | ||||||
| 1 | L1 | 98.5 | 19 | 95.0 | 9.5 | 1.97 × 103 |
| 2 | L2 | 88.2 | 31 | 96.9 | 9.1 | 1.76 × 103 |
| 3 | L3 | 98.4 | 13 | 92.9 | 9.7 | 1.97 × 103 |
| 4 | L4 | 98.9 | 9 | 90.0 | 5.3 | 1.97 × 103 |
| 5 | L5 | 99.7 | 9 | 90.0 | 7.2 | 1.97 × 103 |
Subsequently, we investigated the effects of ligand L2/metal molar ratios, reaction temperature, and the pressure of CO/H2 on the catalytic activity and regioselectivity. As expected, the ratio of ligand L2/Rh(acac)(CO)2 has a great influence on the reaction, increasing the ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 2, entries 1–3) led to the dramatic improvement on the regioselectivity and the ratio of l/b was improved from 31 to 46. The conversion became lower when the ligand/metal ratio was increased to 4
1 (Table 2, entries 1–3) led to the dramatic improvement on the regioselectivity and the ratio of l/b was improved from 31 to 46. The conversion became lower when the ligand/metal ratio was increased to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, although a little higher regioselectivity was obtained (Table 2, entry 4). The further increment of the ligand/metal ratio to 8
1, although a little higher regioselectivity was obtained (Table 2, entry 4). The further increment of the ligand/metal ratio to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 resulted in nearly no reactivity (Table 2, entry 5). The reaction temperature also displayed dramatic effect on the reaction. Decreasing the temperature from 80 °C to 60 °C gave lower reactivity (Table 2, entry 3 vs. entry 6). In addition, we found that the catalytic system is also sensitive to the pressures of CO/H2. Excellent regioselectivity and reactivity were obtained when the pressures of CO/H2 was maintained at 5
1 resulted in nearly no reactivity (Table 2, entry 5). The reaction temperature also displayed dramatic effect on the reaction. Decreasing the temperature from 80 °C to 60 °C gave lower reactivity (Table 2, entry 3 vs. entry 6). In addition, we found that the catalytic system is also sensitive to the pressures of CO/H2. Excellent regioselectivity and reactivity were obtained when the pressures of CO/H2 was maintained at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 bar (up to 93.4% conversion and l/b ratio up to 65, Table 2, entry 7).
5 bar (up to 93.4% conversion and l/b ratio up to 65, Table 2, entry 7).
| Entry | L/Rh | Tb (°C) | Conv.c (%) | l/bd | Lineare (%) | Iso.f (%) | TONg |
|---|---|---|---|---|---|---|---|
a S/C = 2000, [Rh] = 0.2 μM, toluene as solvent, 1-octene as the substrate, decane as internal standard, L2 as the ligand.b Oil bath temperature.c Conversion of 1-octene was determined on the basis of GC analysis.d Linear/branched ratio was determined on the basis of GC analysis.e Percentage of linear aldehyde.f Percentage of the isomerized alkene.g Turn over number (TON) was determined on the basis of the alkene conversion by GC analysis.h H2/CO = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 bar. NA = not available. 5 bar. NA = not available. |
|||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 88.2 | 31 | 96.9 | 9.1 | 1.76 × 103 |
| 2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 86.9 | 35 | 97.2 | 8.5 | 1.72 × 103 |
| 3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 81.8 | 46 | 97.9 | 5.9 | 1.64 × 103 |
| 4 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 70.7 | 47 | 97.9 | 5.6 | 1.41 × 103 |
| 5 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | NA | NA | NA | NA | NA |
| 6 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 | 50.9 | 43 | 97.8 | 3.5 | 1.02 × 103 |
| 7h | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 93.4 | 65 | 98.5 | 6.3 | 1.87 × 103 |
Solvent effects were also investigated and the results were summarized in Table 3. The reactions were performed well in toluene, ethyl acetate and 1,4-dioxane with similar results (Table 3, entries 1, 3, 6). CH2Cl2 as the solvent afforded high l/b ratio (up to 86) and excellent conversion (96.9% conversion, Table 3, entry 2). Moderate conversion was achieved in isopropanol (74.9% conversion, Table 3, entry 5). Compared with CH2Cl2, chloroform and acetonitrile gave similar regioselectivies but with a little lower reactivities (Table 3, entries 4 and 7). As a result, CH2Cl2 was the best choice as the solvent.
| Entry | Solvent | Conv.b (%) | l/bc | Lineard (%) | Iso.e (%) | TONf |
|---|---|---|---|---|---|---|
| a S/C = 2000, [Rh] = 0.2 μM, 1-octene as the substrate, decane as internal standard, L2 as the ligand.b Conversion of 1-octene was determined on the basis of GC analysis.c Linear/branched ratio was determined on the basis of GC analysis.d Percentage of linear aldehyde.e Percentage of the isomerized alkene.f Turn over number (TON) was determined on the basis of the alkene conversion by GC analysis. EA = ethyl acetate. | ||||||
| 1 | Toluene | 93.4 | 65 | 98.5 | 6.3 | 1.87 × 103 |
| 2 | CH2Cl2 | 96.9 | 86 | 98.8 | 6.8 | 1.94 × 103 |
| 3 | EA | 92.6 | 65 | 98.5 | 6.7 | 1.85 × 103 |
| 4 | CHCl3 | 88.6 | 84 | 98.8 | 5.8 | 1.77 × 103 |
| 5 | iPrOH | 74.9 | 68 | 98.6 | 5.3 | 1.50 × 103 |
| 6 | Dioxane | 97.5 | 67 | 98.6 | 6.6 | 1.95 × 103 |
| 7 | CH3CN | 91.4 | 89 | 98.9 | 7.6 | 1.83 × 103 |
| 8 | THF | 96.9 | 59 | 98.3 | 7.3 | 1.94 × 103 |
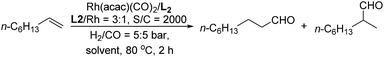
Promoted by these excellent results, we turned our attention to investigate the catalytic system Rh(acac)(CO)2/L2 for the hydroformylation of representative substrates. As shown in Table 4, 1-octene and 1-hexene provided excellent results in the transformations. Conversion was up to 99.2% and the ratio of l/b was up to 90 (Table 4, entries 1–2). In addition, we also applied them into the hydroformylation of styrene, which is a well-known olefinic substrate preferring the branched aldehyde in most Rh-catalyzed hydroformylation transformations. We found that the tetraphosphite ligand L2 displayed moderate reactivity and regioselectivity (Table 4, entry 3). To our delight, the challenging substrate internal olefin 2-octene (trans/cis molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) also proceeded well and obtained good regioselectivity (Table 4, entry 4).
1) also proceeded well and obtained good regioselectivity (Table 4, entry 4).
| Entry | Substrate | Conv.b (%) | l/bc | Lineard (%) | Iso.e (%) | TONf |
|---|---|---|---|---|---|---|
| a S/C = 2000, [Rh] = 0.2 μM, CH2Cl2 as solvent, decane as internal standard, L2 as the ligand.b Conversion was determined on the basis of GC analysis.c Linear/branched ratio was determined on the basis of GC analysis.d Percentage of linear aldehyde.e Percentage of the isomerized alkene.f Turn over number (TON) was determined on the basis of the alkene conversion by GC analysis.g The reaction temperature is 100 °C, and the reaction time is 10 h. ND = not determined. | ||||||
| 1 | 1-Octene | 96.9 | 86 | 98.8 | 6.8 | 1.94 × 103 |
| 2 | 1-Hexene | 99.2 | 90 | 98.9 | 6.9 | 1.98 × 103 |
| 3 | Styrene | 63.4 | 0.6 | 37.5 | ND | 1.28 × 103 |
| 4g | 2-Octene | 60.6 | 16 | 94.1 | ND | 1.20 × 103 |
Conclusions
In conclusion, new tetraphosphite ligands L1–L5 were successfully developed and applied in the Rh-catalyzed hydroformylation of terminal and internal olefins. High catalytic reactivity and excellent regioselectivity for the linear aldehydes were obtained in the Rh-catalyzed hydroformylation of simple and unfunctionalized olefins (l/b ratio up to 90, 98.9% linear selectivity, 99.2% conversion) using the tetraphosphite ligand L2. In addition, the tetraphosphite ligand L2 displayed moderate linear regioselectivity for styrene affording 3-phenylpropanal. And the challenging substrate internal olefin 2-octene also proceeded well and obtained good regioselectivity. Further application of the ligands for related catalytic reactions is underway in our laboratory.Acknowledgements
We thank the grant from Wuhan University (203273463, 203410100064), and “111” Project of the Ministry of Education of China for financial support and the National Natural Science Foundation of China (Grant No. 21372179, 21432007, 21502145).Notes and references
- O. Roelen, (to Chemische Verwertungsgesellschaft Oberhausen m. b. H.) German Pat. DE 849548, 1938/1952, U.S. Pat. 2.327066, 1943Chemical Abstracts, 1944, 38, 3631.
- P. W. N. M. van Leeuwen, Homogeneous Catalysis: Understanding the Art, 2004, p. 424 Search PubMed.
- (a) C. A. Tolman and J. W. Faller, in Homogeneous Catalysiswith Metal Phosphine Complexes, 81, ed. L. H. Pignolet, Plenum, New York, 1983 Search PubMed; (b) C. W. Kohlpaintner and C. D. Frohning in Applied Homogeneous Catalysis with Organometallic Compounds 1, ed. B. Cornils and W. A. Herrmann, VCH, Weinheim, 1996, vol. 1 Search PubMed; (c) R. Franke, D. Selent and A. Börner, Chem. Rev., 2012, 112, 5675 CrossRef CAS PubMed.
- A. Neves, M. Calvete, T. Pinhoe Melo and M. Pereira, Eur. J. Org. Chem., 2012, 32, 6309 CrossRef.
- For reviews, see: (a) B. Breit and W. Seiche, Synthesis, 2001, 1, 1 CrossRef; (b) C. Claver and P. W. N. M. van Leeuwen, Rhodium Catalyzed Hydroformylation, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2002 Search PubMed; (c) F. Ungvary, Coord. Chem. Rev., 2005, 249, 2946 CrossRef CAS.
- (a) T. J. Devon, G. W. Phillips, T. A. Puckette, J. L. Stavinoha and J. J. Vanderbilt, US Pat.4694109, 1987; (b) W. A. Herrmann, C. W. Kohlpaintner, E. Herdtweck and P. Kiprof, Inorg. Chem., 1991, 30, 4271 CrossRef CAS; (c) C. P. Casey, G. T. Whiteker, M. G. Melville, L. M. Petrovich, J. A. Gavney Jr and D. R. Powell, J. Am. Chem. Soc., 1992, 114, 5535 CrossRef CAS; (d) C. P. Casey, E. L. Paulsen, E. W. Beuttenmueller, B. R. Proft, L. M. Petrovich, B. A. Matter and D. R. Powell, J. Am. Chem. Soc., 1997, 119, 11817 CrossRef CAS.
- (a) E. Billig, A. G. Abatjoglou and D. R. Bryant, European Pat. EP 213639, 1987; (b) G. D. Cunny and S. L. Buchwald, J. Am. Chem. Soc., 1993, 115, 2066 CrossRef.
- (a) W. A. Herrmann, R. Schmid, C. W. Kohlpaintner and T. Priermeier, Organometallics, 1995, 14, 1961 CrossRef CAS; (b) H. Klein, R. Jackstell, K.-D. Wiese, C. Borgmann and M. Beller, Angew. Chem., Int. Ed., 2001, 40, 3408 CrossRef CAS.
- (a) M. Kranenburg, Y. E. M. van der Burgt, P. C. J. Kamer, P. W. N. M. van Leeuwen, K. Goubitz and J. Fraanje, Organometallics, 1995, 14, 3081 CrossRef CAS; (b) L. A. Van der Veen, M. D. K. Boele, F. R. Bregman, P. C. J. Kamer, P. W. N. M. van Leeuwen, K. Goubitz, J. Fraanje, H. Schenk and C. Bo, J. Am. Chem. Soc., 1998, 120, 11616 CrossRef CAS; (c) L. A. van der Veen, P. C. J. Kamer and P. W. N. M. van Leeuwen, Angew. Chem., Int. Ed., 1999, 38, 336 CrossRef CAS; (d) L. A. van der Veen, P. C. J. Kamer and P. W. N. M. van Leeuwen, Organometallics, 1999, 18, 4765 CrossRef CAS; (e) J. J. Carbó, F. Maseras, C. Bo and P. W. N. M. van Leeuwen, J. Am. Chem. Soc., 2001, 123, 7630 CrossRef; (f) P. C. J. Kamer, P. W. N. M. van Leeuwen and J. N. H. Reek, Acc. Chem. Res., 2001, 34, 895 CrossRef CAS PubMed.
- R. Paciello, L. Siggel and M. Röer, Angew. Chem., Int. Ed., 1999, 38, 1920 CrossRef CAS.
- (a) R. Jackstell, H. Klein, M. Beller, K.-D. Wiese and D. Rottger, Eur. J. Org. Chem., 2001, 3871 CrossRef CAS; (b) S. C. van der Slot, J. Duran, J. Luten, P. C. J. Kamer and P. W. N. M. van Leeuwen, Organometallics, 2002, 21, 3873 CrossRef CAS; (c) Y. Yan, X. Zhang and X. Zhang, J. Am. Chem. Soc., 2006, 128, 16058 CrossRef CAS PubMed.
- (a) V. F. Slagt, J. N. H. Reek, P. C. J. Kamer and P. W. N. M. van Leeuwen, Angew. Chem., Int. Ed., 2001, 40, 4271 CrossRef CAS; (b) V. F. Slagt, P. W. N. M. van Leeuwen and J. N. H. Reek, Angew. Chem., Int. Ed., 2003, 42, 5619 CrossRef CAS PubMed; (c) V. F. Slagt, P. C. J. Kamer, P. W. N. M. van Leeuwen and J. N. H. Reek, J. Am. Chem. Soc., 2004, 126, 1526 CrossRef CAS PubMed; (d) B. Breit and W. Seiche, J. Am. Chem. Soc., 2003, 125, 6608 CrossRef CAS PubMed; (e) B. Breit and W. Seiche, Angew. Chem., Int. Ed., 2005, 44, 1640 CrossRef CAS PubMed; (f) W. Seiche, A. Schuschkowski and B. Breit, Adv. Synth. Catal., 2005, 347, 1488 CrossRef CAS; (g) M. Kuil, T. Soltner, P. W. N. M. van Leeuwen and J. N. H. Reek, J. Am. Chem. Soc., 2006, 128, 11344 CrossRef CAS PubMed; (h) P. Dydio, W. I. Dzik and M. Lu, Angew. Chem., Int. Ed., 2011, 50, 396 CrossRef CAS PubMed; (i) U. Gellrich, W. Seiche, M. Keller and B. Breit, Angew. Chem., Int. Ed., 2012, 51, 11033 CrossRef CAS PubMed.
- (a) Y. Yan, X. Zhang and X. Zhang, J. Am. Chem. Soc., 2006, 128, 16058 CrossRef CAS PubMed; (b) Y. Yan, X. Zhang and X. Zhang, Adv. Synth. Catal., 2007, 349, 1582 CrossRef CAS; (c) S. Yu, Y. Chie and X. Zhang, Adv. Synth. Catal., 2009, 351, 537 CrossRef CAS; (d) S. Yu, X. Zhang, Y. Yan, C. Cai, L. Dai and X. Zhang, Chem.–Eur. J., 2010, 16, 4938 CrossRef CAS PubMed; (e) S. Yu, Y. Chie, Z. Guan and X. Zhang, Org. Lett., 2008, 10, 3469 CrossRef CAS PubMed; (f) S. Yu, Y. Chie, Z. Guan, Y. Zou, W. Li and X. Zhang, Org. Lett., 2009, 11, 241 CrossRef CAS PubMed.
- (a) C. Chen, Y. Qiao, H. Geng and X. Zhang, Org. Lett., 2013, 15, 1048 CrossRef CAS PubMed; (b) C. Chen, P. Li, Z. Hu, H. Wang, H. Zhu, X. Hu, Y. Wang, H. Lv and X. Zhang, Org. Chem. Front., 2014, 1, 947 RSC.
- (a) H. Kakei, R. Tsuji, T. Ohshima, H. Morimoto, S. Matsunaga and M. Shibasaki, Chem.–Asian J., 2007, 2, 257 CrossRef CAS PubMed; (b) F. Fang, F. Xie, H. Yu, H. Zhang, B. Yang and W. Zhang, Tetrahedron Lett., 2009, 50, 6672 CrossRef CAS; (c) J. Ruchti and E. M. Carreira, J. Am. Chem. Soc., 2014, 136, 16756 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra of compounds. See DOI: 10.1039/c5ra23683e |
| ‡ Zongpeng Zhang and Caiyou Chen contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |