Improved photo-luminescence behaviour of Eu3+ activated CaMoO4 nanoparticles via Zn2+ incorporation†
B. P. Singh*a,
Maheshwaryb,
P. V. Ramakrishnac,
Saurabh Singha,
V. K. Sonud,
Santosh Singhe,
P. Singha,
A. Bahadurf,
R. A. Singhb and
S. B. Raif
aDepartment of Physics, IIT (BHU), Varanasi-221005, India. E-mail: bheeshmapratap@gmail.com; bpsingh.rs.app@itbhu.ac.in
bDepartment of Physics, Dr. H. S. Gour Central University, Sagar, M. P.-470003, India
cDepartment of Physics, Andhra University, Visakhapatanam-530003, India
dCentre for Advanced Studies in Chemistry, North Eastern Hill University, Shillong-793022, India
eDepartment of Pure and Applied Physics, Guru Ghasidas University, Bilaspur, India-495009
fDepartment of Physics, Banaras Hindu University, Varanasi-221005, India
First published on 22nd May 2015
Abstract
Zn2+ (0, 2, 5, 7 and 10 at%) co-doped CaMoO4:2Eu3+ nanophosphors have been synthesized via the polyol method using ethylene glycol (EG) as both capping agent and reaction medium at 150 °C. From XRD analysis, all 900 °C annealed Zn co-doped CaMoO4:Eu3+ nanophosphors have a tetragonal scheelite phase. Some extra phase evolution has been observed for the as-prepared Zn doped samples. The intensity and crystallinity of XRD patterns increase as heat treatment increases to 900 °C. The valence states of the involved compositions (Zn co-doped CaMoO4:Eu) were investigated by X-ray photoelectron spectroscopy (XPS) and it was found that Ca, Mo, Eu and Zn are in their +2, +6, +3 and +2 oxidation states, respectively. TG-DSC studies of the as-prepared samples corroborate their thermal stability. A TEM (Transmission electron microscopy) study reveals that the particles have spherical morphology. Photoluminescence studies have been carried out under ∼266, and 395 nm excitation wavelengths. Zn co-doping in the CaMoO4:Eu matrix produces a high distortion and modifies the crystal field around the Eu3+ ion and improves the PL intensity. CIE co-ordinates of the 900 °C annealed 10 at% Zn co-doped CaMoO4:Eu sample under 266 nm excitation is x = 0.64 and y = 0.35, which are close to the standard of NTSC (x = 0.67 and y = 0.33). These investigations reveal that Zn co-doped CaMoO4:Eu3+ nano-materials can be used as potential red emitting phosphors, an area which is a bottleneck in the development of low cost LEDs.
1. Introduction
White light-emitting diodes (w-LEDs) are promising and are supposed to be the next generation in illumination due to their high energy efficiencies, long lifetimes, good reliability and environmental friendliness compared to conventional incandescent lamps. It is well known that w-LEDs are produced by mixing red, green and blue (RGB) LEDs, combing a blue LED with a yellow phosphor of (Y,Gd)3(Al,Ga)5O12:Ce3+, or using near-ultraviolet (UV) LED-stimulated RGB phosphors. The major challenge in developing near-UV LEDs is to explore highly efficient tri-color phosphors that possess an excitation wavelength that matches well with the emission spectrum of the near-UV LED chips.1–3 The current red phosphors based on Eu3+ activated oxides, sulphides and nitrides have disadvantages such as low reliability, high toxicity and luminous efficacy, compared with blue and green phosphors. Therefore, it is necessary and urgent to explore novel red-emitting phosphors that can be efficiently excited in the near UV range. The scheelite structured molybdates have a vast number of industrial applications and are used in scintillators, solid state lasers, fluorescent lamps and photocatalysis. One of the most fascinating aspects of these materials is their ability to generate white light from a single phasic phosphor material.4–6 Calcium molybdate, CaMoO4 is a self activated blue-green host with a phonon energy ∼815 cm−1.7 Photoluminescence properties can be tuned by doping with different rare earth ions. In the CaMoO4 lattice, Ca atoms have 8 co-ordinations and make [CaO8] polyhedra while Mo atoms have 4-co-ordinations building [MoO4] polyhedra.7,8 MoO42− units in the CaMoO4 lattice have broad and intense absorption bands. This occurs due to charge transfer from oxygen to metal in the near-UV region. A blue-green light emission is observed due to a transition into MoO42− units in the lattice host. Eu3+-doped CaMoO4 phosphors can be efficiently excited in the near-UV region, spanning from 250 to 400 nm.9 Yan et al. has reported a 3 times improvement in red emission intensity in CaMoO4:Eu via bismuth co-doping.10 Recently, enhancements in luminescent intensity in CaMoO4:Eu and in Y2WO6:Ln (Ln = Sm, Eu and Dy) through Gd3+ co-doping have been reported.11,12 A single-component white-light phosphor is normally produced by co-doping a sensitizer and an activator into the same host matrix. A few prominent investigations have been performed to improve the luminescent properties of the CaMoO4 phosphor by co-doping other metal ions. However, the low efficacy of these nanomaterials has limited their general application. Consequently, it is of great interest to significantly enhance the emission intensity of phosphors in order to enhance their potential applications.The energy transfer mechanism from a sensitizer to an activator such as in Eu2+/Mn2+, Ce3+/Mn2+, and Ce3+/Eu2+ couples has been investigated in many inorganic hosts, and an effective resonance-type multi polar interaction has been verified in NaSr4(BO3)3:Ce3+/Mn2+, NaBa4(BO3)3:Ce3+/Mn2+, Sr3Sc(PO4)3:Eu2+/Mn2+, Sr3B2O6:Ce3+/Eu2+, and so on. Moreover, some single-phase phosphors, such as Ca10K(PO4)7:Eu2+/Mn2+, Ca9Y(PO4)7:Eu2+/Mn2+, Ca9Y(PO4)7:Ce3+/Eu2+, and Ca9Y(PO4)7:Ce3+/Mn2+, are used to improve the emission intensity for n-UV LED (light emitting diode) applications.11,13,14 Also an improvement in photoluminescence intensity has been reported with co-doping of charge compensators such as Li+, K+, Na+ and Bi3+ in phosphors or with a SiO2 coating over phosphor particles. In this direction, non-radiative rates are reduced by either charge compensation, improved crystallinity or the extent of the decrease in surface dangling bonds/OH bonds from the inner core phosphor by using a shell.15 Su and co-workers also reported enhanced photoluminescence in a CaWO4 based red phosphor via Eu and Na co-doping.16
There are some reports of Zn doped nano-phosphors and their improvement in photoluminescence intensity in the literature17–21 but no such study on Eu3+–Zn2+ co-doped CaMoO4 materials has been found to the best of our knowledge.
Many reports have been published on synthesis routes as well as luminescent properties of molybdates doped with different lanthanide ions. Synthesis routes such as solid state reaction, auto-combustion, sol–gel, and solvo-thermal have been reported for CaMoO4 and/or Ln3+ doped CaMoO4 in the literature, with an emphasis of controlling the crystal size, morphology, and the composition, which are crucial for a high quantum efficiency.22 Among these polyol synthesis has been effectively been used to prepare the metal-nanoparticles. In this methodology, ethylene glycol (EG) is used as a capping agent with a low synthesis temperature (∼150 °C). The use of poly alcohols, like ethylene glycol, as reducing agents for obtaining metallic nanoparticles has some advantages, as the by-products obtained during this process are ketones or carboxylic acids, they can be easily removed from the reaction mixture.23
Herein we have prepared a series of CaMoO4 based red phosphors including CaMoO4:Eu3+ (Eu3+ = 2 at%) and Ca1−x−yMoO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xEu
xEu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yZn (x = 2, y = 2, 5, 7 and 10 at%). The detailed photoluminescence properties of Eu3+ doped CaMoO4 with different concentrations of Zn2+ ions that emit light in the visible range were studied for ASP and 900 °C annealed samples. The detailed structural and downshifting phenomenon and involved decay kinetics have been studied for Zn co-doping in the Eu3+ activated CaMoO4 phosphor matrix.
yZn (x = 2, y = 2, 5, 7 and 10 at%). The detailed photoluminescence properties of Eu3+ doped CaMoO4 with different concentrations of Zn2+ ions that emit light in the visible range were studied for ASP and 900 °C annealed samples. The detailed structural and downshifting phenomenon and involved decay kinetics have been studied for Zn co-doping in the Eu3+ activated CaMoO4 phosphor matrix.
2. Synthesis
Zn2+ (Zn2+ = 2, 5, 7 and 10 at%) co-doped CaMoO4:Eu (here the concentration of Eu3+ is taken as 2 at% optimal) was prepared using ethylene glycol (EG) as capping agent with a reaction medium at 150 °C. Zinc oxide (ZnO, AR grade), calcium carbonate (CaCO3, AR grade), europium oxide (Eu2O3, 99.99%, Sigma Aldrich) and ammonium molybdate ((NH4)2Mo7·4H2O, AR grade) were used as sources of Ca2+, Eu3+ and MoO42−. In a typical synthesis procedure of a 1 g sample of 5 at% Zn2+ co-doped CaMoO4:Eu nanoparticles, 0.3312 g of CaCO3 and 0.0189 g of Eu2O3 and 0.0146 g of ZnO were dissolved together in concentrated nitric acid (HNO3). The mixture was heated at 80 °C to remove the excess acid this process of removal of excess acid was repeated five times after addition of de-ionized water (5 ml). To this solution, 0.6351 g of (NH4)2Mo7·2H2O was added followed by 50 ml of EG. The pH of the solution was adjusted to 8–9 using urea. The resulting solution was then stirred for 1 hour. This solution was then transferred to a two neck round bottom flask and was heated up to 150 °C for 3 hours under refluxing conditions in a condenser until precipitation was complete. The white precipitate so obtained was collected by centrifugation and washed 5 times in methanol to remove excess of EG and finally it was washed with acetone and dried at 90 °C for 2 hours in ambient conditions to yield the final white product. Finally, the as prepared samples were divided in 2 parts. One part of the sample was annealed at 900 °C in an ambient atmosphere at a heating rate of 2 °C min−1 for 4 hours in an alumina crucible and the other part was left untreated.2.1 Characterization techniques
Phase confirmation of the synthesized samples was examined by Rigaku-Miniflex-II X-ray diffractometer with Ni filtered Cu Kα radiation (λ = 1.5405 Å) at 30 kV and 15 mA. All patterns were recorded over the range 10 ≤ 2θ ≤ 70° with a step size of 0.02°. The chemical composition and valence state of the elements were analysed by X-ray photoelectron spectroscopy (XPS) using a monochromatic AlKα (hν = 1486.6 eV) X-ray source and a hemispherical analyzer (SPECS, HSA3500). The recorded spectra were charge-corrected to the C 1s ∼284.6 eV as the reference. The size and morphology of the samples were inspected using a Transmission Electron Microscope (TEM) JEOL JSM 100CX operating at an accelerating voltage of 200 kV. The samples for TEM were prepared by depositing a drop of a colloidal ethanol solution of the powder sample onto a carbon-coated copper grid. Simultaneous DSC/TGA spectra were recorded using NETZSCH STA 449 F1 Jupiter. DSC and TG analyses were carried out using 10 mg of the sample at a heating rate of 10 °C min−1 from 35 to 800 °C, in nitrogen atmosphere under a flow of 60 cm3 min−1. The Raman spectra of the as prepared and annealed samples were measured with Renishaw micro-Raman spectrometer attached with 633 nm laser as an excitation source. Photoluminescence excitation (PLE), emission (PL) and lifetime measurements were performed using a Fluorolog-3 spectrofluorometer (Model: FL3-11, Horiba Jobin Yvon). The 266 nm excitation wavelength of a Nd:YAG laser and CCD (charged coupled device) detector (Ocean Optics, QE 65000) were also used for emission measurement.3. Results and discussion
3.1 Structural studies
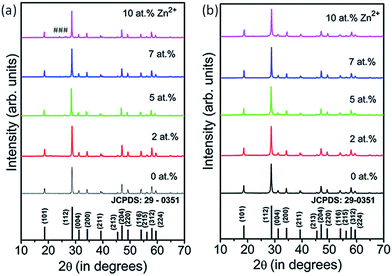 | ||
| Fig. 1 XRD patterns of Zn2+ (0, 2, 5, 7 and 10 at%) doped CaMoO4:Eu3+ nanoparticles for (a) ASP and (b) 900 °C annealed samples. Symbols marked as (#) show the extra phase evolution. | ||
All diffraction peaks for 900 °C annealed samples match well with JCPDS card no. 29-0351 (a = 5.226 Å, c = 11.43 Å and V = 312.17 Å3). The lattice parameters for ASP 2 at% Zn2+-doped CaMoO4 samples are a = 5.230 Å, c = 11.462 Å, V = 313.56 Å3 and for 900 °C annealed samples they are a = 5.231 Å, c = 11.46 Å, V = 313.57 Å3. Overall, cell volume decreases on annealing the samples as compared to ASP samples with Zn2+ co-doping in CaMoO4:Eu host matrix. The unit-cell constants and the calculated average crystallite sizes of the ASP and 900 °C annealed samples of CaMoO4 with different concentrations of Zn2+ ions are summarized in Table S1 (ESI†).
The diffraction pattern intensities for 5, 7 and 10 at% Zn2+-doped CaMoO4:Eu were found to be slightly less than those for 2 at% doped samples, which may be due to defects created at higher concentrations of Zn2+-doping in CaMoO4:Eu (Fig. 1(a)). Samples annealed at ∼900 °C show a slightly higher degree of crystalline behaviour than the ASP samples, which is shown in Fig. 1(b).
The average crystallite sizes were estimated with the Scherrer formula,
 | (1) |
Rietveld analyses was performed for the Zn (0, 2, 5, 7 and 10 at%) co-doped CaMoO4:Eu annealed at 900 °C samples using the FullProf software.25 Typical Rietveld fitting for undoped and 2 at% Zn co-doped CaMoO4:Eu are shown in Fig. 2(a) and (b). The Wyckoff positions of atoms based on space group I41/a (88) and Z = 4 (number of CaMoO4 formula units per unit cell) in CaMoO4 unit cell are:7,8 Ca: (4b: 0, 0.25, 0.0625), Mo: (4a: 0, 0.025, 0.125) and O: (16f: x, y, z) with angles (α = β = γ = 90°).
 | ||
| Fig. 2 Rietveld XRD patterns demonstrating observed and calculated difference and corresponding Bragg positions of (a) un-doped and (b) 2 at% Zn co-doped CaMoO4:Eu. | ||
The Pseudo-Voigt function was used to model the peak profiles and six coefficient polynomial was used to describe the background.
ASP samples show high agglomeration (shown in Fig. S1 (ESI†)) and Zn inclusion improves the grain growth of the particle shape evolution. During the heating process, nucleation and crystal growth occurred. This results in irregular shapes and agglomerated particles.26 The particle sizes estimated from TEM are ∼25–52 nm, which is in agreement with the calculated sizes from XRD studies. The typical particle size (shown in Fig. 3 itself) has been estimated to be ∼25 nm.
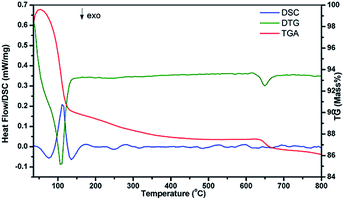
To study the heat flow as a function of temperature in the inert gas (N2) atmosphere associated with transitions in the 2 at% Zn2+ co-doped CaMoO4:Eu3+ ASP sample DSC was recorded (Fig. 4). The curve shows only endothermic peaks. The peak around 110 °C represents the mass loss due to evaporation of water and methanol. These results show that the prepared nano-phosphors are thermally stable and can be used in lighting and display devices.
 | ||
| Fig. 5 Room temperature Raman spectra of Zn free and 10 at% Zn co-doped CaMoO4:Eu samples annealed at 900 °C. | ||
All Raman-active modes of CaMoO4 crystals reported in this work are in good agreement with those reported in the literature.33,34 In fact, the shifts observed on these bands can be attributed to the degree of interaction between the O–Mo–O bonds and distortions on the [MoO4] clusters induced by the structural order/disorder in the lattice.
A peak at ∼141.1 eV, which corresponds to Eu3+ (4d3/2) and an absence of a peak at ∼127.1 eV corresponding to Eu2+ (4d5/2) is observed. This confirms the high probability of Eu3+ in the sample (shown in Fig. S5 (ESI†)). It is also confirmed from the photoluminescence study (discussed later). Typical XPS spectra of Eu3+ showing core binding energy and intensity with Zn (0, 2 and 10 at%) co-doped CaMoO4:Eu annealed at 900 °C samples are shown in Fig. S5 (ESI†). Intensity of these Eu3+ peaks are very small for annealed samples and improves with increasing Zn2+ concentration.
In addition, O 1s spectral regions have been used to obtain information regarding the presence of oxygen vacancies in the sample (Fig. 6(c)). Peaks were de-convoluted using Gaussian function. Two peaks are well fitted at BE ∼529.7 (P1) and 531.43 eV (P2) having FWHM ∼1.7 and 1.79 eV, respectively. Typical peak fitting of O 1s spectra for the 10 at% Zn doped at 900 °C annealed sample has been shown in Fig. S6 (ESI†). On increasing Zn2+ co-doping the peak position changes slightly by ±0.1–0.2 eV (Fig. 6(c)). Overall peaks show an asymmetric nature in the higher BE side. This may be due to defects and the oxygen vacancies that were created by Zn2+ doping. There are few reports which indicate that the high energy side of the O(1s) peak arises due to hydroxyl groups –OH or other radicals on the sample surface such as CO or CO2.36 However, the asymmetric behaviour of high energy peaks (∼530.5 eV) in an O 1s spectrum is the signature of the presence of an oxygen ion vacancy in the lattice.37 Vacancies decrease on annealing of the sample surface.7 The Core BE peaks of 10 at% Zn doped CaMoO4:Eu were observed at ∼1024.56 and ∼1048.4 eV and correspond to Zn2p3/2 and Zn2p1/2 (Fig. 6(d)).38
4. Optical studies
4.1 Excitation study
Excitation spectra of ASP Zn co-doped (0, 2, 5, 7, and 10 at%) CaMoO4:Eu nano-phosphors at an emission wavelength of 615 nm are shown in Fig. 7. A broad band from 230 to 320 nm is observed which arises due to a combination of the ligand to metal charge transfer O2− → Mo6+ and charge transfer band (CTB) from the completely filled 2p orbitals of O2− to the partially filled f–f orbitals of the Eu3+ ions (O2− → Eu3+),9,22,32 and the intra f–f transitions of Eu3+ around 360 (7F0 → 5D4), 376 nm (7F0 → 5G3), (382 (7F0 → 5G4), 395 (7F0 → 5L6), 415 (7F0 → 5D3), 464 (7F0 → 5D2) and 532 nm (7F0 → 5D1) are observed. The wavelength corresponding to the peak of Eu/Mo–O CTB band around 230–320 nm decreases from 280 to 266 nm and the corresponding FWHM decreases from 51 to 37 nm with an increase in Zn2+ ion concentration up to 2 at%. This blue shift in the Mo/Eu–O CT band with Zn2+ ion concentration can be related to some Mo/Eu based compound formation which may result in phase segregation, (also observed from ASP XRD data of Zn doped samples). The excitation spectra of 900 °C annealed Zn co-doped (0, 2, and 10 at%) CaMoO4:Eu3+ nano-phosphors at an emission wavelength of 615 nm has been given in Fig. S7 (ESI†).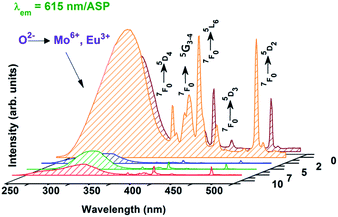 | ||
| Fig. 7 Excitation spectra of ASP, Zn (0, 2, 5, 7 and 10 at%) co-doped CaMoO4:Eu samples monitoring emission at a wavelength of 615 nm. | ||
The intensity of the bands increases up to 10 at% Zn2+ co-doping and the position of the Eu/Mo–O charge-transfer band (CTB) is shifted to a higher wavelength by ∼2–5 nm due to annealing of the samples at 900 °C compared with the ASP samples; similar observations have been reported for the Mo–O CTB in CaWO4 and W–O CTB in CaMoO4 host.29,32
When an electron is transferred from oxygen to Mo, an electronic transition takes place which gives rise to the Mo–O charge transfer band (CTB). In ASP samples, there are a relatively large number of dangling bonds over the particle surface and the lattice is less ordered as compared to that of annealed samples. There is also a higher degree of ionic character between Mo and O for the as-prepared samples as compared to that for the 900 °C annealed samples, and this results in lower energy absorption for annealed samples. On annealing the degree of co-valent character increases. Consequently, the position of the Mo–O charge-transfer band is shifted to a higher wavelength by ∼2–5 nm upon annealing the samples as compared to the as-prepared samples.28
4.2 Emission study
The Eu3+ ion has generally been selected as the activator ion to investigate the luminescence properties of rare earth tungstate/molybdate materials as it shows emissions in the visible region. Since the ground electronic state configuration of the Eu3+ ion has 7F0 non-degenerate and non-overlapping 2S+1LJ multiplets. Therefore the Eu3+ ion can be used as a structural probe for investigating the local environment in a host matrix.39 It is well documented that the symmetry of the crystal sites of doped Eu3+ ions will determine the relative intensity of the 5D0 → 7F1 and 5D0 → 7F2 transitions. If the 5D0 → 7F1 magnetic dipole transition is dominant in the spectrum, this indicates that europium is located in a site with inversion symmetry. If the 5D0 →7F2 electric dipole transition is dominant this means that Eu3+ is located in a site without inversion symmetry.40Fig. 8(a) shows the PL emission spectra of ASP Zn (0, 2, 5, 7 and 10 at%) co-doped CaMoO4:Eu3+ under 266 nm excitation. All the samples show strong 5D0 →7F2 (615 nm), 5D0 → 7F1 (590 nm), 5D0 → 7F3 (654 nm) and 5D0 → 7F4 (705 nm) emission lines upon 266 nm excitation. It is observed that the electric dipole transition at 615 nm is dominant over the magnetic dipole transition at 590 nm. It is well documented that the electric dipole transition is hypersensitive to its environment and the parity allowed transition originating from 5D0 → 7F1 is insensitive to the crystal field environment. It is suggested that most of the Eu3+ enters into the lattice site centres without inversion symmetry.11,23,40 Emission intensity increases up to 2 at% Zn2+ doped CaMoO4:Eu may be due to substitutional and crystal field effects. After 2 at% Zn doping, intensity decreases. Zn2+ co-doping may create vacancies that act as a sensitizer, mixing the charge-transfer states. Zn2+ addition increased the PL intensity by increasing the radiative transition probability. However, an increase in the Zn2+ concentration over a certain limit generates a significant amount of oxygen ion vacancies in the lattice. Consequently, the crystal lattice collapses, and the luminescence intensity decreases.42
 | ||
| Fig. 8 Emission spectra of Zn2+ (0, 2, 5, 7 and 10 at%) co-doped CaMoO4:Eu for (a) ASP and (b) 900 °C annealed samples at a 266 nm excitation wavelength. | ||
Similar behaviour has been reported for an Li+ doped Y2O3:Eu system.43 The PL band positions with different luminescence intensities have been observed for 900 °C annealed samples as compared to ASP Zn doped samples (shown in Fig. 8(b)). Photoluminescence intensity increases ∼3 times for 10 at% Zn2+ co-doped CaMoO4:Eu as compared to Zn free sample annealed at 900 °C samples. Emission intensity increases up to 10 at% Zn2+ doping. On annealing the sample crystallinity increases, non-radiative decay rate decreases and thus radiative rate is improved. This means that co-doping of Zn2+ improves luminescence. Improvement of luminescence is due to the substitution of Ca2+ sites by Zn2+ ions. Zn2+ doping may change the crystal field and asymmetricity around the Eu3+ ion which leads electric dipole transitions. Also the energy absorbed by the Zn2+ fully or partly transfers into Eu3+, raising the activation energy of Eu3+ and increasing the transition processes, leading to an improvement of the emitting intensity and red color purity. It is likely that the dipole moment of the transitions increases upon co-doping of Zn2+ ions. In addition, improved crystallinity as well as removal of organic moieties (such as PEG) gives rise to enhanced luminescence after heat treatment at 900 °C. Also PL study of Zn doped CaMoO4:Eu for the ASP and annealed at 900 °C samples under 395 nm excitation has been carried out and it showed a similar pattern intensity as under 266 nm excitation (shown in Fig. S8 and S9 (ESI†)).
The ratio of the integrated area of electric dipole transition (5D0 → 7F2) to magnetic dipole transition (5D0 → 7F1) has been probed to study the structural distortion around the Eu3+ ion, and is known as the asymmetric ratio (A21).11,23 In our case the asymmetric ratio is represented as,
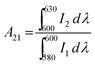 | (2) |
4.3 Decay analysis
The decay curves of the level 5D0 (615 nm) of Eu3+ have been measured and are shown in Fig. 9. The excitation wavelength is fixed at 395 nm. The decay curves for Eu3+ emissions can be well fitted by using bi-exponential curve fitting which is expressed as:
 | (3) |
 | (4) |
 | ||
| Fig. 9 Decay curve of 10 at% Zn co-doped CaMoO4:Eu (a) ASP and (b) 900 °C annealed sample under 395 nm excitation. | ||
The life time values obtained using bi-exponential decay equation for ASP and 900 °C annealed 10 at% Zn2+ co-doped CaMoO4:Eu are ∼0.58 and 0.76 ms, respectively under 395 nm excitation.
These values are in good agreement with the reported values for other Eu3+ doped compounds.9,11 As for Mo–O CTB (∼266 nm) excitation, luminescence decay follows a non-exponential equation because the initial stage energy transfer from Mo–O or Zn2+ to the excited states of Eu3+ occurs and then the decays from the 5D0 level. The details of energy transfer rate and mechanism have been reported in literature.11,22 In the case of samples annealed at 900 °C, the lifetime values are higher than those of the as-prepared samples. This may be due to a reduction in non-radiative rates (R0) as compared to the radiative rate. Non-radiative rate R0 is expressed as:
 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–15
000–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and the value is comparable with the third overtone stretching vibrations of the –OH functional group (∼3500 cm−1). This functional group arises from the water molecules absorbed or associated during the synthesis of the nanomaterials. EG and aqueous media are used and are the source of the water. R0 values become large when ΔE ∼ 2hνmax. In the case of the ASP samples, a significant extent of non-radiative transfer of energy from the excited states of the Eu3+ ions to the different vibrational modes of –OH species occurs which leads to a reduction in the Eu3+ emission. Similar reports have been documented for Eu3+/Mn2+ doped CaF2 and Fe3O4 hybrid structures.44 Recently the radiative and non-radiative decay rates of CdSe nanorods were found to be modified in Au/CdSe tetrapod structures and the non-radiative rate changes from 1.91 × 107 s−1 to 9.33 × 109 s−1 were observed for CdSe nanorod to Au/CdSe tetrapod structures.45
000 cm−1 and the value is comparable with the third overtone stretching vibrations of the –OH functional group (∼3500 cm−1). This functional group arises from the water molecules absorbed or associated during the synthesis of the nanomaterials. EG and aqueous media are used and are the source of the water. R0 values become large when ΔE ∼ 2hνmax. In the case of the ASP samples, a significant extent of non-radiative transfer of energy from the excited states of the Eu3+ ions to the different vibrational modes of –OH species occurs which leads to a reduction in the Eu3+ emission. Similar reports have been documented for Eu3+/Mn2+ doped CaF2 and Fe3O4 hybrid structures.44 Recently the radiative and non-radiative decay rates of CdSe nanorods were found to be modified in Au/CdSe tetrapod structures and the non-radiative rate changes from 1.91 × 107 s−1 to 9.33 × 109 s−1 were observed for CdSe nanorod to Au/CdSe tetrapod structures.45
The radiative decay rate constants are defined as:11
| (kr) = 1/τav | (6) |
Calculated values of radiative rate constants for 10 at% Zn2+ co-doped CaMoO4:Eu3+ for ASP and 900 °C annealed sample are 1.724 × 103 and 1.315 × 103 s−1, respectively.
4.4 CIE studies
Fig. 10 shows the Commission Internationale de l’Eclairage (CIE) chromaticity diagram for Zn2+ (0 and 10 at%) co-doped CaMoO4:Eu3+ annealed at 900 °C phosphors excited at 266 nm. CIE coordinates vary for ASP and 900 °C annealed samples at 266 and 395 nm excitation. Typical CIE coordinates for 10 at% ASP Zn2+ co-doped sample is (0.58, 0.34) at 266 nm excitation. CIE color coordinates for Zn free and 10 at% Zn2+ co-doped CaMoO4:Eu annealed samples at 266 nm excitation are (x1 = 0.58, 0.36) and (x2 = 0.64, 0.35), which lie well within the red region. It is worthwhile to observe that under 266 and 395 nm (see Table S2 (ESI†), the color coordinates are located in the shallow red for ASP and lie in red region, for 900 °C annealed samples, respectively. Red emission color is due to the characteristic emission of Eu3+ ion. Detailed CIE coordinates have been calculated for Zn co-doped samples for ASP and samples annealed at 900 °C under 266 and 395 nm excitation, given in Table S2 (see ESI†).5. Conclusion
Highly nano-crystalline nanoparticles of Zn co-doped CaMoO4:Eu have been prepared using a polyol synthesis. Tetragonal scheelite single phase has been confirmed through XRD study. Characteristics valence states for Ca, Mo, Zn and Eu have been probed through an XPS study and they were in their formal +2, +6, +2 and +3 oxidation states. TEM study confirms the spherical morphology of the Zn doped samples. Characteristic Raman modes of vibrations have been observed for the CaMoO4 host. Enhanced photoluminescence for 900 °C annealed samples has been observed as compared to ASP via Zn-doping, it may be due to the reduction of –OH ions and organic moieties at higher temperature. Also Zn2+ co-doping in CaMoO4:Eu matrix produces high asymmetricity. The asymmetric ratio is ∼7.4 to 10.6 and ∼6.1 to 9.3 under 266 and 395 nm excitation wavelengths, which reflects that it is a high red emitter. Zn2+ co-doping favours the crystallinity and changes the crystal field around the Eu3+ ion, resulting in a significant enhancement in PL intensity. CIE coordinates for ASP, it is lie in the shallow red region while for annealed samples, it is lie in red region under 266 and 395 nm excitation. Studies corroborate the potentiality of these samples as a promising red phosphor for w-LED applications at much lower cost.Acknowledgements
One of the authors BPS is thankful for financial assistantship to Council of Scientific and Industrial Research (CSIR), New Delhi, India for providing the Senior Research Fellowship. Also author Maheshwary acknowledges the Central Research Fellowship provided by University Grants Commission (UGC), India. BPS gratefully acknowledges Dr R. S. Ningthoujam, Chemistry Division, BARC, India for his support and encouragements.References
- S. Neeraj, N. Kijima and A. K. Cheetham, Chem. Phys. Lett., 2004, 387, 2–6 CrossRef CAS PubMed.
- G. S. R. Raju, H. C. Jung, J. Y. Park, B. K. Moon, R. Balakrishnaiah, J. H. Jeong and J. J. H. Kim, Sens. Actuators, B, 2010, 146, 395–402 CrossRef PubMed.
- M. Yamada, T. Naitou, K. Izuno, H. Tamaki, Y. Murazaki, M. Kameshima and T. Mukai, Jpn. J. Appl. Phys., 2003, 42, 20–23 CrossRef.
- L. S. Cavalcante, V. M. Longo, J. C. Sczancoski, M. A. P. Almeida, A. A. Batista, J. A. Varela, M. Orlandi, E. Longo and M. S. Liu, CrystEngComm, 2012, 14853–868 Search PubMed.
- V. M. Longo, L. S. Cavalcante, E. C. Paris, J. C. Sczancoski, P. S. Pizani, M. S. Li, J. Andres, E. Longo and J. A. Varela, J. Phys. Chem. C, 2011, 115, 5207–5219 CAS.
- J. Guo, D. Zhou, Y. Li, T. Shao, Z.-M. Qi, B.-B. Jinc and H. Wang, Dalton Trans., 2014, 11888–11896 RSC.
- B. P. Singh, A. K. Parchur, R. S. Ningthoujam, A. A. Ansari, P. Singh and S. B. Rai, Dalton Trans., 2014, 4770–4778 RSC.
- A. K. Parchur and R. S. Ningthoujam, Dalton Trans., 2011, 7590–7594 RSC.
- A. K. Parchur, R. S. Ningthoujam, S. B. Rai, G. S. Okram, R. A. Singh, M. Tyagi, S. C. Gadkari, R. Tewari and R. K. Vatsa, Dalton Trans., 2011, 7595–7601 RSC.
- S. Yan, J. Zhang, X. Zhang, S. Lu, X. Ren, Z. Nie and X. Wang, J. Phys. Chem. C, 2007, 111, 13256–13260 CAS.
- B. P. Singh, A. K. Parchur, R. S. Ningthoujam, A. A. Ansari, P. Singh and S. B. Rai, Dalton Trans., 2014, 4779–4789 RSC.
- A. M. Kaczmarek, K. V. Hecke and R. V. Deun, Inorg. Chem., 2014, 53, 9498–9508 CrossRef CAS PubMed.
- N. Guo, Y. Jia, W. Lü, W. Lv, Q. Zhao, M. Jiao, B. Shao and H. You, Dalton Trans., 2013, 5649–5654 RSC.
- C. K. Chang and T. M. Chen, Appl. Phys. Lett., 2007, 91, 081902 CrossRef PubMed.
- A. Xie, X. Yuan, S. Hai, J. Wang, F. Wang and L. Li, J. Phys. D: Appl. Phys., 2009, 42, 105107 CrossRef.
- Y. G. Su, L. P. Li and G. S. Li, Chem. Mater., 2008, 20, 6060–6067 CrossRef CAS.
- R. Dey, V. K. Rai and A. Pandey, Spectrochim. Acta, Part A, 2012, 99, 288–291 CrossRef CAS PubMed.
- V. Singh, V. K. Rai, I. Ledoux-Rak, L. Badie and H. Y. Kwak, Appl. Phys. B, 2009, 97, 805–809 CrossRef CAS.
- A. Pandey and V. K. Rai, Dalton Trans., 2013, 11005–11011 RSC.
- S. M. Chung, S. Y. Kang, J. H. Shin, W. S. Cheong, C. S. Hwang, K. I. Cho, S. J. Lee and Y. J. Kim, J. Cryst. Growth, 2011, 326, 94–97 CrossRef CAS PubMed.
- D. Hartnath, A. F. Khan and H. Chander, J. Phys. D: Appl. Phys., 2006, 39, 4956–4960 CrossRef.
- A. K. Parchur, A. I. Prasad, S. B. Rai, A. A. Ansari and R. S. Ningthoujam, Dalton Trans., 2012, 11032–11045 RSC.
- Maheshwary, B. P. Singh, J. Singh and R. A. Singh, RSC Adv., 2014, 4, 32605–32621 CAS.
- R. D. Shanon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32751 Search PubMed.
- J. R. Carvajal, Introduction to the program FullProf, Laboratoire Leon Brillouin (CEA-CRNS), France Search PubMed.
- N. S. Gajbhiye and R. S. Ningthoujam, Mater. Res. Bull., 2006, 41, 1612–1621 CrossRef CAS PubMed.
- N. V. Jadhav, A. I. Prasad, A. Kumar, R. Mishra, S. Dhara, K. R. Babu, C. L. Prajapat, N. L. Misra, R. S. Ningthoujam, B. N. Pandey and R. K. Vatsa, Colloids Surf., B, 2013, 108, 158–168 CrossRef CAS PubMed.
- M. N. Luwang, R. S. Ningthoujam, S. K. Srivastava and R. K. Vatsa, J. Am. Chem. Soc., 2011, 133, 2998–3004 CrossRef CAS PubMed.
- Maheshwary, B. P. Singh and R. A. Singh, New. J. Chem, 2015 10.1039/c4nj01911c.
- A. Golubovic, R. Gajic, Z. D. -Mitrovic and S. Nikolic, J. Alloys Compd., 2006, 415, 16–22 CrossRef CAS PubMed.
- Z. C. Ling, H. R. Xia, D. G. Ran, F. Q. Liu, S. Q. Sun, J. D. Fan, H. J. Zhang, J. Y. Wang and L. L. Yu, Chem. Phys. Lett., 2006, 426, 85–90 CrossRef CAS PubMed.
- A. K. Parchur, A. A. Ansari, B. P. Singh, T. N. Hasan, F. N. Syed, S. B. Rai and R. S. Ningthoujam, Integr. Biol., 2014, 6, 53–64 RSC.
- P. G. Zverev, Phys. Status Solidi C, 2004, 1, 3101–3105 CrossRef CAS PubMed.
- A. P. A. Marques, F. V. Motta, E. R. Leite, P. S. Pizani, J. A. Varela, E. Longo and D. M. A. de Melo, J. Appl. Phys., 2008, 104, 043505–043510 CrossRef PubMed.
- S. I. Woo, J. S. Kim and H. K. Jun, J. Phys. Chem. B, 2004, 108, 8941–8946 CrossRef CAS.
- L. R. Shah, B. Ali, H. Zhu, W. G. Wang, Y. Q. Song, H. W. Zhang, S. I. Shah and J. Q. Xiao, J. Phys.: Condens. Matter, 2009, 21, 486004 CrossRef PubMed.
- A. K. Parchur, A. I. Prasad, S. B. Rai, R. Tewari, R. K. Sahu, G. S. Okram, R. A. Singh and R. S. Ningthoujam, AIP Adv., 2012, 2, 032119 CrossRef PubMed.
- Y. Vahidshad, M. N. Tahir, A. I. Zad, S. M. Mirkazemi, R. Ghazemzadeh and W. Tremel, J. Mater. Chem. C, 2015, 3, 889–898 RSC.
- C. Hsu and R. C. Powell, Phys. Rev. Lett., 1975, 35, 734–737 CrossRef CAS.
- G. S. R. Raju, E. Pavitra, Y. H. Ko and J. S. Yu, J. Mater. Chem., 2012, 22, 15562–15569 RSC.
- J. Yu, K. Huang, L. Yuan and S. Feng, New J. Chem., 2014, 38, 1441–1445 RSC.
- T. Jia, Y. Liu, H. Zhao, H. Du, J. Sun and G. Ge, J. Solid State Chem., 2010, 183, 584–589 CrossRef PubMed.
- L. Sun, C. Qian, C. Liao, X. Wang and C. Yan, Solid State Commun., 2001, 119, 393–396 CrossRef CAS.
- L. P. Singh, S. K. Srivastava, R. Mishra and R. S. Ningthoujam, J. Phys. Chem. C, 2014, 118, 18087–18096 CAS.
- K. K. Haldar, S. Kundu and A. Patra, Appl. Phys. Lett., 2014, 104, 063110 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06692a |
| This journal is © The Royal Society of Chemistry 2015 |