Soluble squaraine derivatives for 4.9% efficient organic photovoltaic cells†
Hisahiro Sasabe*ab,
Tsukasa Igrashia,
Yusuke Sasakia,
Guo Chenbc,
Ziruo Hongbd and
Junji Kido*ab
aDepartment of Organic Device Engineering, Yamagata University, 4-3-16 Jonan, Yonezawa, Yamagata 992-8510, Japan. E-mail: h-sasabe@yz.yamagata-u.ac.jp; kid@yz.yamagata-u.ac.jp; Fax: +81 238 26 3412; Tel: +81 238 26 3052
bResearch Center for Organic Electronics, Yamagata University, 4-3-16 Jonan, Yonezawa, Yamagata 992-8510, Japan
cKey Laboratory of Advanced Display and System Applications, Ministry of Education, Shanghai University, Yanchang Road 149, Shanghai 200072, China
dDepartment of Materials Science and Engineering, University of California, Los Angeles, CA 90095, USA
First published on 5th September 2014
Abstract
A series of soluble squaraine derivatives has been designed and developed as a donor material for solution-processible organic photovoltaic cells. In combination with [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) as an acceptor, we obtained a power conversion efficiency of 4.86% from bulk-heterojunction photovoltaic cells.
Recently, small molecule based organic photovoltaic (OPV) cells have attracted increasing attention owing to their features such as facile synthesis and purification, and tunable molecular structures and energy levels.1 By using small molecules as donors for OPVs, a power conversion efficiency (PCE) of over 10% has been already obtained from a tandem device structure.2 However, considering the simple processing in mass production, it is more attractive to make simple single-heterojunction devices. Therefore it is important to tune the bandgap of donor materials to 1.5 eV or even smaller, from which maximal product of photocurrent and electrical potential can be obtained. Among many small molecule OPV materials, squaraine (SQ) dyes have shown promising potential for OPV due to their high absorption coefficients of ∼105 M−1 cm−1 and intense absorption in visible and near-infrared (NIR) spectral regions.3,4 In spite of the small bandgap, the open circuit voltage (VOC) is relatively high, making SQ materials a good model system for OPV research. Among SQ derivatives, amino-SQs with four hydroxyl (OH) groups exhibit outstanding performance for OPV devices.5–7 For example, Wei et al. have reported a solution-processed bulk-heterojunction (BHJ) cell with a PCE of 5.5% by using a SQ named 2,4-bis[4-(N,N-diisobutylamino)-2,6-dihydroxyphenyl]squaraine (DIB-SQ).5b Wang et al. have developed a series of N,N-diarylamino-SQ derivatives to achieve a PCE of 3.2%.5c They have pointed out that N-aryl group deepens highest occupied molecular orbital (HOMO) levels relative to SQ with N-alkyl groups thus increasing VOC as well as improving charge carrier transport. Recently, Yang et al. have developed an asymmetric SQ bearing a carbazole substituent, and reached a PCE of 2.8% with a VOC of 1.12 V.5d By using DIB-SQ as a donor material, we have reported a vacuum co-evaporated BHJ cell with a PCE greater than 6%, and a solution-processed BHJ cell with a PCE 5.0%.7a,b Most recently, we have investigated the effect of the side chains and the number of OH groups in a series of amino-SQs on physical properties and photovoltaic performance.7f We have clearly showed that OH group deepens the HOMO level of amino-SQ, and both the side chains and OH groups control the aggregation behavior in the thin solid film. As mentioned above, SQ derivatives are promising candidates as donor materials for high-performance OPV cells, and thus more molecular structures that are suitable for OPV applications are yet to be explored.1e
In this study, we synthesized a series of amino-SQ derivatives with SQ(OH)4 skeleton named SQ-RP (SQ-BP, SQ-OP and SQ-DP) containing phenyl- and different length of alkyl chains (Fig. 1).
These SQs had absorption maxima at 650 nm with a high extinction coefficient (ε) around 3.0 × 105 M−1 cm−1 in the solution. Typically, the conventional SQ derivative is hardly soluble in ordinary solvents except halogenated solvents, however, these SQ derivatives showed very high solubility up to 15 mg mL−1 in non-halogenated solvents, such as THF and toluene. Further, these SQs have deep HOMO level of −5.3 eV suggesting high VOC in BHJ type OPV with a combination of fullerene derivatives. By using a combination of [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) as an acceptor in OPV devices, we obtained a PCE of 4.86% under AM 1.5 G solar illumination at 100 mW cm−2 at room temperature. We also investigated the relationship among the chemical structure, optical and electronic properties, and revealed an effective molecular design of SQs materials for high efficiency OPV devices.
Synthetic route of SQ-RP is shown in Scheme S1 (see ESI†). As a reference, SQ-MP was prepared according to the literature.5c From synthesis point of view, SQs studied in this work have several advantages: (i) facile preparation via a condensation reaction between electron-rich aromatic amines and squaric acid, and thus the by-product is only water and easily removed via distillation; (ii) no use of expensive and hardly-separable metal-catalysts and ligands in the final reaction; (iii) easy separation and purification from the reaction mixture; (iv) high yield of over 80%. These features make this SQ family an attractive donor for OPV. The materials were characterized using 1H-NMR, mass spectrometry, and elemental analyses (see ESI†). The purity of SQs thus obtained was confirmed to be >99.5% by HPLC analysis. Among these four SQs, SQ-MP is hardly soluble (<1 mg mL−1) even in halogenated solvents, however, the other SQs with longer alkyl chains are highly soluble in halogenated solvents. Interestingly, SQ-DP is even soluble in non-halogenated solvents suggesting potential for OPV cells processed without halogenated solvents (see Table S1†).
The thermal properties of SQ-RP were estimated by a differential scanning calorimetry (DSC) and a thermogravimetric analysis (TGA). The glass transition temperatures (Tg) of SQ-RP were not detected, and the weight loss of 5% (Td5) was found at around 300 °C indicating high thermal stability (Fig. S1†). The photophysical properties were determined by UV-vis absorption and PL measurements in the solution and the solid states (Fig. 2, S2 and S3†). All the novel SQs exhibited the single strong absorption peak maxima at 650 nm with a high extinction coefficient (ε) over 3.0 × 105 M−1 cm−1 in 10−6 M CHCl3 solution (Fig. 2a). These SQs showed weaker photoluminescence (PL) emission at wavelength of 690 nm compared with dialkylamino-SQs such as DIB-SQ. DIB-SQ reportedly shows a strong emission with a PL quantum yield (PLQY) of 0.80.5c Therefore, introduction of phenyl moieties greatly lowers PLQY and causes larger Stokes shift. Additionally, no detectable emission was observed from these SQ films suggesting aggregation-induced loss in PLQY. A red-shifted and broadened absorption band compared to that in the solution was observed at around 665 nm in the thin films cast from CHCl3 solution on a quartz substrate (Fig. 2b). At the meantime, SQ-DP with longer alkyl chains exhibits another peak at ∼580 nm, which SQ-BP and SQ-OP with shorter alkyl chains do not have obvious blue-shift. Therefore, we deduced that the steric hindrance of longer alkyl chains could induce the formation of thermodynamically stable dimer state, in other words, H-aggregates.3 Upon heating the SQ-DP film at 70 °C for 10 minutes, the 580 nm peak slightly increased (Fig. S3†). Eventually, all the SQs films show similar peak upon heating at 120 °C for 10 minutes, suggesting there occurs aggregation in the films. It is ascribed to the formation of H-aggregates. The HOMO and the lowest unoccupied molecular orbital (LUMO) levels were determined by using cyclic voltammetry in 0.5 mM CH2Cl2 solution (Fig. S4†). All these SQs showed the same HOMO/LUMO levels (−5.3/−3.6 eV) irrespective of the different length of alkyl chains. The deep HOMO level of −5.3 eV is favorable for a high VOC in OPV devices. On the other hand, the LUMO level of SQs is 0.4 eV shallower than that of PC71BM (−4.0 eV) indicating that efficient exciton dissociation and charge separation are achievable. All the physical properties of these SQs are summarized in Table 1 and S2.†
 | ||
| Fig. 2 UV-vis and PL spectra of SQ-RP in (a) solution (10−6 M), (b) as cast film on quartz substrate. | ||
| Compound | εa (M−1 cm−1) | λmaxa (nm) | Tm/Tdb (°C) | HOMO/LUMOc |
|---|---|---|---|---|
| a Absorption spectra were measured in CHCl3 (10−6 M).b Measured by a DSC.c Measured by a CV (0.5 mM in CH2Cl2).d Solubility is too low to measure. | ||||
| SQ-MP | 3.2 × 105 | 645 | n.d./321 | n.d.d |
| SQ-BP | 3.6 × 105 | 650 | n.d./322 | 5.3/3.6 (eV) |
| SQ-OP | 3.5 × 105 | 650 | 230/290 | 5.3/3.6 (eV) |
| SQ-DP | 3.5 × 105 | 650 | 177/298 | 5.3/3.6 (eV) |
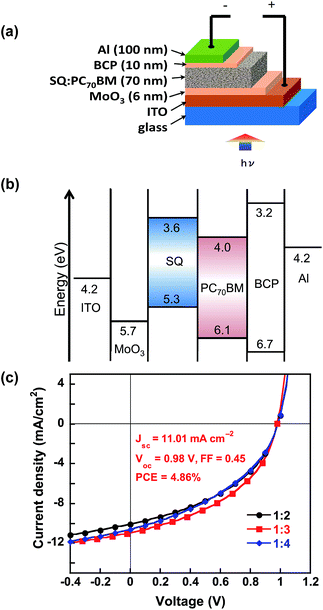
As mentioned above, the HOMO/LUMO levels of these SQs are same in solution. Then, we investigated their photovoltaic performance in thin film OPV devices. To study the effects of the side chain on the photovoltaic performance, we fabricated solution-processed BHJ cells using SQ as a donor combined with PC71BM as an acceptor. As shown in Fig. 3a, the SQ:PC71BM blend films were sandwiched between molybdenum oxide (MoO3) modified indium-tin-oxide (ITO)8 anode and bathocuproine (BCP)/Al cathode.9 The device structure is ITO/MoO3 (6 nm)/SQ:PC71BM (70 nm, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x)/BCP (10 nm)/Al (100 nm) (x = 2–4). Energy level diagram is shown in Fig. 3b. MoO3 effectively deepens the surface work function of ITO to −5.7 eV, and therefore delivers VOC which corresponds to HOMO level (−5.3 eV) of SQs.5a,7a The active layer SQ:PC71BM was spin-coated from chloroform solution. Based on our previous study,7a,b we found the blend ratio of the donor and acceptor significantly affected the carrier mobility in the photoactive layer and the OPV performance. In this work, we tuned the blend ratio of SQ and PC71BM from 1
x)/BCP (10 nm)/Al (100 nm) (x = 2–4). Energy level diagram is shown in Fig. 3b. MoO3 effectively deepens the surface work function of ITO to −5.7 eV, and therefore delivers VOC which corresponds to HOMO level (−5.3 eV) of SQs.5a,7a The active layer SQ:PC71BM was spin-coated from chloroform solution. Based on our previous study,7a,b we found the blend ratio of the donor and acceptor significantly affected the carrier mobility in the photoactive layer and the OPV performance. In this work, we tuned the blend ratio of SQ and PC71BM from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to optimize the ratio with respect to device performance. The key OPV parameters are listed in Table S3.† Fig. 3c shows the J–V characteristics of the SQ-BP-based BHJs with various SQ-BP/PC71BM blend ratios. A 1
4 to optimize the ratio with respect to device performance. The key OPV parameters are listed in Table S3.† Fig. 3c shows the J–V characteristics of the SQ-BP-based BHJs with various SQ-BP/PC71BM blend ratios. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio between SQ-BP and PC71BM based OPV device yielded the maximum PCE of 4.86%, with a short circuit current density (JSC) = 11.01 mA cm−2, VOC = 0.98 V, and a fill factor (FF) = 0.45 (Fig. S5†). The efficiency shows strong light intensity dependence as seen in Fig. S6,† indicating considerable non-germinated recombination.7a,b The PCE at low light intensity reaches over 6.0% corresponding to weak non-germinated recombination at low carrier density. Also the PCE is not sensitive to blend ratio in the range from 1
3 ratio between SQ-BP and PC71BM based OPV device yielded the maximum PCE of 4.86%, with a short circuit current density (JSC) = 11.01 mA cm−2, VOC = 0.98 V, and a fill factor (FF) = 0.45 (Fig. S5†). The efficiency shows strong light intensity dependence as seen in Fig. S6,† indicating considerable non-germinated recombination.7a,b The PCE at low light intensity reaches over 6.0% corresponding to weak non-germinated recombination at low carrier density. Also the PCE is not sensitive to blend ratio in the range from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. It suggests that charge transport is relatively independent on the blend ratio. Next, we looked into the effect of the length of alkyl chains on the photovoltaic performance. We compared three OPV cells based on SQ-BP, SQ-OP and SQ-DP as a donor, respectively, with the same SQ:PC71BM blend ratio of 1
4. It suggests that charge transport is relatively independent on the blend ratio. Next, we looked into the effect of the length of alkyl chains on the photovoltaic performance. We compared three OPV cells based on SQ-BP, SQ-OP and SQ-DP as a donor, respectively, with the same SQ:PC71BM blend ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. As shown in Fig. S7 and Table S3,† SQ-DP-based device gave a much lower PCE of 1.97%, with a JSC = 5.72 mA cm−2, a VOC = 0.96 V, and a FF = 0.36. SQ-BP with shorter alkyl chains based device generated higher PCE than that of SQ-DP with longer alkyl chains. To clarify these differences, UV-vis spectra of the SQ/PC71BM active layers were observed (Fig. S8†). A SQ-BP/PC71BM (1
3. As shown in Fig. S7 and Table S3,† SQ-DP-based device gave a much lower PCE of 1.97%, with a JSC = 5.72 mA cm−2, a VOC = 0.96 V, and a FF = 0.36. SQ-BP with shorter alkyl chains based device generated higher PCE than that of SQ-DP with longer alkyl chains. To clarify these differences, UV-vis spectra of the SQ/PC71BM active layers were observed (Fig. S8†). A SQ-BP/PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) blend film showed a strong red-shifted and broadened peak for enhancing photoresponse in NIR region. On the other hand, SQ-DP/PC71BM (1
3) blend film showed a strong red-shifted and broadened peak for enhancing photoresponse in NIR region. On the other hand, SQ-DP/PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) blend film showed an additional blue-shifted peak. SQ with longer linear alkyl chains demonstrated a stronger blue-shifted peak suggesting that the steric hindrance of longer alkyl chains could induce the formation of H-aggregates even in the SQ/PC71BM blend film. This feature is sharply different from dialkylamino-SQ system that shows only a red-shifted peak in the SQ/PC71BM blend film.7f As shown in the external quantum efficiency (EQE) spectra (Fig. S7†), photocurrent in SQ-DP-based OPV was greatly decreased due to the weaker photoresponse in NIR region.
3) blend film showed an additional blue-shifted peak. SQ with longer linear alkyl chains demonstrated a stronger blue-shifted peak suggesting that the steric hindrance of longer alkyl chains could induce the formation of H-aggregates even in the SQ/PC71BM blend film. This feature is sharply different from dialkylamino-SQ system that shows only a red-shifted peak in the SQ/PC71BM blend film.7f As shown in the external quantum efficiency (EQE) spectra (Fig. S7†), photocurrent in SQ-DP-based OPV was greatly decreased due to the weaker photoresponse in NIR region.
In conclusion, we report a series of soluble SQs as donor materials for OPVs. We found that shorter alkyl chains induces a strong red-shifted and broadened peak for enhanced photoresponse in NIR region thus favorable to realize a high OPV performance.
Acknowledgements
We thank the Japan Science and Technology Agency (JST) for financial support via the Japan Regional Innovation Strategy Program by the Excellence (J-RISE) and the Adaptable and Seamless Technology Transfer Program (A-STEP, AS251Z00216M).Notes and references
- (a) Y. Li, Q. Guo, Z. Li, J. Pei and W. Tian, Energy Environ. Sci., 2010, 3, 1427 RSC; (b) B. Walker, C. Kim and T.-Q. Nguyen, Chem. Mater., 2011, 23, 470 CrossRef CAS; (c) P. M. Beaujuge and J. M. J. Fréchet, J. Am. Chem. Soc., 2011, 133, 20009 CrossRef CAS PubMed; (d) A. Mishra and P. Bäuerle, Angew. Chem., Int. Ed., 2012, 51, 2020 CrossRef CAS PubMed; (e) J. Roncali, P. Leriche and P. Blanchard, Adv. Mater., 2014, 26, 3821 CrossRef CAS PubMed.
- (a) http://www.heliatek.com; (b) Y. S. Liu, C.-C. Chen, Z. R. Hong, J. Gao, Y. Yang, H. P. Zhou, L. T. Dou, G. Li and Y. Yang, Sci. Rep., 2013, 3, 3365 Search PubMed.
- (a) A. Ajayaghosh, Acc. Chem. Res., 2005, 38, 449 CrossRef CAS PubMed; (b) S. Sreejith, P. Carol, P. Chithra and A. Ajayabhosh, J. Mater. Chem., 2008, 18, 264 RSC; (c) Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564 RSC; (d) L. Becerina and P. Salice, Eur. J. Org. Chem., 2010, 1207 CrossRef PubMed; (e) F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376 CrossRef PubMed; (f) L. Becerina and P. Salice, Synlett, 2014, 477 CrossRef PubMed.
- (a) F. Silvestri, M. D. Irwin, L. Beverina, A. Facchetti, G. A. Pagani and T. J. Marks, J. Am. Chem. Soc., 2008, 130, 17640 CrossRef CAS PubMed; (b) U. Mayerfoffer, K. Deing, K. Grub, H. Braunschweig, K. Meerholz and F. Wurthner, Angew. Chem., Int. Ed., 2009, 48, 8776 CrossRef PubMed; (c) S. So, H. Choi, H. M. Ko, C. Kim, S. Paek, N. Cho, K. Song, J. K. Lee and J. Ko, Sol. Energy Mater. Sol. Cells, 2012, 92, 224 CrossRef PubMed; (d) M. Sassi, M. Crippa, R. Ruffo, M. Drees, U. K. Pandey, R. Terrmine, A. Golemme, A. Facchetti and L. Beverina, J. Mater. Chem. A, 2013, 2631, 2631 RSC; (e) S. L. Lam, X. Liu, F. Zhao, C. K. Lee and W. L. Kwan, Chem. Commun., 2013, 49, 4543 RSC; (f) B. A. Rao, K. Yesudas, G. S. Kumar, K. Bhanuprakash, V. J. Rao, G. D. Sharama and S. P. Singh, Photochem. Photobiol. Sci., 2013, 12, 1688 RSC.
- (a) S. Wang, E. I. Mayo, M. D. Perez, L. Griffe, G. Wei, P. I. Djurovich, S. R. Forrest and M. E. Thomson, Appl. Phys. Lett., 2009, 94, 233304 CrossRef PubMed; (b) G. Wei, S. Wang, K. Sun, M. E. Thomson and S. R. Forrest, Adv. Energy Mater., 2010, 4, 1927 CAS; (c) S. Wang, L. Hall, V. V. Diev, R. Haiges, G. Wei, X. Xiao, P. I. Djurovich, S. R. Forrest and M. E. Thompson, Chem. Mater., 2011, 23, 4789 CrossRef CAS; (d) D. Yang, Q. Yang, L. Yang, Q. Luo, Y. Huang, Z. Lu and S. Zhao, Chem. Commun., 2013, 49, 10465 RSC; (e) J. W. Ryan, T. Kirchartz, A. Viterisi, J. Nelson and E. Palomares, J. Phys. Chem. C, 2013, 117, 19866 CrossRef CAS; (f) S. Brück, C. Krause, R. Turrisi, L. Beverina, S. Wilken, W. Saak, A. Lützen, H. Borchert, M. Scheiek and J. Parisi, Phys. Chem. Chem. Phys., 2014, 16, 1067 RSC; (g) A. M. D. Pelle, P. J. Homnick, Y. Bae, P. M. Lahti and S. Thayumanavan, J. Phys. Chem. C, 2014, 118, 1793 CrossRef.
- J. Huang, T. Goh, X. Li, M. Y. Sfeir, E. A. Bielinski, S. Tomasulo, M. L. Lee, N. Hazari and A. D. Taylor, Nat. Photonics, 2013, 7, 479 CrossRef CAS.
- (a) G. Chen, H. Sasabe, Z. Wang, X. Wang, Z. Hong, Y. Yang and J. Kido, Adv. Mater, 2012, 24, 2768 CrossRef CAS PubMed; (b) G. Chen, H. Sasabe, X. Wang, Z. Hong, J. Kido and Y. Yang, Phys. Chem. Chem. Phys., 2012, 14, 14661 RSC; (c) G. Chen, D. Yokoyama, H. Sasabe, Z. Hong, Y. Yang and J. Kido, Appl. Phys. Lett., 2012, 101, 083904 CrossRef PubMed; (d) G. Chen, H. Sasabe, W. Lu, X. Wang, J. Kido, Z. Hong and Y. Yang, J. Mater. Chem. C, 2013, 1, 6547 RSC; (e) G. Chen, H. Sasabe, X. Wang, Z. Hong and J. Kido, Synth. Met., 2014, 192, 10 CrossRef CAS PubMed; (f) G. Chen, H. Sasabe, Y. Sasaki, H. Katagiri, X. Wang, T. Sano, Z. Hong, Y. Yang and J. Kido, Chem. Mater., 2014, 26, 1356 CrossRef CAS.
- V. Shrotriya, G. Li, Y. Yao, C. Chu and Y. Yang, Appl. Phys. Lett., 2006, 88, 073508 CrossRef PubMed.
- P. Peumans, A. Yamov and S. R. Forrest, J. Appl. Phys., 2003, 93, 3693 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures; synthetic procedure and spectroscopic characterizations of SQ-RP; device performances of SQ-RP. See DOI: 10.1039/c4ra08171d |
| This journal is © The Royal Society of Chemistry 2014 |